3D Printing and Virtual Surgical Planning in Craniofacial and Thoracic Surgery: Applications to Personalised Medicine
Abstract
1. Introduction
2. Materials and Methods
2.1. Approach
2.2. Process
3. Virtual Surgical Planning, Computer-Aided Design and Manufacturing Technologies in Clinical Cases
3.1. Case 1: Skull Trauma Sequel
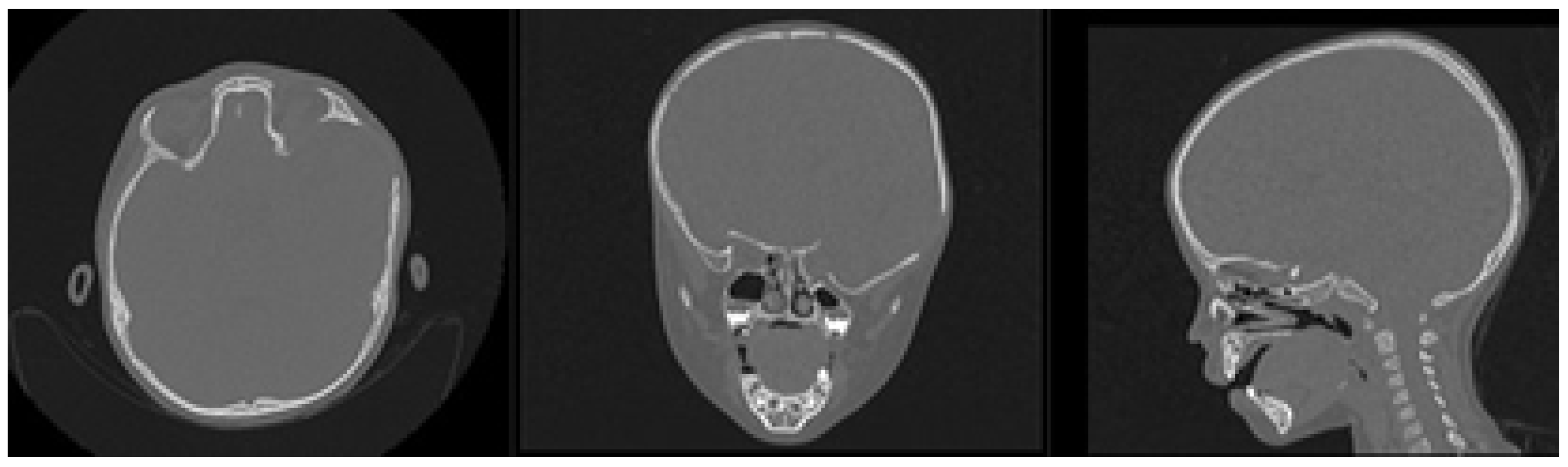
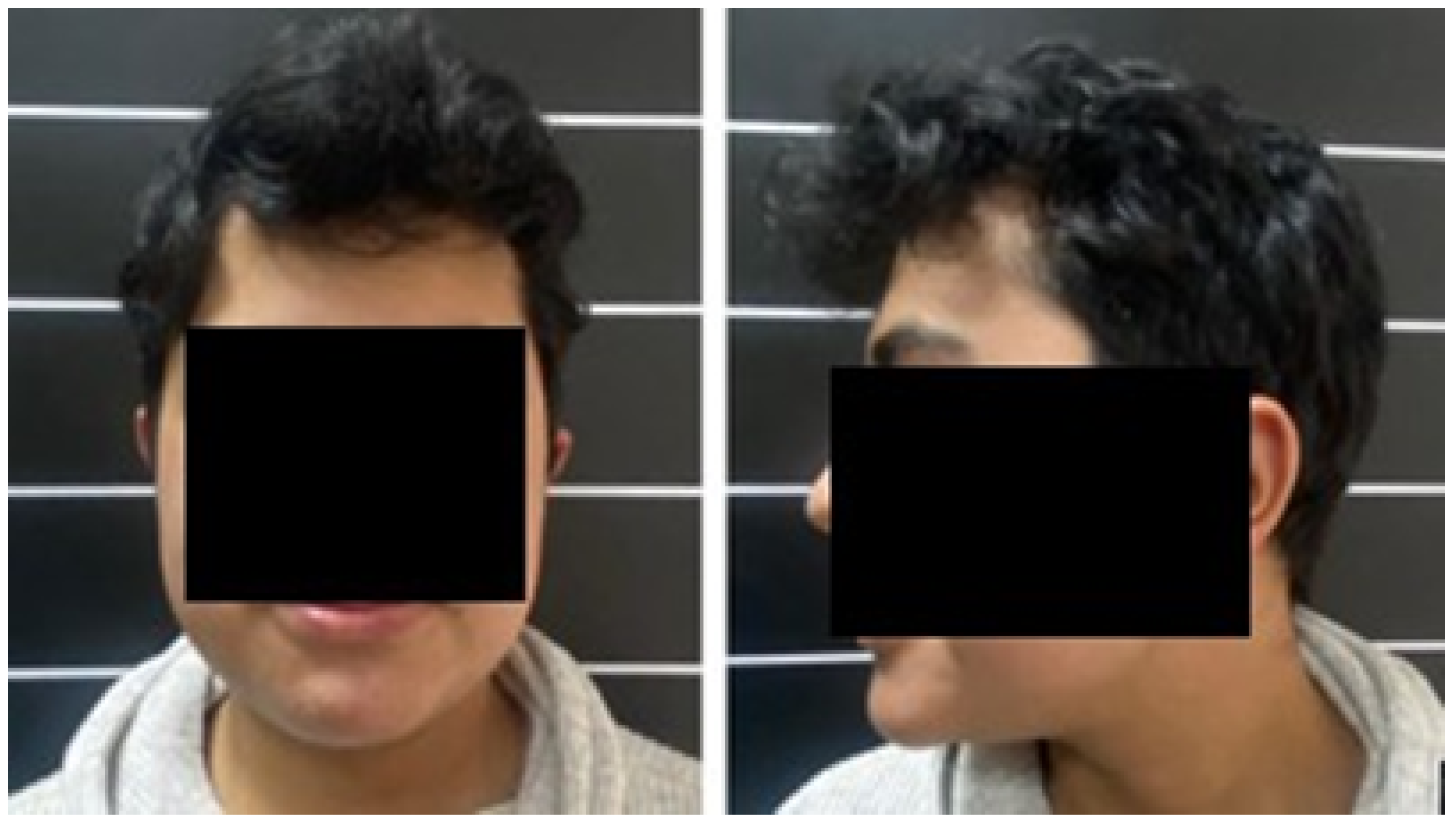
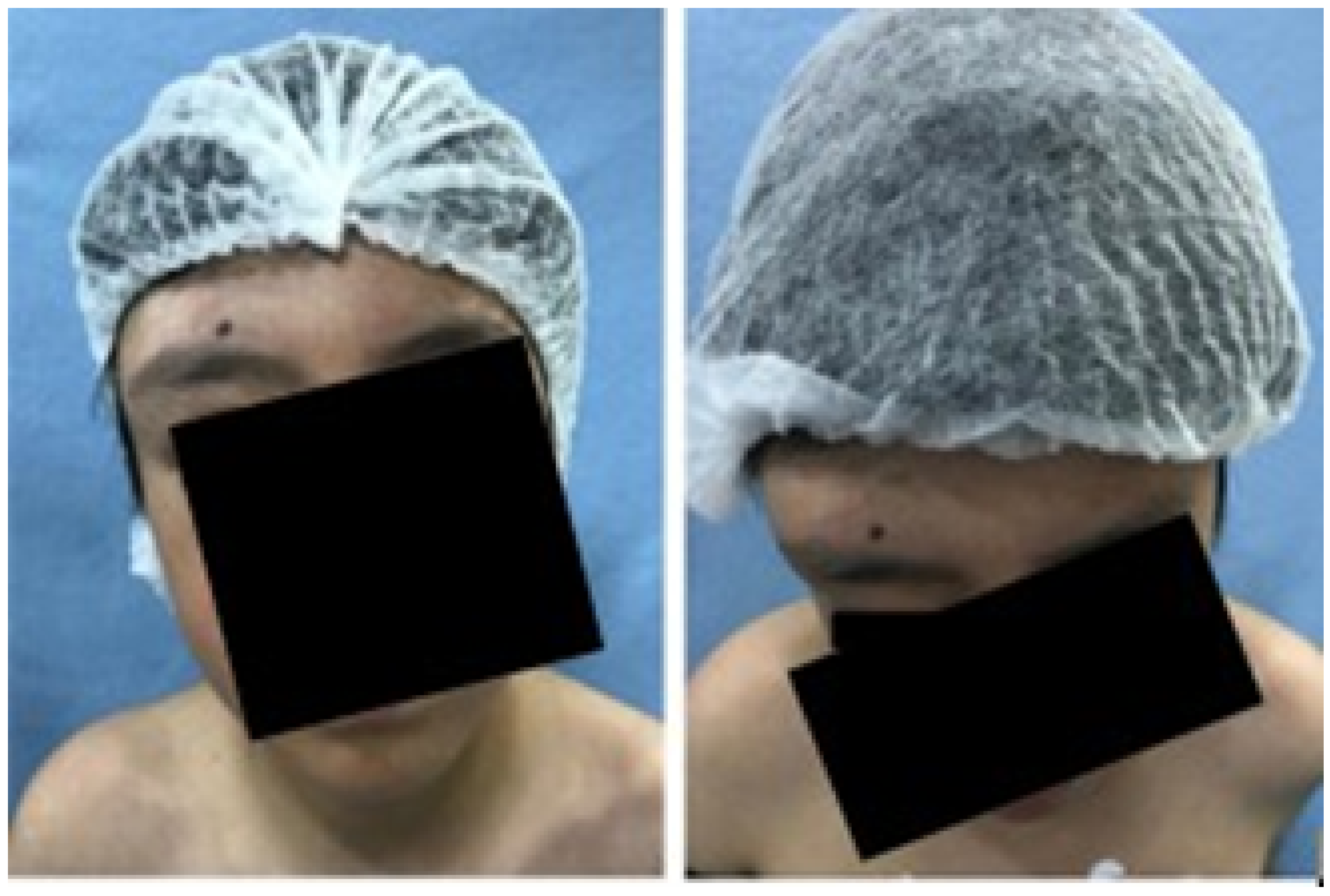
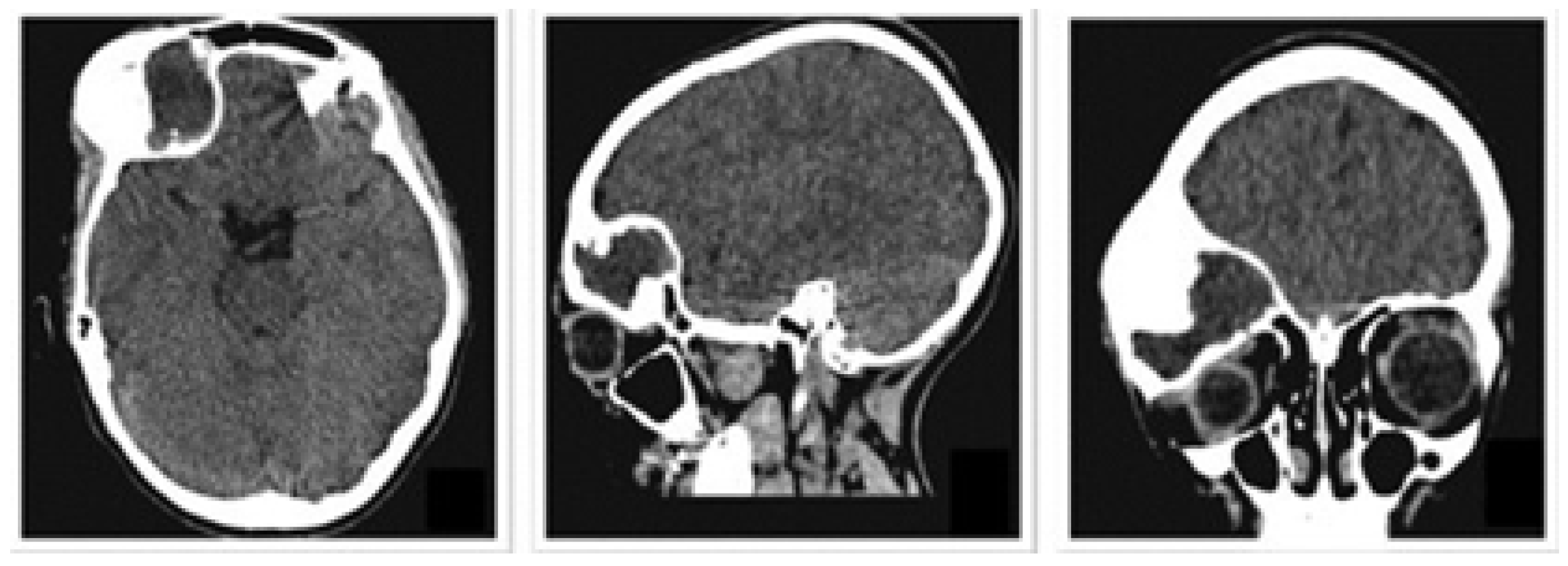
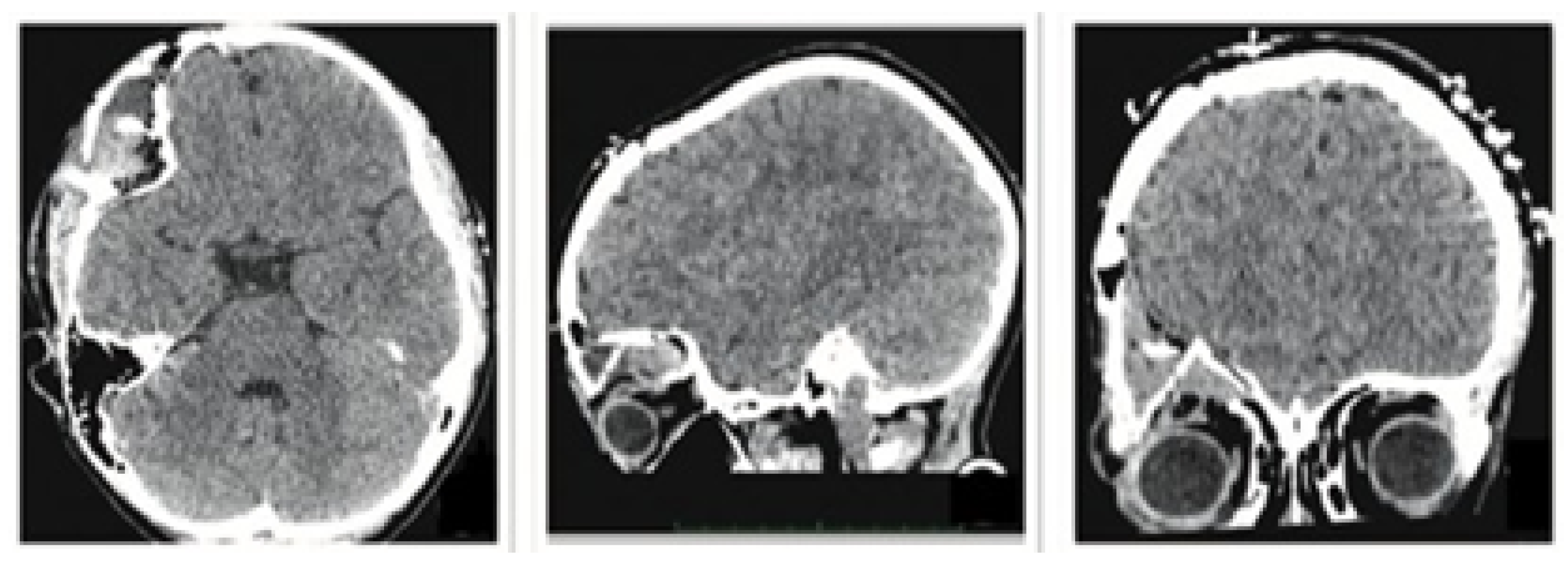
3.1.1. Diagnosis and Analysis
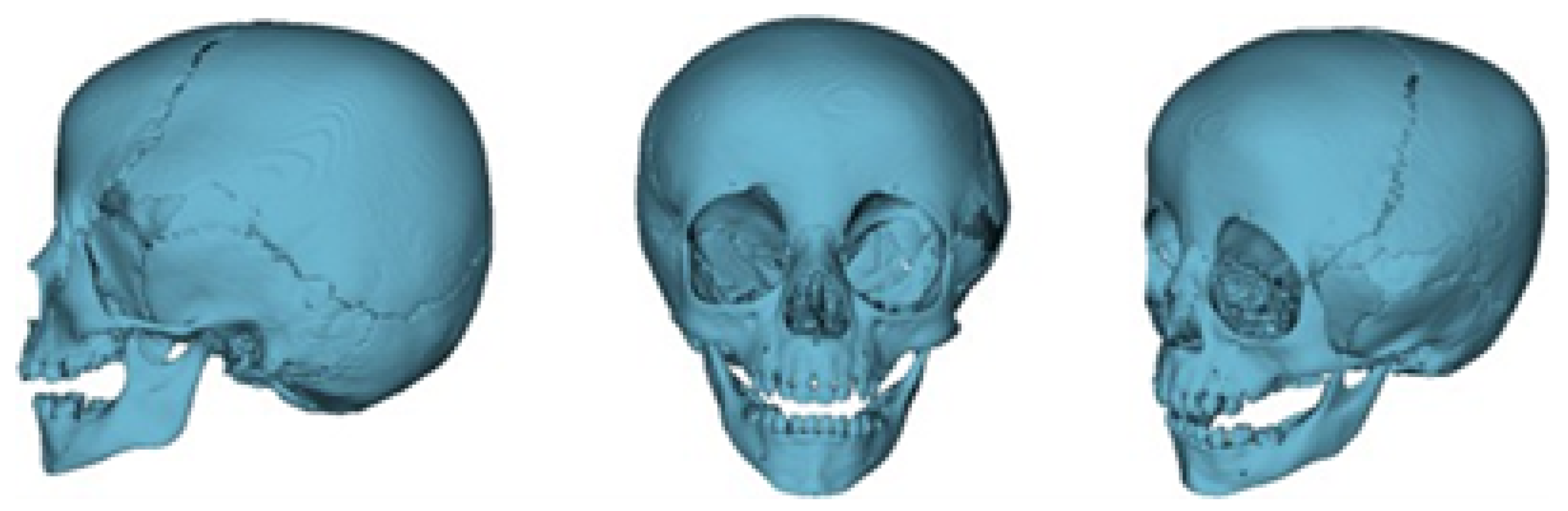
3.1.2. Surgical Planning
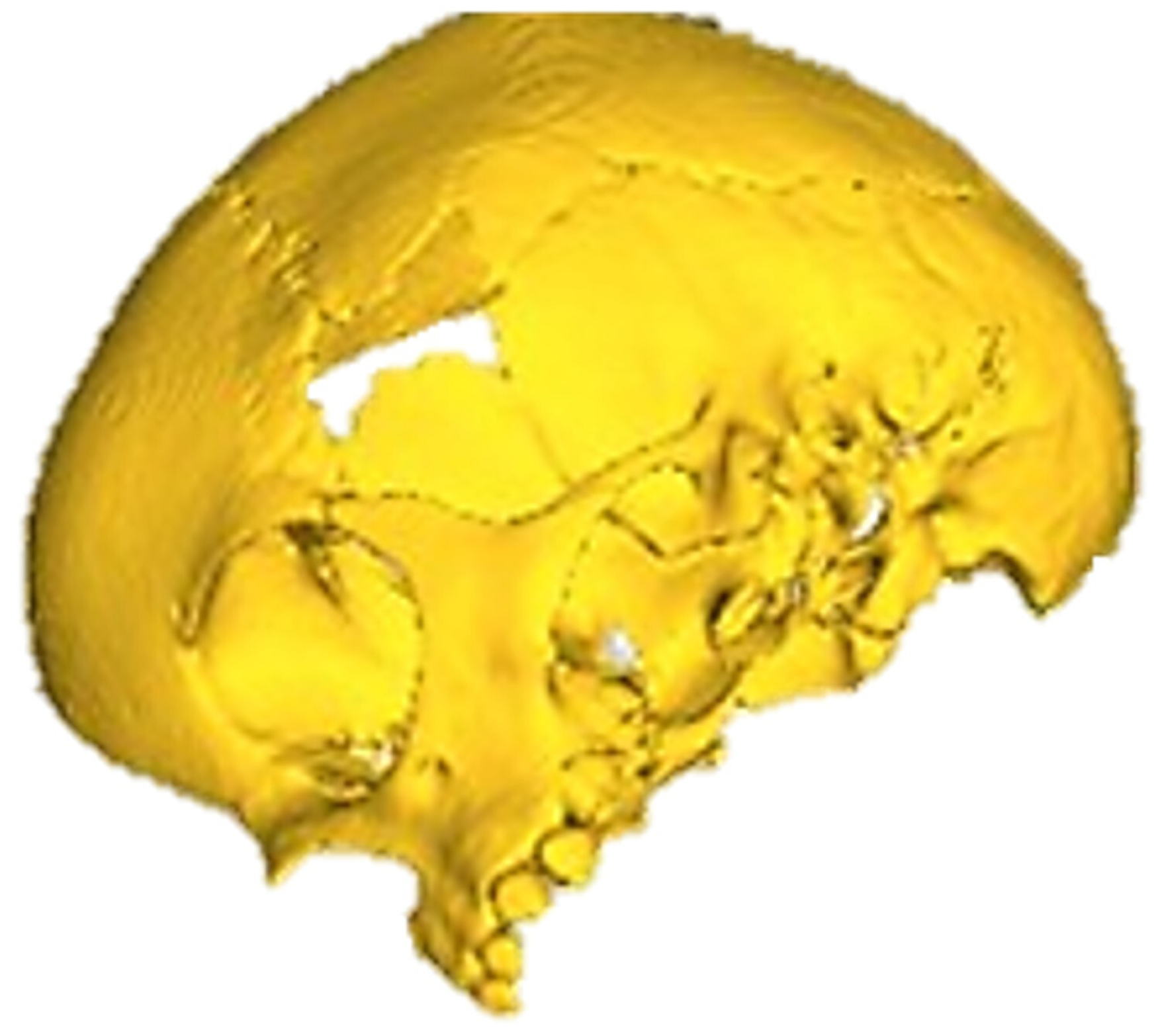
3.1.3. Planning and Printing Anatomical Model
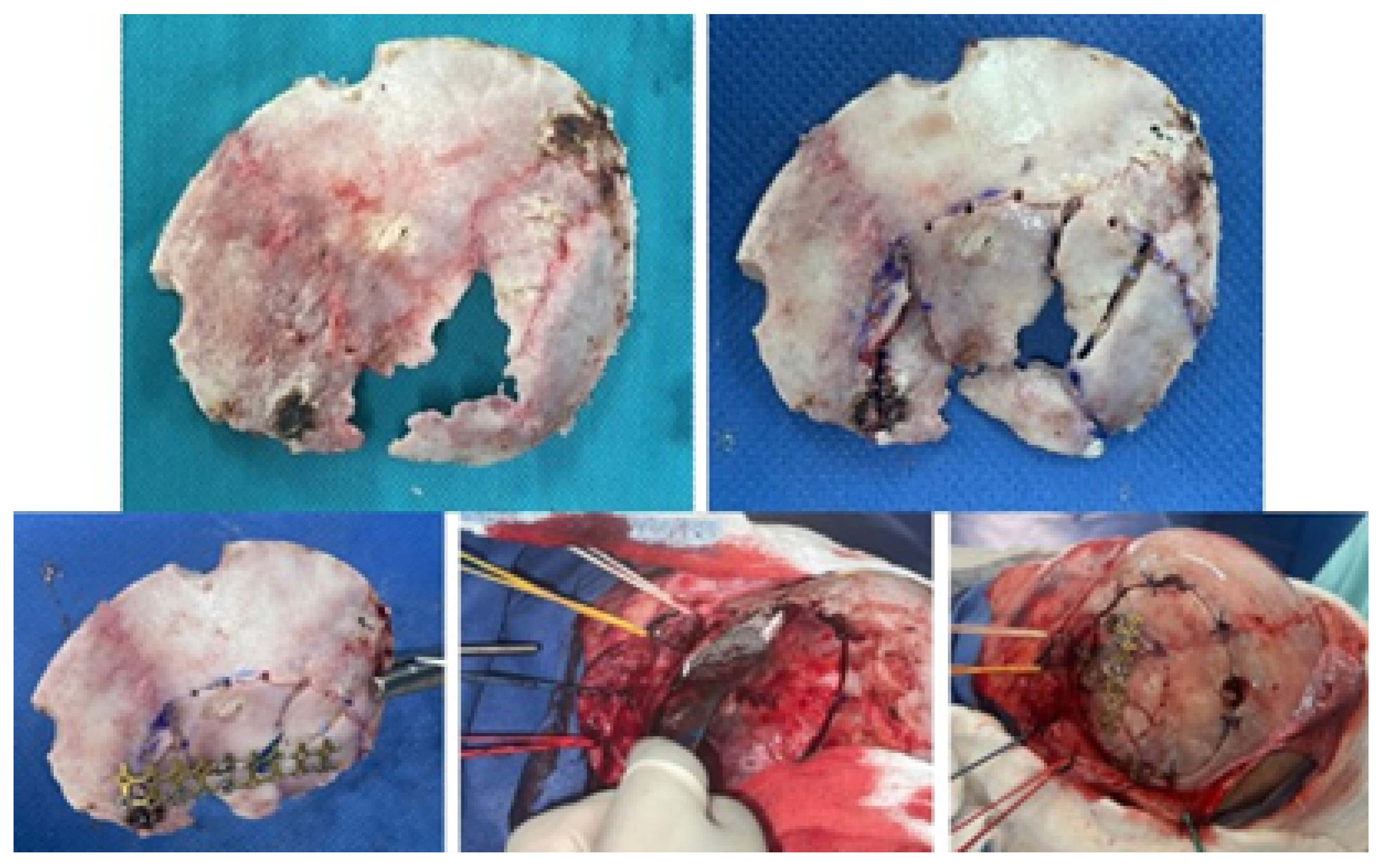
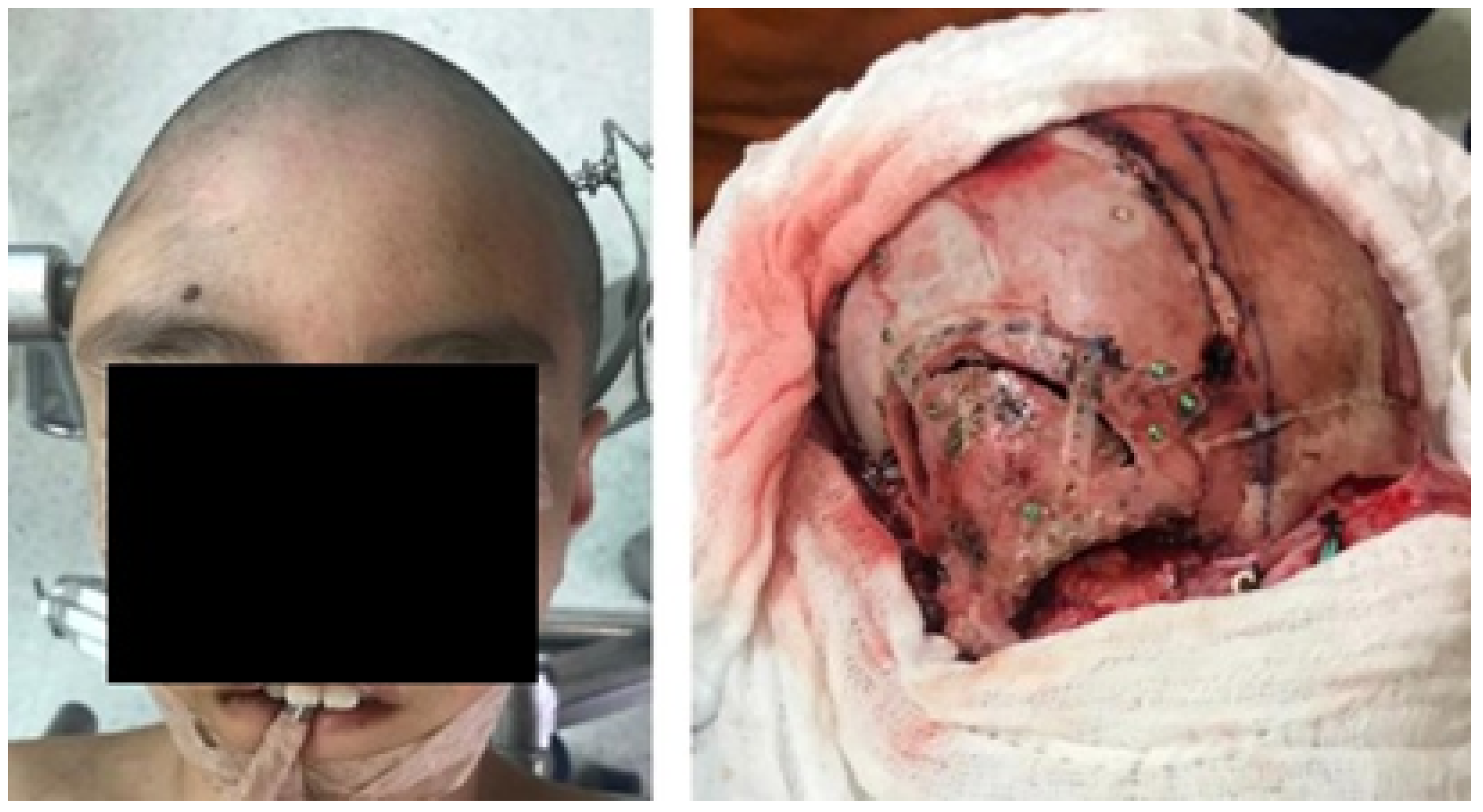
3.1.4. Intraoperative Approach
3.1.5. Postoperative
3.2. Case 2: Skull Trauma Sequel
3.2.1. Diagnosis and Analysis
3.2.2. Surgical Planning
3.2.3. Anatomical Model Printing
3.2.4. Intraoperative Approach
3.2.5. Postoperative Results
3.3. Case 3: Osteofibrous Dysplasia
3.3.1. Diagnosis and Analysis
3.3.2. Surgical Planning
3.3.3. Planning and Printing Anatomical Models
3.3.4. Intraoperative Approach
3.3.5. Postoperative Results
3.4. Case 4: Right Sternoclavicular Joint Tumour
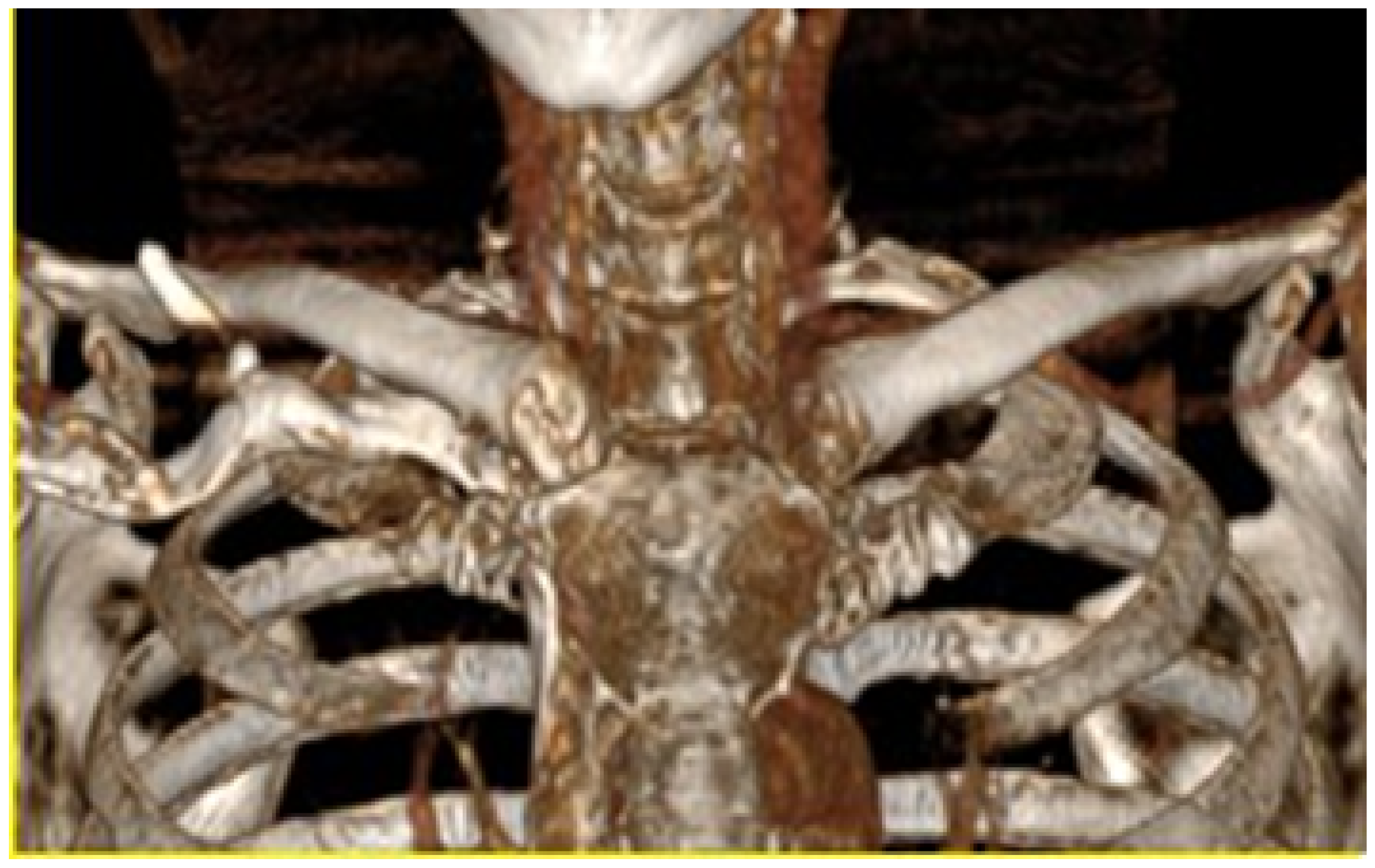
3.4.1. Diagnosis and Analysis
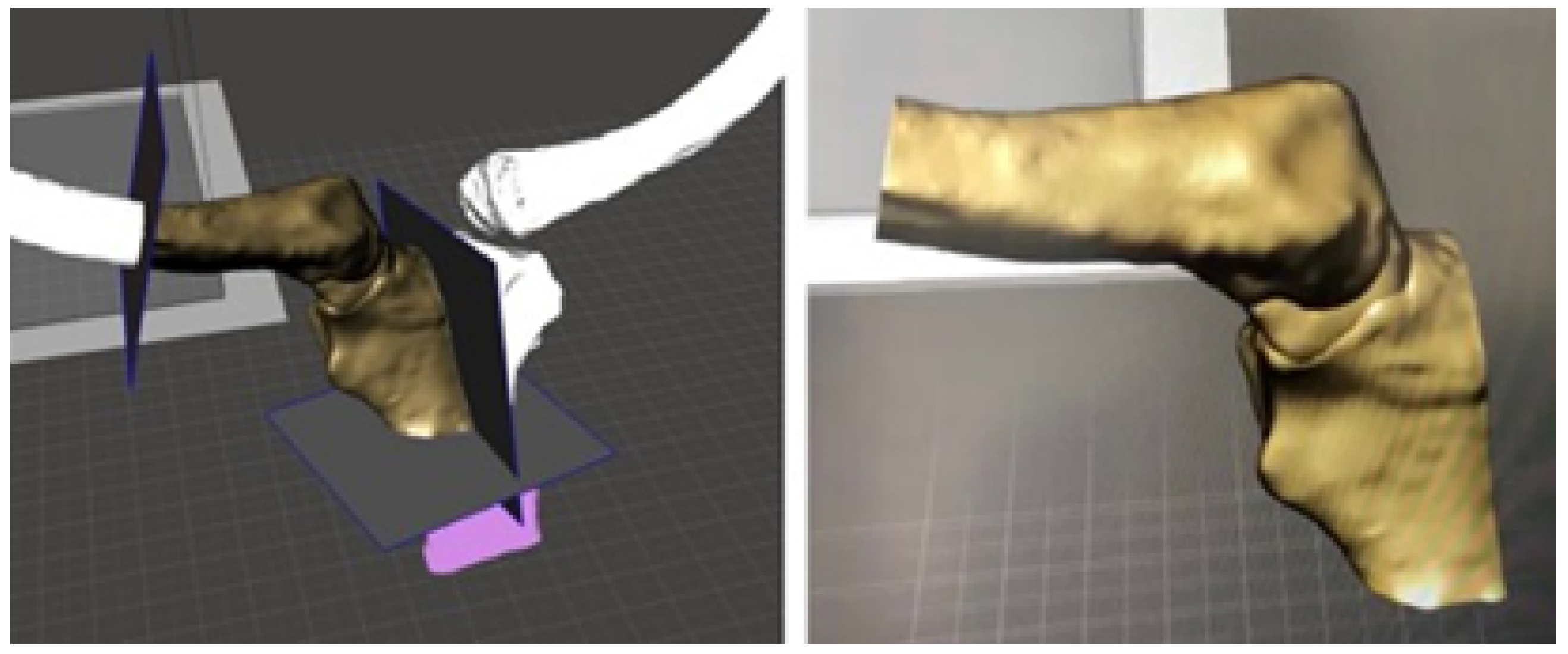
3.4.2. Surgical Planning
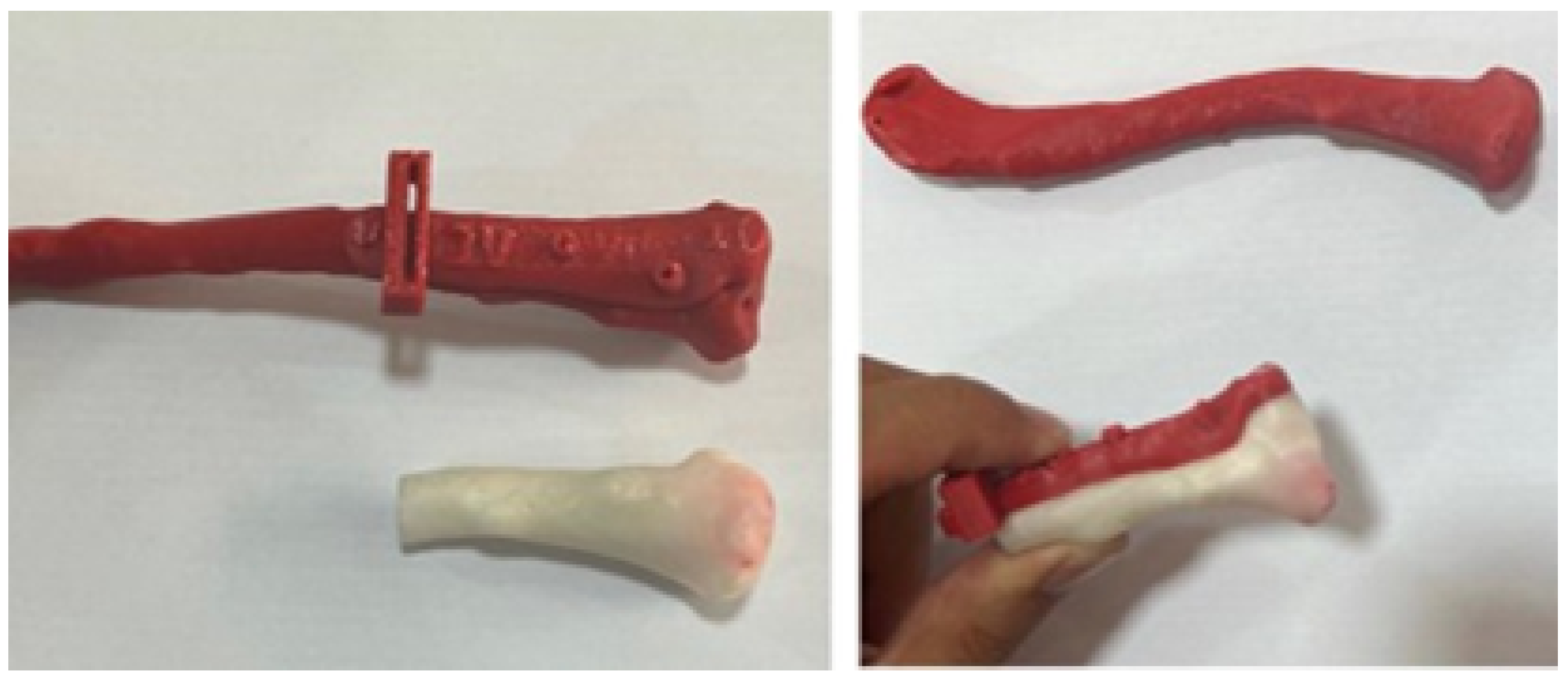
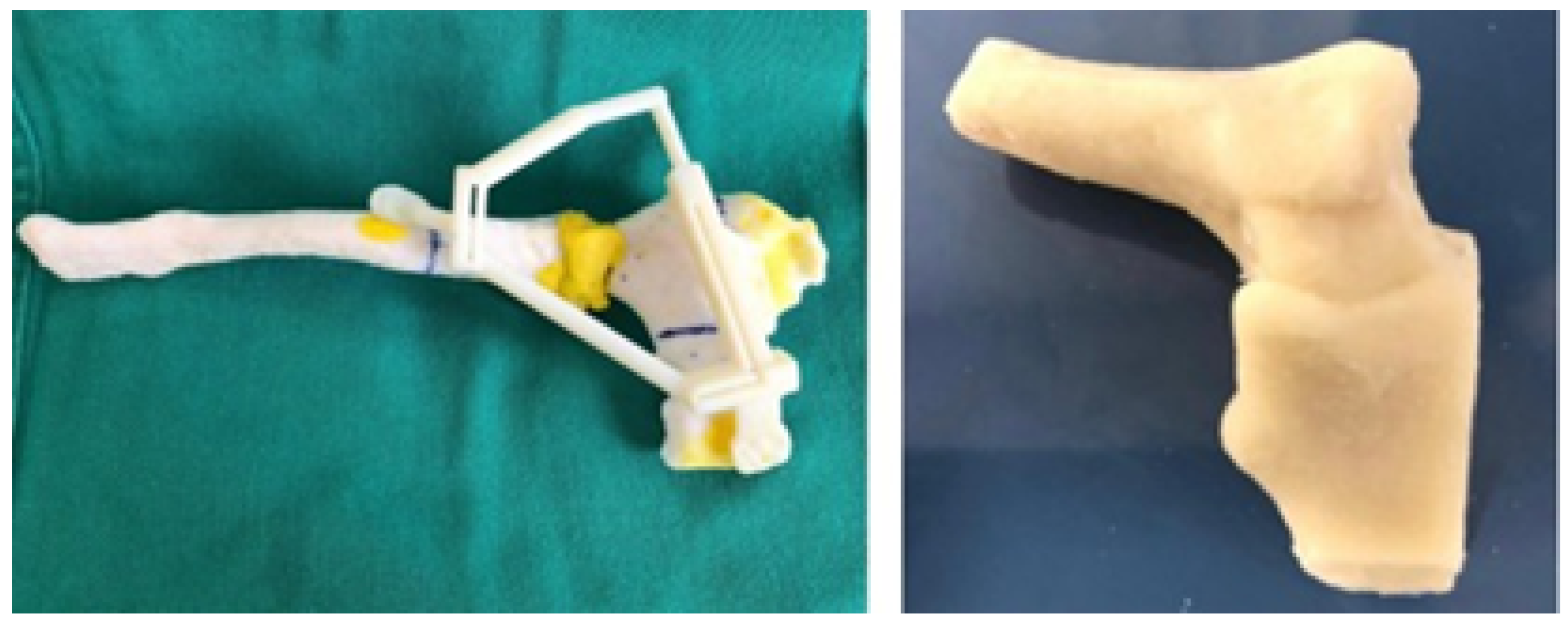
3.4.3. Design and Printing of Anatomical Models
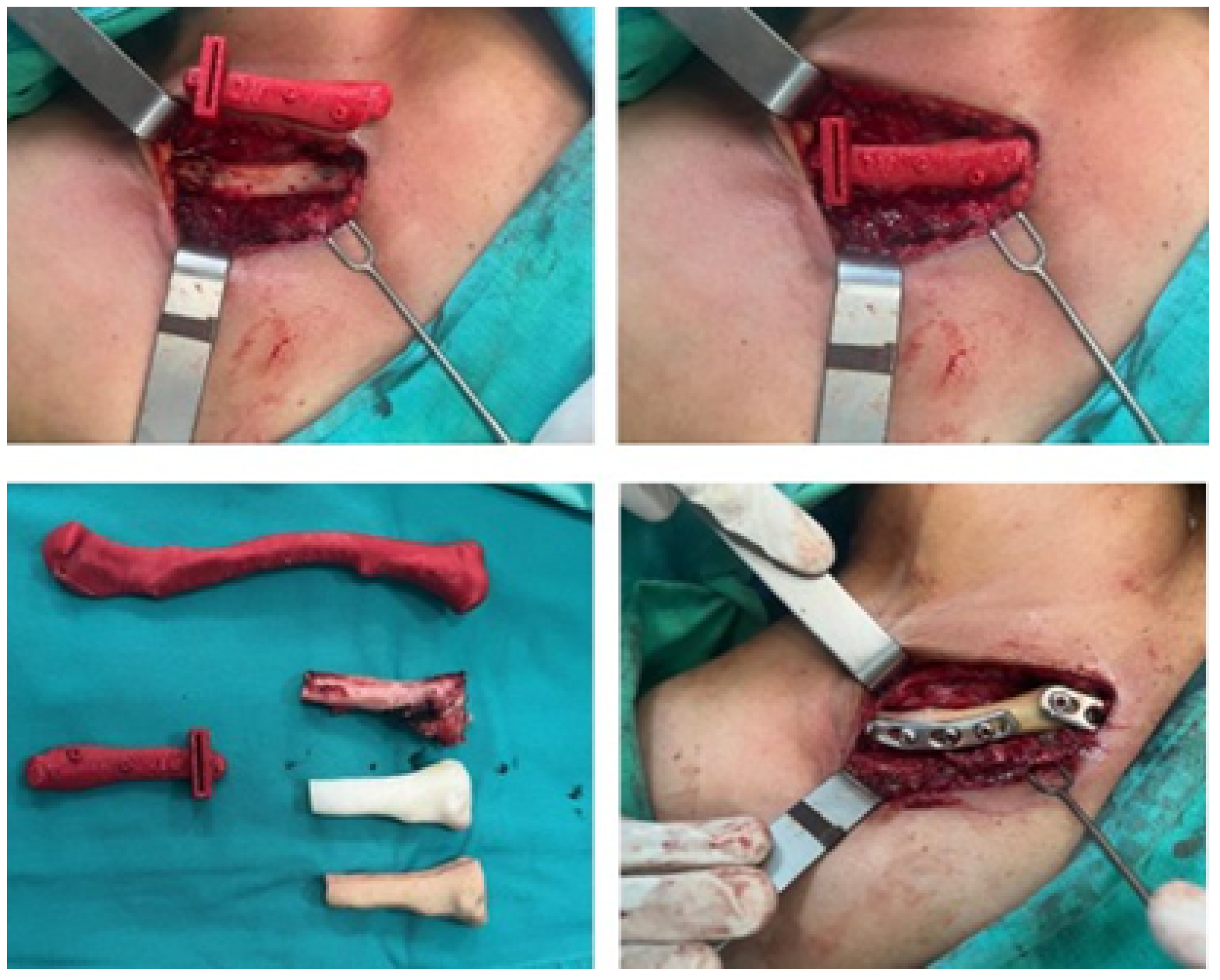
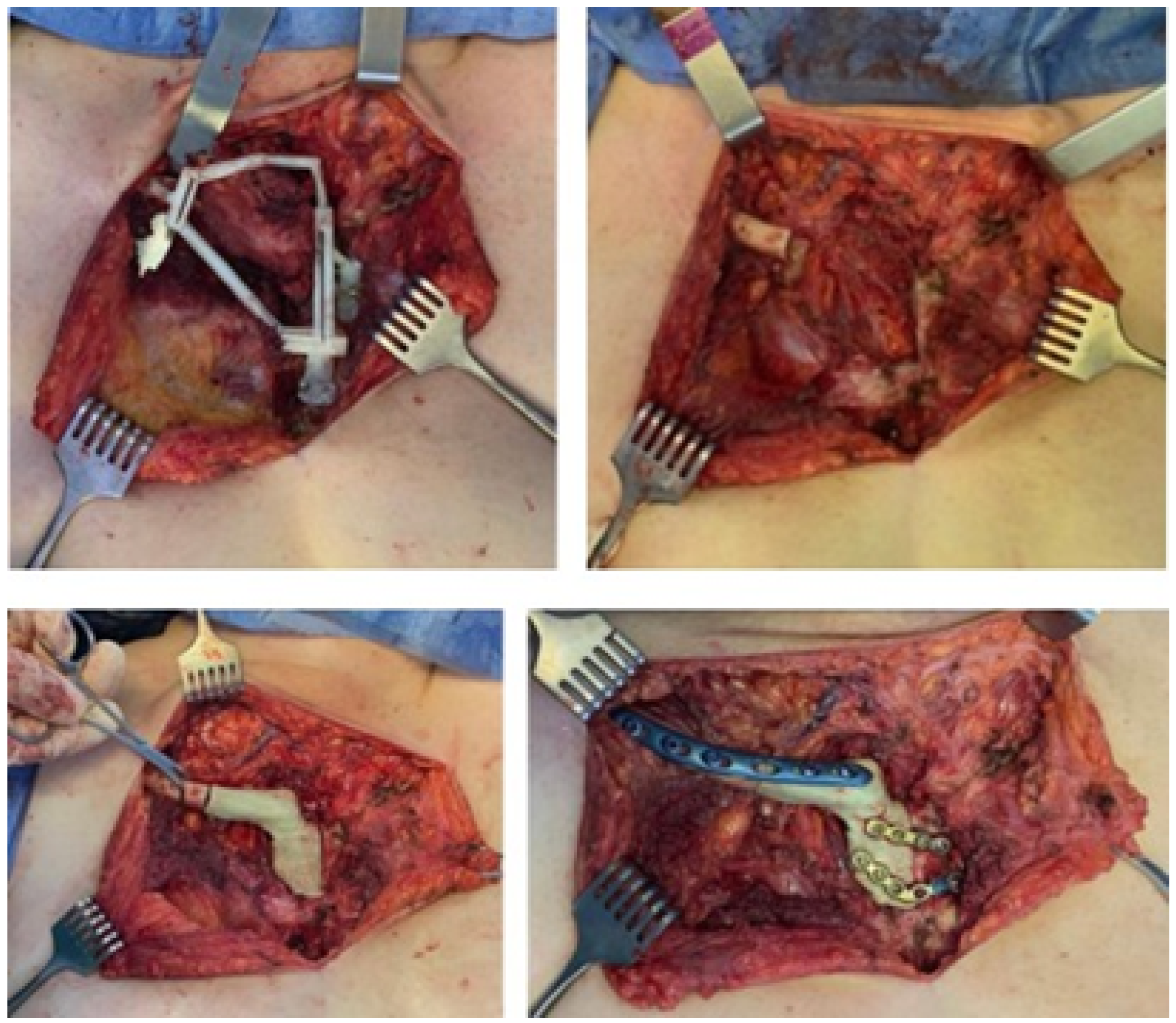
3.4.4. Intraoperative Approach
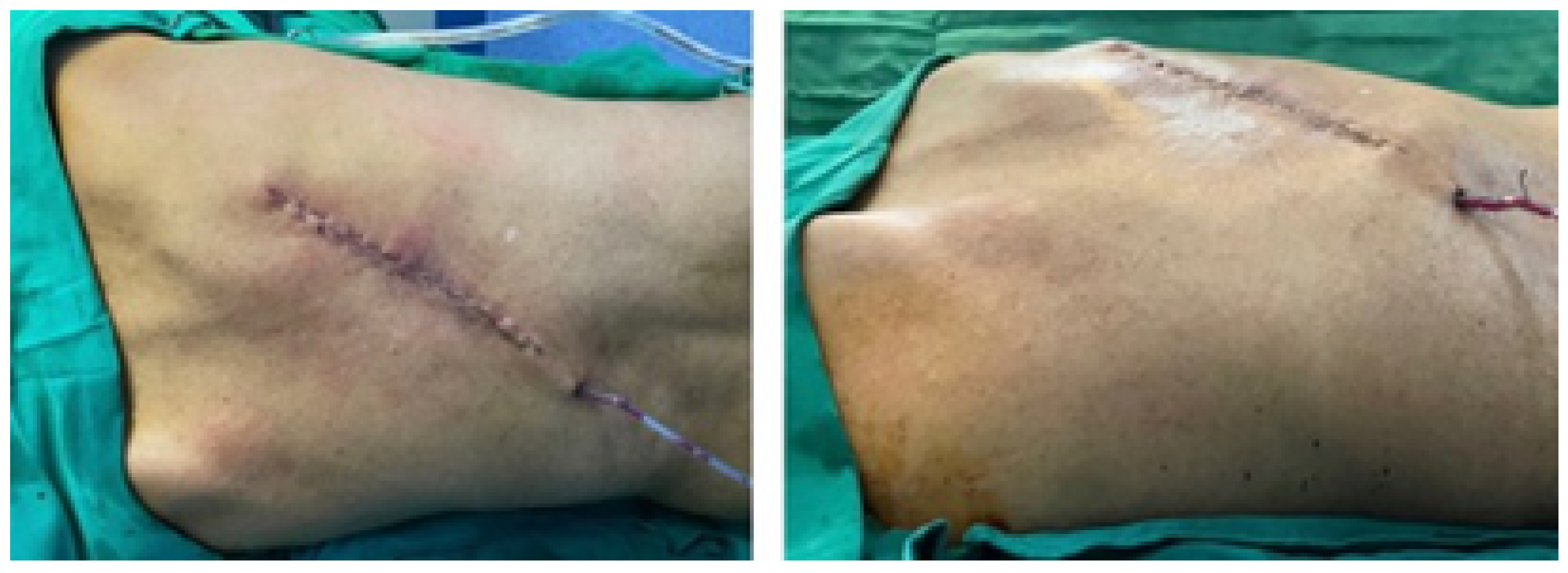
3.4.5. Postoperative Results
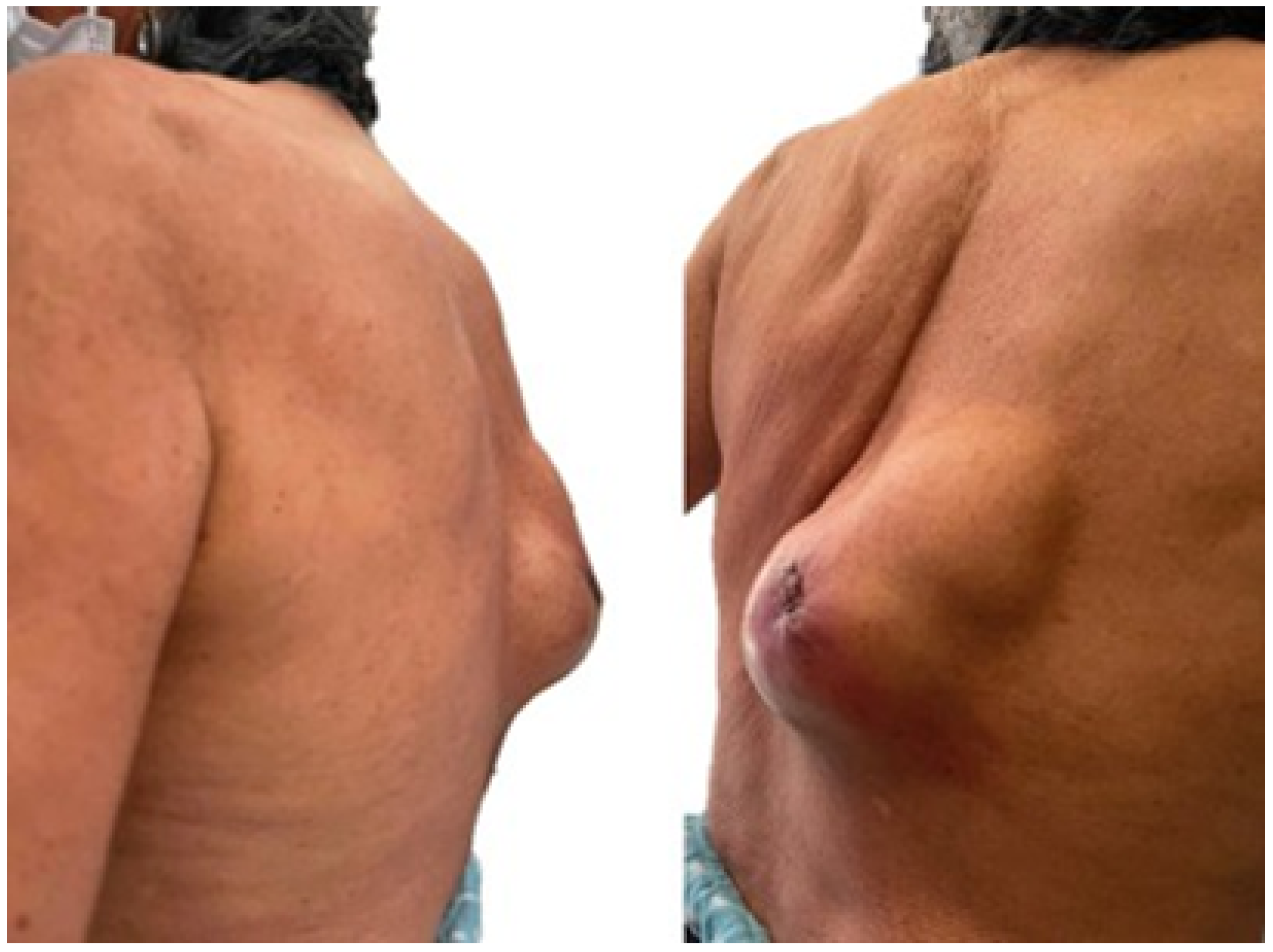
3.5. Case 5: Posterior Chest Tumour
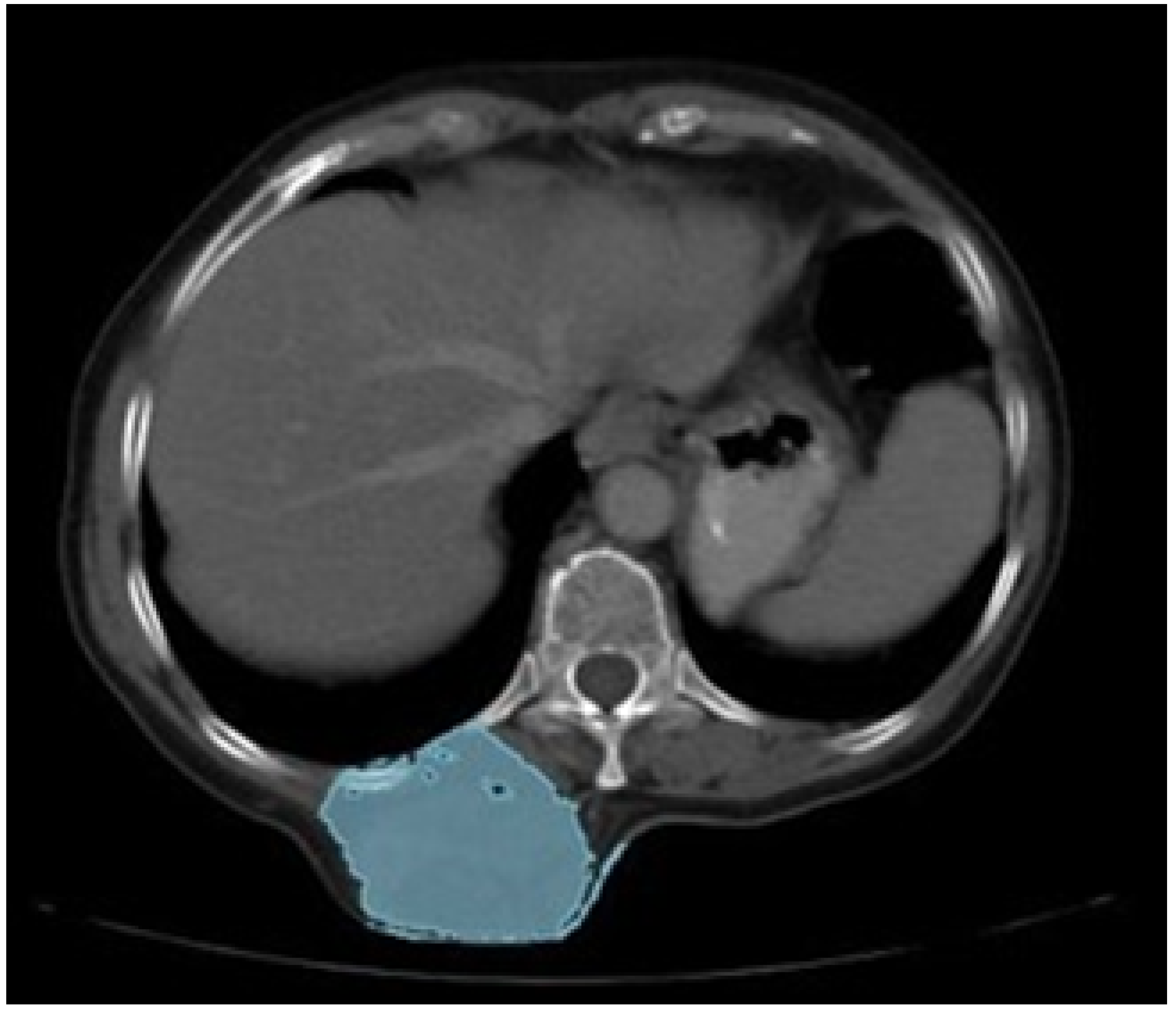
3.5.1. Diagnosis and Analysis
3.5.2. Surgical Planning
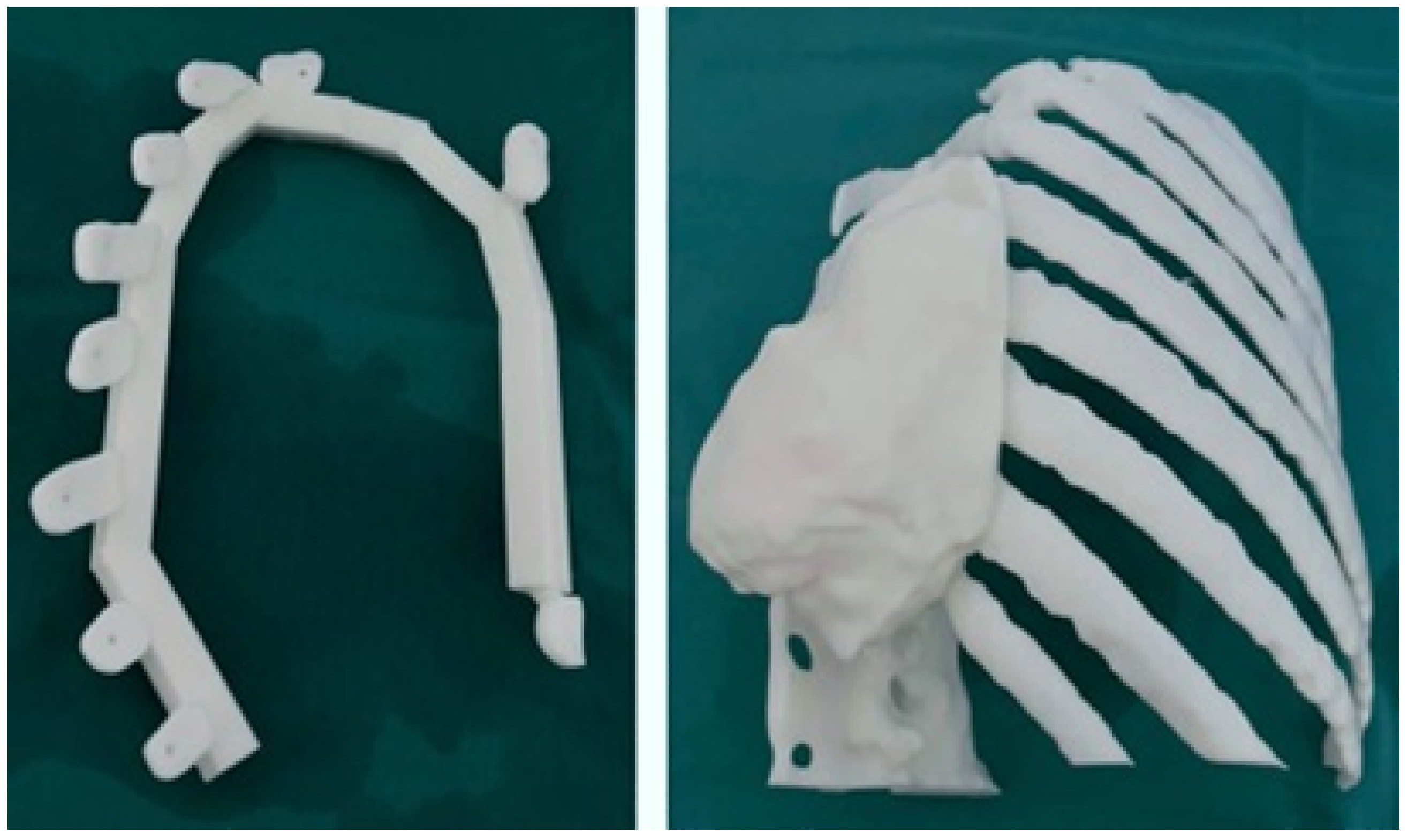
3.5.3. Design and Printing of Anatomical Models
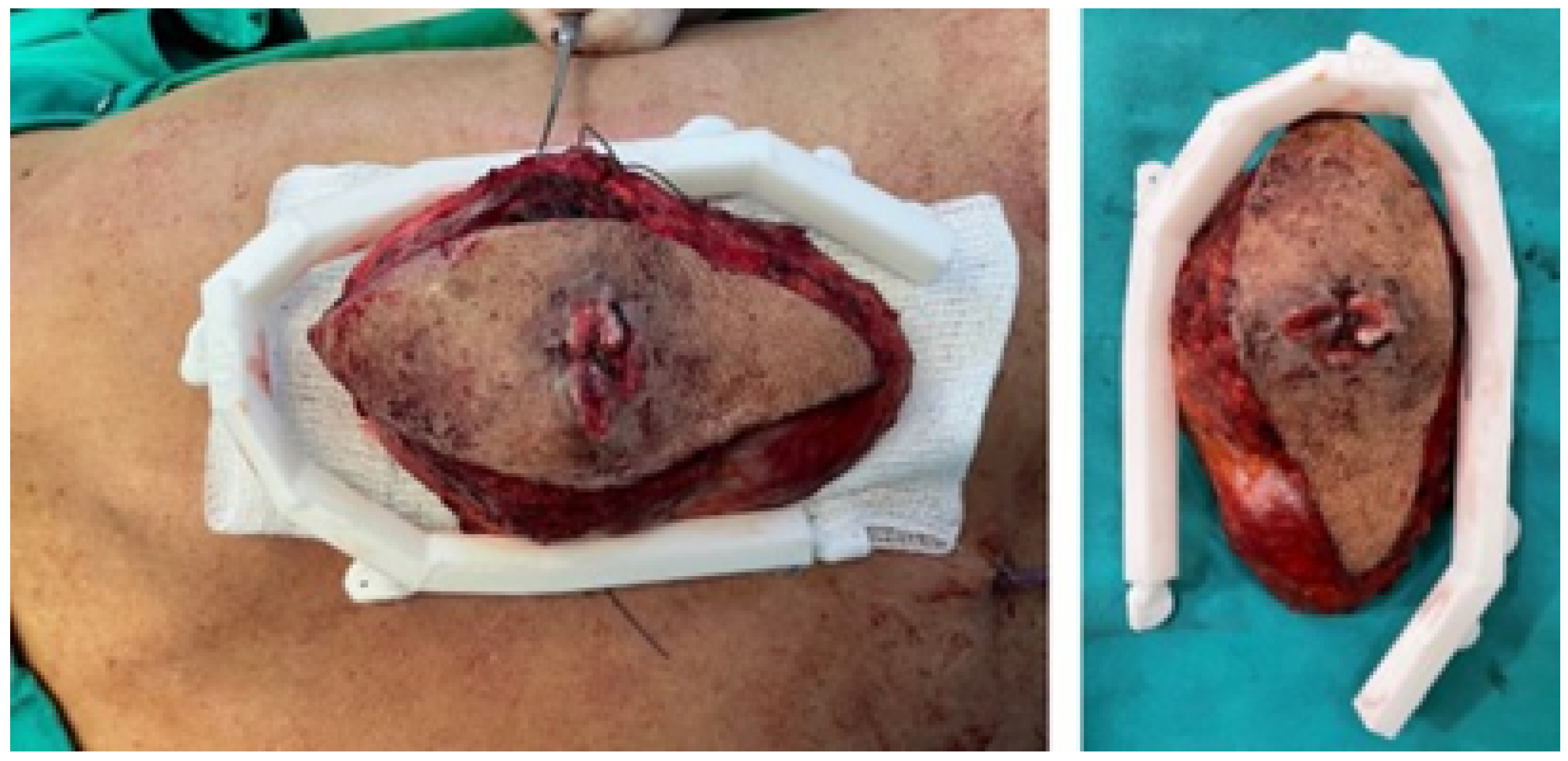
3.5.4. Intraoperative Approach
3.5.5. Postoperative Results
3.6. Case 6: Right Sternoclavicular Tumour
3.6.1. Diagnosis and Analysis
3.6.2. Surgical Planning
3.6.3. Design and Printing of Anatomical Models
3.6.4. Intraoperative Approach
3.6.5. Postoperative Results
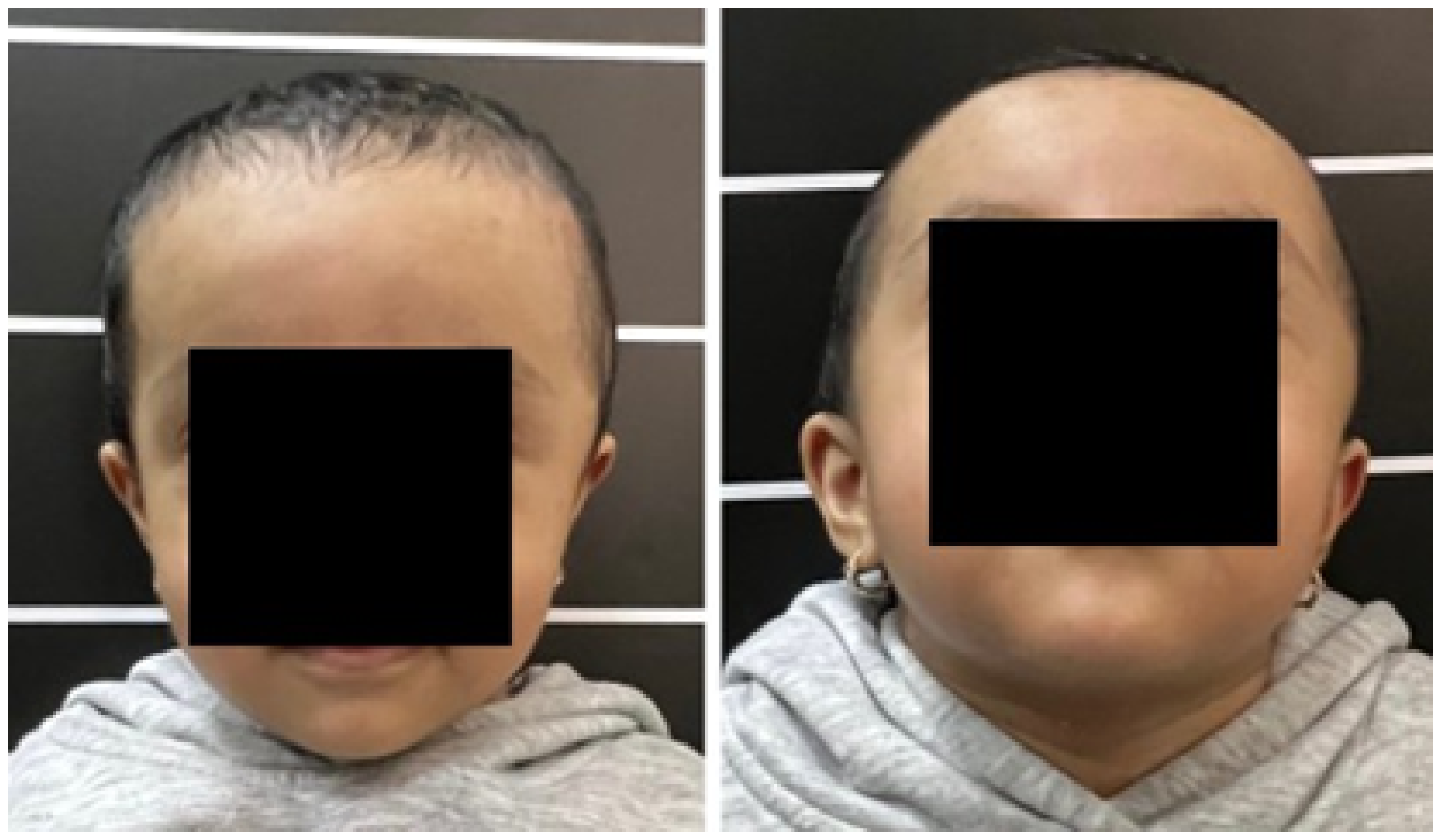
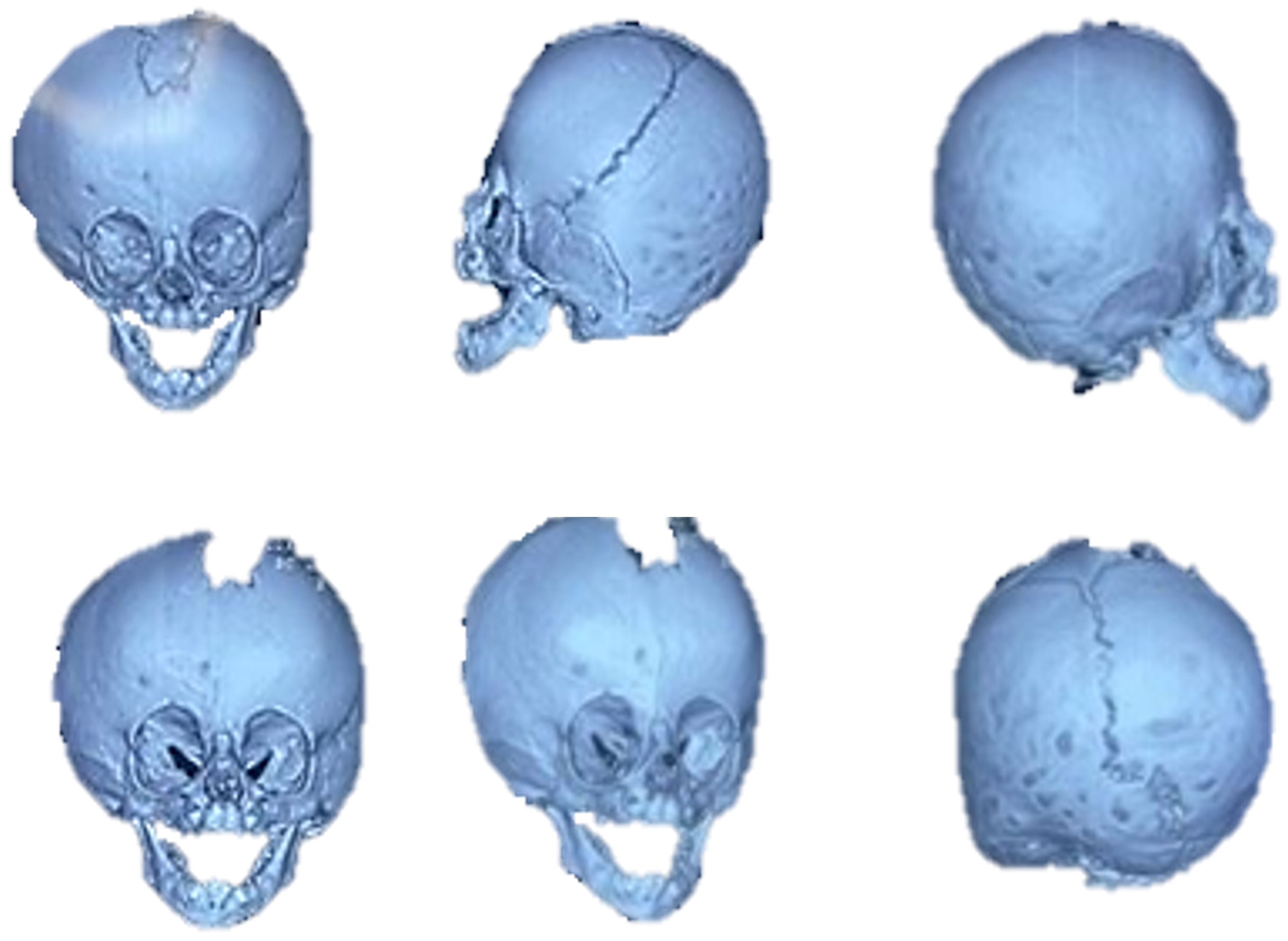
3.7. Case 7: Metopic Craniosynostosis and Trigonocephaly
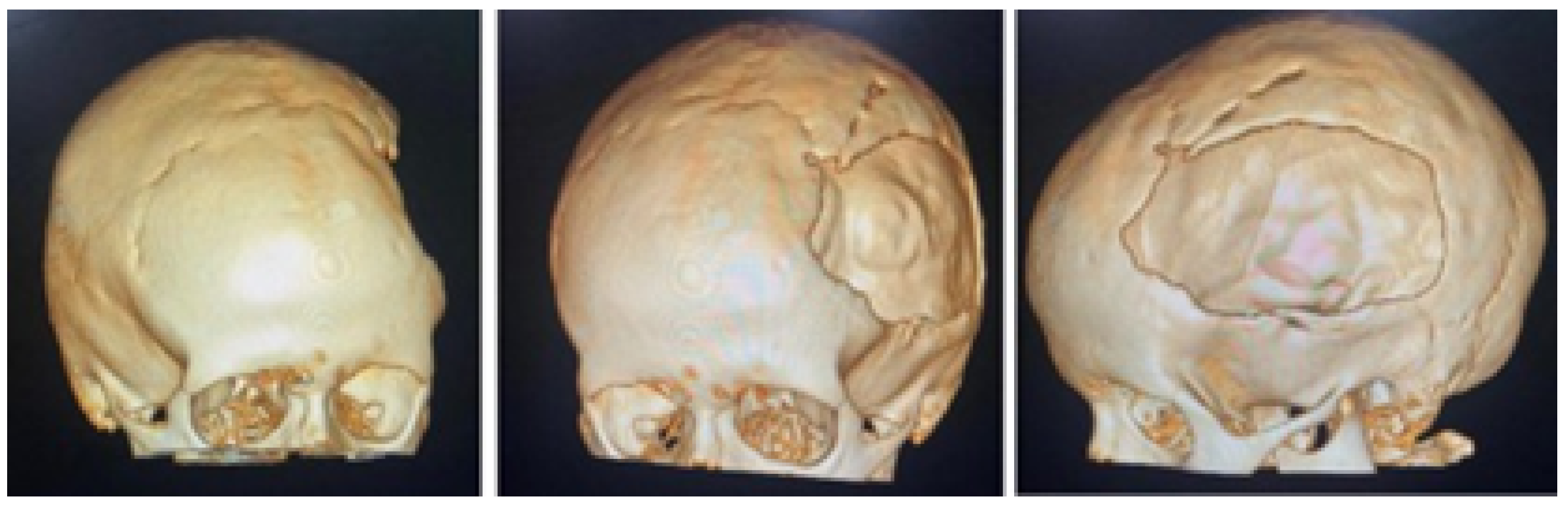
3.7.1. Diagnosis and Analysis
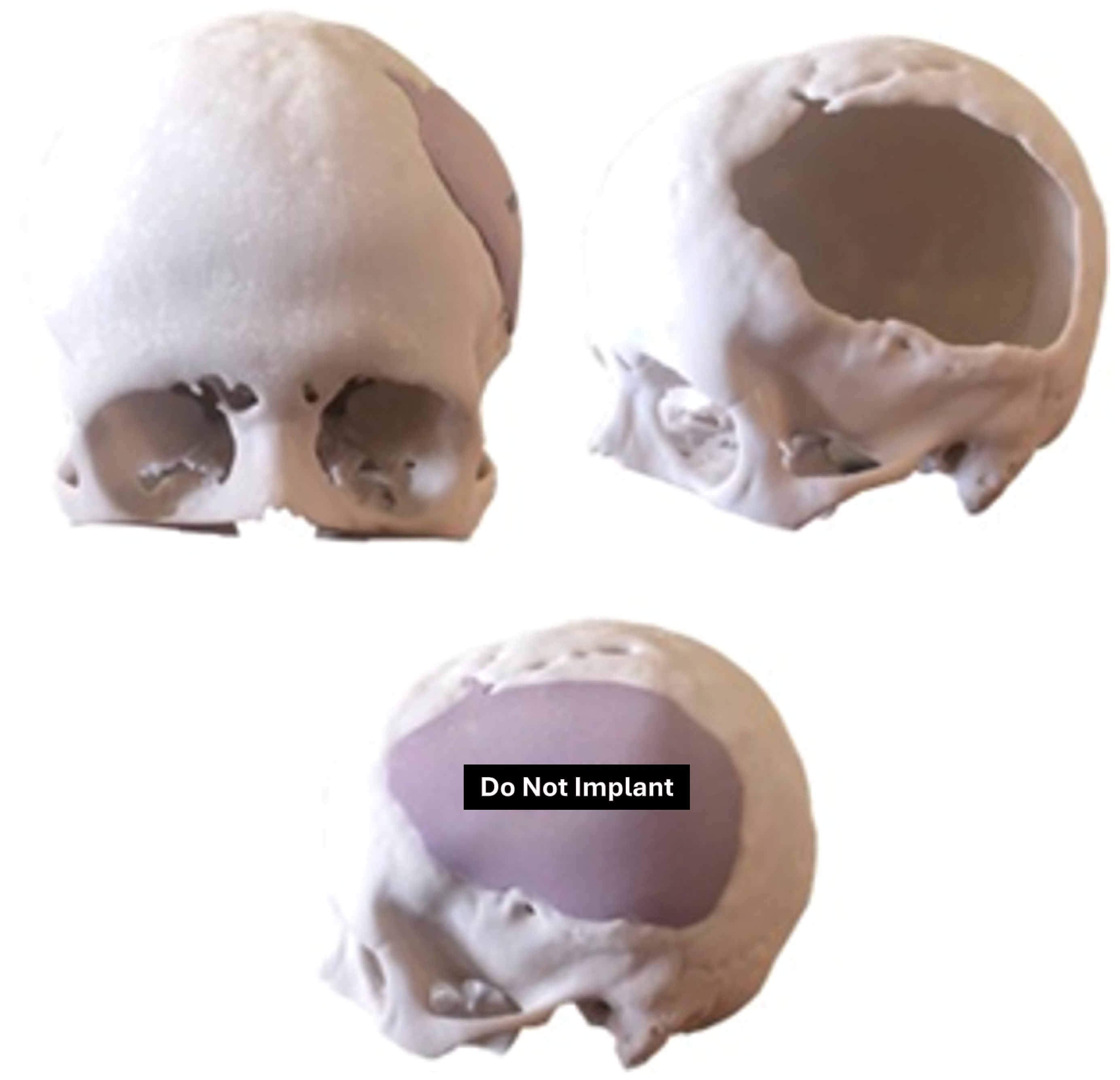
3.7.2. Surgical Planning
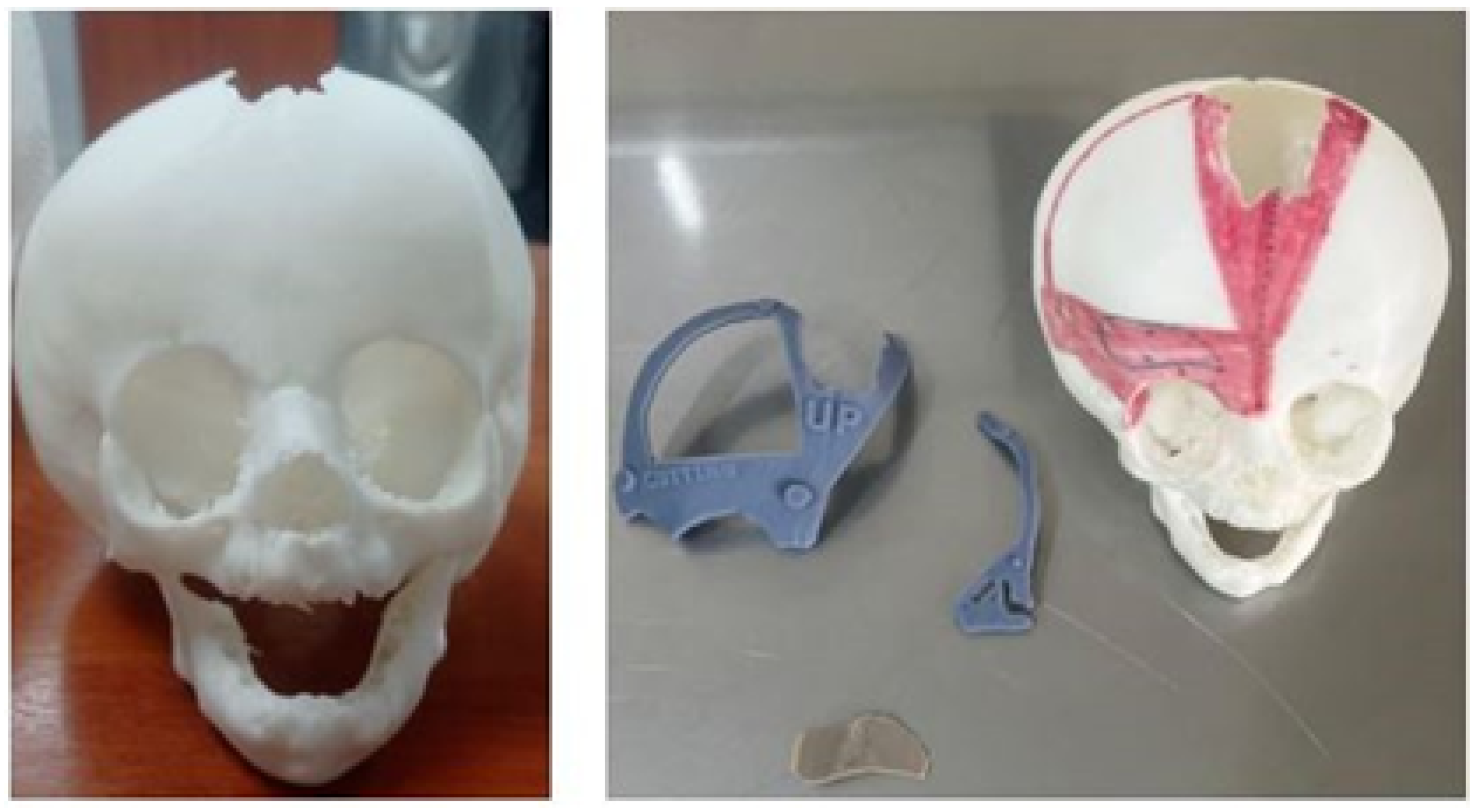
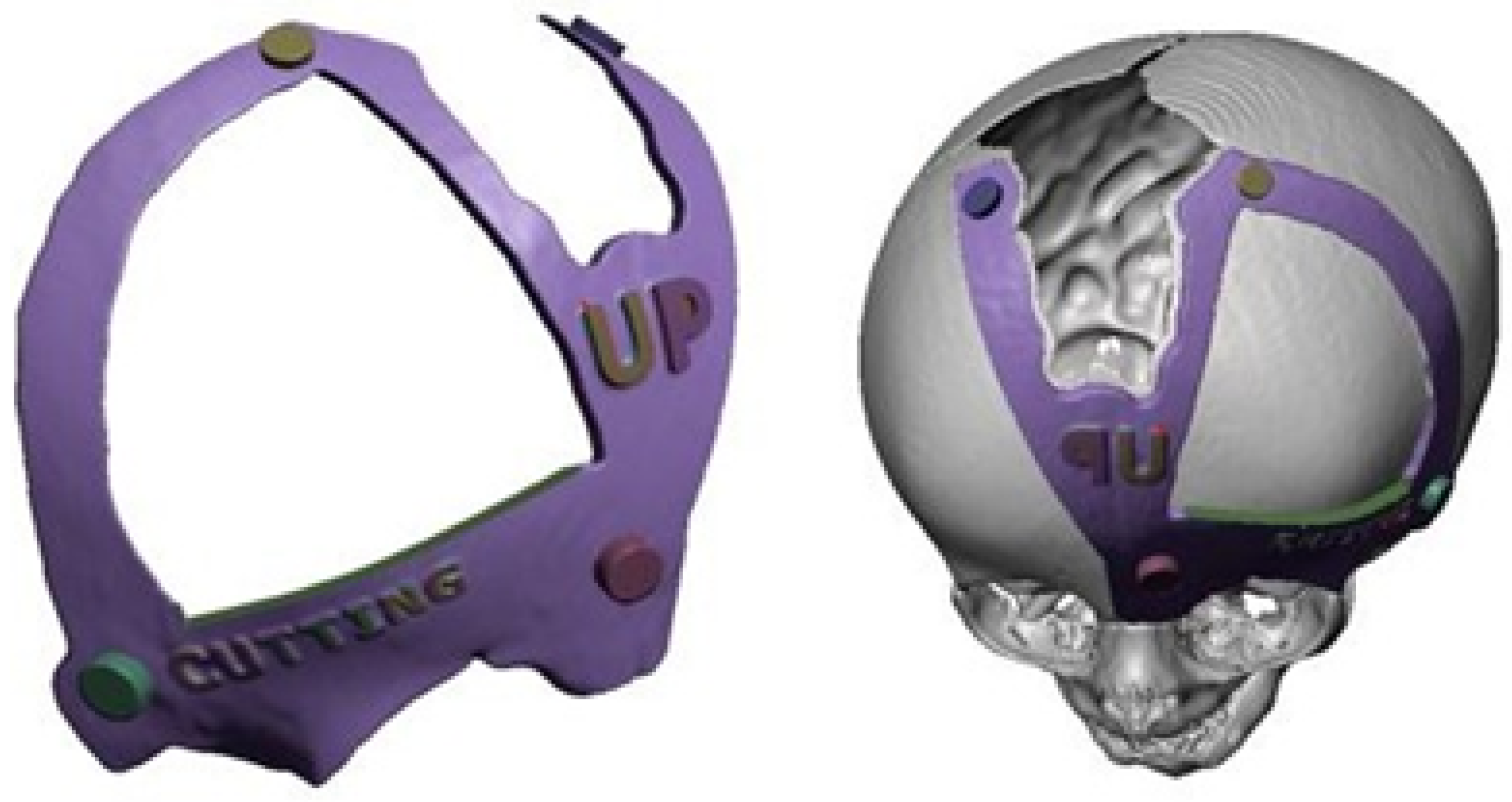
3.7.3. Design and Printing of Anatomical Models
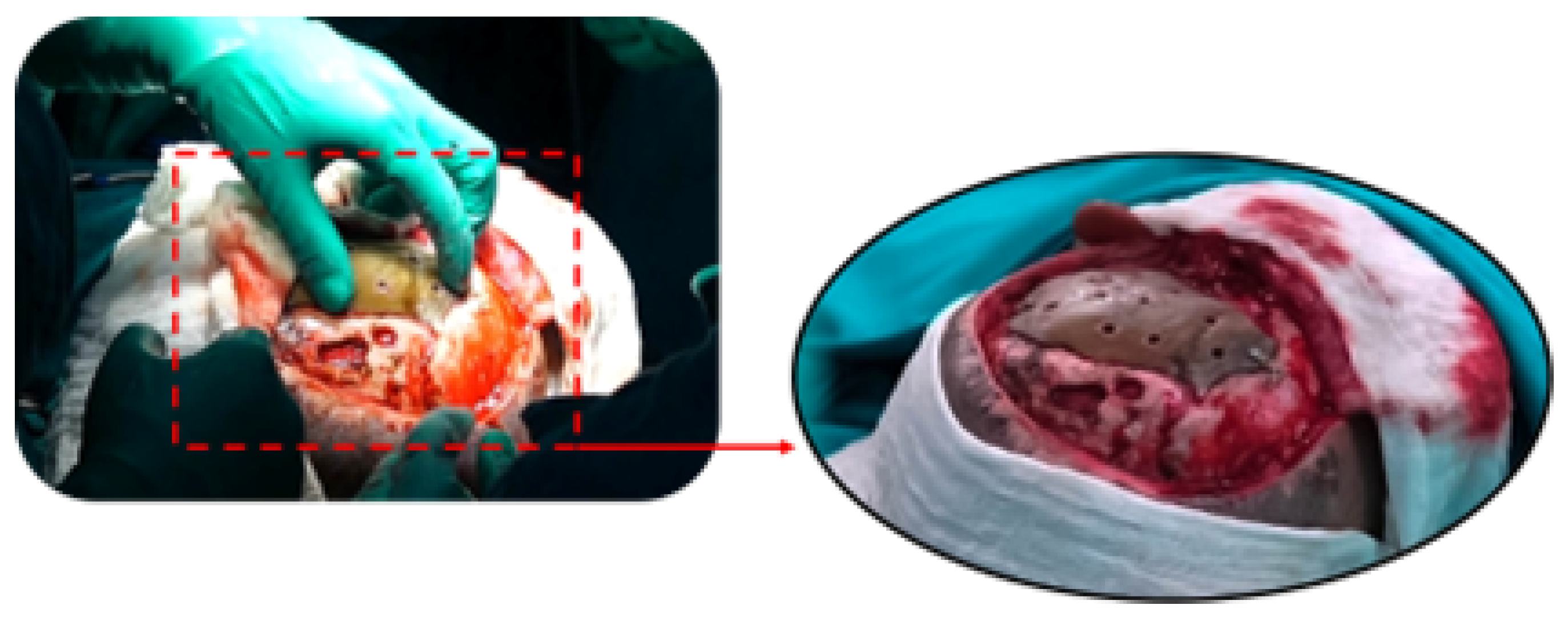
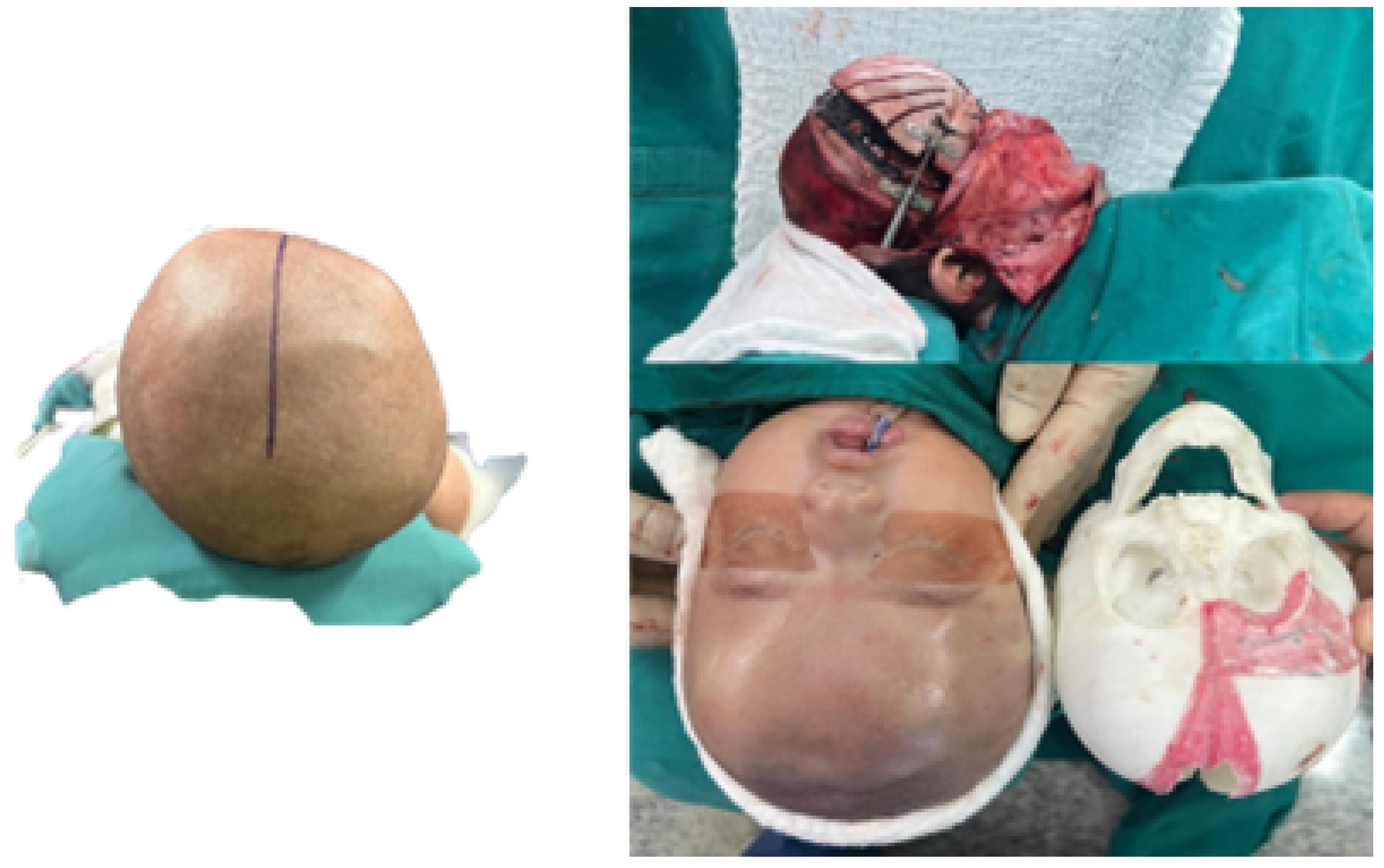
3.7.4. Intraoperative Approach
3.7.5. Posoperative Results
4. Results and Discussion
- 1.
- The utilisation of scale 1:1 models for simulated surgery and trial-and-error testing with the corresponding anatomical models is pivotal in appropriate surgical approach selection and the individualised planning of surgery for each patient.
- 2.
- The additive manufacturing of surgical guides facilitates the execution of precise incisions, with sufficient margins to ensure both oncological efficacy and defect reconstruction.
- 3.
- The fabrication of custom-made prostheses for bone reconstruction and restoration circumvents the morbidity associated with the donor site and the augmented anaesthetic-surgical time, which is concomitant with freehand manufacturing. The prosthesis is custom-made to fit each patient’s specific defect, offering both anatomical and aesthetic benefits.
4.1. Economic Analysis of 3D Surgical Planning
Implementation Costs
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Di Bella, S.; Mineo, R. The Engineer’s Point of View. In 3D Printing in Bone Surgery; Springer: Berlin/Heidelberg, Germany, 2022; pp. 39–51. [Google Scholar]
- Soni, N.; Leo, P. Artificial recreation of human organs by additive manufacturing. InMechanical Engineering in Biomedical Applications: Bio-3D Printing, Biofluid Mechanics, Implant Design, Biomaterials, Computational Biomechanics, Tissue Mechanics; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2024; pp. 23–42. [Google Scholar]
- Glennon, A.; Esposito, L.; Gargiulo, P. Implantable 3D printed devices—Technologies and applications. In Handbook of Surgical Planning and 3D Printing; Elsevier: Amsterdam, The Netherlands, 2023; pp. 383–407. [Google Scholar]
- Geng, Z.; Bidanda, B. Medical applications of additive manufacturing. In Bio-Materials and Prototyping Applications in Medicine; Springer: New York, NY, USA, 2021; pp. 97–110. [Google Scholar]
- Delgado, J.; Blasco, J.R.; Portoles, L.; Ferris, J.; Hurtos, E.; Atorrasagasti, G. FABIO project: Development of innovative customized medical devices through new biomaterials and additive manufacturing technologies. In Annals of DAAAM & Proceedings; DAAAM International Vienna: Vienna, Austria, 2010; pp. 1541–1543. [Google Scholar]
- Wolf, C.; Juchem, D.; Koster, A.; Pilloy, W. Generation of Customized Bone Implants from CT Scans Using FEA and AM. Materials 2024, 17, 4241. [Google Scholar] [CrossRef]
- Hsieh, T.y.; Dedhia, R.; Cervenka, B.; Tollefson, T.T. 3D printing: Current use in facial plastic and reconstructive surgery. Curr. Opin. Otolaryngol. Head Neck Surg. 2017, 25, 291–299. [Google Scholar] [CrossRef]
- Tack, P.; Victor, J.; Gemmel, P.; Annemans, L. 3D-printing techniques in a medical setting: A systematic literature review. Biomed. Eng. Online 2016, 15, 1–21. [Google Scholar] [CrossRef]
- Velásquez-García, L.F.; Kornbluth, Y. Biomedical applications of metal 3D printing. Annu. Rev. Biomed. Eng. 2021, 23, 307–338. [Google Scholar] [CrossRef]
- Larosa, M.; Jardini, A.; Bernardes, L.; Wolf Maciel, M.; Maciel Filho, R.; Zaváglia, C.; Zaváglia, F.; Calderoni, D.; Kharmandayan, P. Custom-built implants manufacture in titanium alloy by Direct Metal Laser Sintering (DMLS). In Proceedings of the High Value Manufacturing: Advanced Research in Virtual and Rapid Prototyping-Proceedings of the 6th International Conference on Advanced Research and Rapid Prototyping, VR@ P, Leiria, Portugal, 1–5 October 2013; Volume 2014, pp. 297–301. [Google Scholar]
- Germaini, M.M.; Belhabib, S.; Guessasma, S.; Deterre, R.; Corre, P.; Weiss, P. Additive manufacturing of biomaterials for bone tissue engineering–A critical review of the state of the art and new concepts. Prog. Mater. Sci. 2022, 130, 100963. [Google Scholar] [CrossRef]
- Sharma, S.K.; Saxena, K.K.; Dixit, A.K.; Singh, R.; Mohammed, K.A. Role of additive manufacturing and various reinforcements in MMCs related to biomedical applications. Adv. Mater. Process. Technol. 2024, 10, 231–248. [Google Scholar] [CrossRef]
- Singh, A.B. Transforming healthcare: A review of additive manufacturing applications in the healthcare sector. Eng. Proc. 2024, 72, 2. [Google Scholar]
- Cano-Vicent, A.; Tambuwala, M.M.; Hassan, S.S.; Barh, D.; Aljabali, A.A.; Birkett, M.; Arjunan, A.; Serrano-Aroca, Á. Fused deposition modelling: Current status, methodology, applications and future prospects. Addit. Manuf. 2021, 47, 102378. [Google Scholar] [CrossRef]
- Jardini, A.; Larosa, M.; Macedo, M.; Bernardes, L.; Lambert, C.; Zavaglia, C.; Maciel Filho, R.; Calderoni, D.; Ghizoni, E.; Kharmandayan, P. Improvement in cranioplasty: Advanced prosthesis biomanufacturing. Procedia Cirp 2016, 49, 203–208. [Google Scholar] [CrossRef]
- Radovan, H.; Jozef, Ž.; Teodor, T.; Jaroslav, M.; Martin, L. Evaluation of custom-made implants using industrial computed tomography. In Proceedings of the 10th International Conference on Digital Technologies 2014, Zilina, Slovakia, 9–11 July 2014; pp. 82–86. [Google Scholar]
- Hudák, R.; Živčák, J.; Tóth, T.; Majerník, J.; Lisỳ, M. Usage of industrial computed tomography for evaluation of custom-made implants. In Applications of Computational Intelligence in Biomedical Technology; Springer: Berlin/Heidelberg, Germany, 2015; pp. 29–45. [Google Scholar]
- Rontescu, C.; Amza, C.G.; Bogatu, A.M.; Cicic, D.T.; Anania, F.D.; Burlacu, A. Reconditioning by welding of prosthesis obtained through additive manufacturing. Metals 2022, 12, 1177. [Google Scholar] [CrossRef]
- Jardini, A.L.; Larosa, M.A.; de Carvalho Zavaglia, C.A.; Bernardes, L.F.; Lambert, C.S.; Kharmandayan, P.; Calderoni, D.; Maciel Filho, R. Customised titanium implant fabricated in additive manufacturing for craniomaxillofacial surgery: This paper discusses the design and fabrication of a metallic implant for the reconstruction of a large cranial defect. Virtual Phys. Prototyp. 2014, 9, 115–125. [Google Scholar] [CrossRef]
- Munsch, M. Laser additive manufacturing of customized prosthetics and implants for biomedical applications. In Laser Additive manufacturing; Elsevier: Amsterdam, The Netherlands, 2017; pp. 399–420. [Google Scholar]
- Abouel Nasr, E.; Al-Ahmari, A.M.; Moiduddin, K.; Al Kindi, M.; Kamrani, A.K. A digital design methodology for surgical planning and fabrication of customized mandible implants. Rapid Prototyp. J. 2017, 23, 101–109. [Google Scholar] [CrossRef]
- Jardini, A.L.; Larosa, M.A.; Maciel Filho, R.; de Carvalho Zavaglia, C.A.; Bernardes, L.F.; Lambert, C.S.; Calderoni, D.R.; Kharmandayan, P. Cranial reconstruction: 3D biomodel and custom-built implant created using additive manufacturing. J.-Cranio-Maxillofac. Surg. 2014, 42, 1877–1884. [Google Scholar] [CrossRef]
- Haleem, A.; Javaid, M. Role of CT and MRI in the design and development of orthopaedic model using additive manufacturing. J. Clin. Orthop. Trauma 2018, 9, 213–217. [Google Scholar] [CrossRef]
- Mendonça, C.J.A.; Setti, J.A.P. 3D printing in orthopedic surgery. In Personalized Orthopedics: Contributions and Applications of Biomedical Engineering; Springer: Berlin/Heidelberg, Germany, 2022; pp. 375–409. [Google Scholar]
- Wong, K.C. 3D-printed patient-specific applications in orthopedics. Orthop. Res. Rev. 2016, 8, 57–66. [Google Scholar] [CrossRef]
- Almeida, H.A.; Vasco, J.; Correia, M.; Ruben, R. Layer Thickness Evaluation Between Medical Imaging and Additive Manufacturing. In Proceedings of the VipIMAGE 2019: Proceedings of the VII ECCOMAS Thematic Conference on Computational Vision and Medical Image Processing, Porto, Portugal, 16–18 October 2019; Springer: Berlin/Heidelberg, Germany, 2019; pp. 693–701. [Google Scholar]
- Peel, S.; Bhatia, S.; Eggbeer, D.; Morris, D.S.; Hayhurst, C. Evolution of design considerations in complex craniofacial reconstruction using patient-specific implants. Proc. Inst. Mech. Eng. Part H J. Eng. Med. 2017, 231, 509–524. [Google Scholar] [CrossRef]
- Biscaccianti, V.; Fragnaud, H.; Hascoët, J.Y.; Crenn, V.; Vidal, L. Digital chain for pelvic tumor resection with 3D-printed surgical cutting guides. Front. Bioeng. Biotechnol. 2022, 10, 991676. [Google Scholar] [CrossRef] [PubMed]
- Popescu, D.; Laptoiu, D.; Marinescu, R.; Botezatu, I. Design and 3D printing customized guides for orthopaedic surgery–lessons learned. Rapid Prototyp. J. 2018, 24, 901–913. [Google Scholar] [CrossRef]
- Pereira, C.; Ventura, F.; Gaspar, M.; Fontes, R.; Mateus, A. Customized implant development for maxillo-mandibular reconstruction. In Virtual and Rapid Manufacturing; CRC Press: Boca Raton, FL, USA, 2007; pp. 159–166. [Google Scholar]
- Cholkar, A.; Kinahan, D.J.; Brabazon, D. Topology and FEA modeling and optimization of a patient-specific zygoma implant. In Proceedings of the 24th International Conference on Material Forming (ESAFORM 2021), Liege, Belgium, 14–16 April 2021. [Google Scholar]
- Larosa, M.A.; Jardini, A.L.; Zavaglia, C.A.d.C.; Kharmandayan, P.; Calderoni, D.R.; Maciel Filho, R. Microstructural and mechanical characterization of a custom-built implant manufactured in titanium alloy by direct metal laser sintering. Adv. Mech. Eng. 2014, 6, 945819. [Google Scholar] [CrossRef]
- Mian, S.H.; Moiduddin, K.; Abdo, B.M.; Sayeed, A.; Alkhalefah, H. Modelling and evaluation of meshed implant for cranial reconstruction. Int. J. Adv. Manuf. Technol. 2022, 118, 1967–1985. [Google Scholar] [CrossRef]
- Fedorov, A.; Beichel, R.; Kalpathy-Cramer, J.; Finet, J.; Fillion-Robin, J.C.; Pujol, S.; Bauer, C.; Jennings, D.; Fennessy, F.; Sonka, M.; et al. 3D Slicer as an image computing platform for the Quantitative Imaging Network. Magn. Reson. Imaging 2012, 30, 1323–1341. [Google Scholar] [CrossRef] [PubMed]
- Moncayo-Matute, F.P.; Pena-Tapia, P.G.; Vázquez-Silva, E.; Torres-Jara, P.B.; Abad-Farfán, G.; Moya-Loaiza, D.P.; Andrade-Galarza, A.F. Description and application of a comprehensive methodology for custom implant design and surgical planning. Interdiscip. Neurosurg. 2022, 29, 101585. [Google Scholar] [CrossRef]
- Serrano, C. Impression 3D de Dispositifs Médicaux Utilisés en Chirurgie: Quelles Recommandations Pour l’élaboration d’un Modèle d’évaluation Médico-Économique? Ph.D. Thesis, Université Paris-Saclay, Orsay, France, 2020. [Google Scholar]
- Sazzad, F.; Ramanathan, K.; Moideen, I.S.; Gohary, A.E.; Stevens, J.C.; Kofidis, T. A systematic review of individualized heart surgery with a personalized prosthesis. J. Pers. Med. 2023, 13, 1483. [Google Scholar] [CrossRef] [PubMed]
- Moncayo-Matute, F.P.; Peña-Tapia, P.G.; Vázquez-Silva, E.; Torres-Jara, P.B.; Moya-Loaiza, D.P.; Abad-Farfán, G. Surgical planning for the removal of cranial espheno-orbitary meningioma through the use of personalized polymeric prototypes obtained with an additive manufacturing technique. In Proceedings of the 2023 3rd International Conference on Electrical, Computer, Communications and Mechatronics Engineering (ICECCME), Canary Islands, Spain, 19–21 July 2023; pp. 1–7. [Google Scholar]
- Daniels, A.H.; Singh, M.; Knebel, A.; Thomson, C.; Kuharski, M.J.; De Varona, A.; Nassar, J.E.; Farias, M.J.; Diebo, B.G. Preoperative Optimization Strategies in Elective Spine Surgery. JBJS Rev. 2025, 13, e24. [Google Scholar] [CrossRef]
- Han, H.H.; Shim, J.H.; Lee, H.; Kim, B.Y.; Lee, J.S.; Jung, J.W.; Yun, W.S.; Baek, C.H.; Rhie, J.W.; Cho, D.W. Reconstruction of complex maxillary defects using patient-specific 3D-printed biodegradable scaffolds. Plast. Reconstr.-Surg. -Glob. Open 2018, 6, e1975. [Google Scholar] [CrossRef]
- Kämmerer, P.W.; Müller, D.; Linz, F.; Peron, P.F.; Pabst, A. Patient-specific 3D-printed cutting guides for high oblique sagittal osteotomy—an innovative surgical technique for nerve preservation in orthognathic surgery. J. Surg. Case Rep. 2021, 2021, rjab345. [Google Scholar] [CrossRef]
- Sharma, A.; Rickey, D.; Hayakawa, T.; Pathak, A.; Dubey, A.; Sasaki, D.; Harris, C.; Buchel, E.; Alpuche, J.; McCurdy, B.M.; et al. Utilization of 3D Printing in Surgical Oncology: An Institutional Review of Cost and Time Effectiveness. Cureus J. Med. Sci. 2017. [Google Scholar]
- Van den Broeck, J.; Wirix-Speetjens, R.; Vander Sloten, J. Preoperative analysis of the stability of fit of a patient-specific surgical guide. Comput. Methods Biomech. Biomed. Eng. 2015, 18, 38–47. [Google Scholar] [CrossRef]
- Caiti, G.; Dobbe, J.G.; Strijkers, G.J.; Strackee, S.D.; Streekstra, G.J. Positioning error of custom 3D-printed surgical guides for the radius: Influence of fitting location and guide design. Int. J. Comput. Assist. Radiol. Surg. 2018, 13, 507–518. [Google Scholar] [CrossRef]
- Teoh, S. Fatigue of biomaterials: A review. Int. J. Fatigue 2000, 22, 825–837. [Google Scholar] [CrossRef]
- Zoabi, A.; Redenski, I.; Oren, D.; Kasem, A.; Zigron, A.; Daoud, S.; Moskovich, L.; Kablan, F.; Srouji, S. 3D printing and virtual surgical planning in oral and maxillofacial surgery. J. Clin. Med. 2022, 11, 2385. [Google Scholar] [CrossRef]
- Guzman-Bautista, A.; López-Arrabal, A.; Sanchez-Oro-Aguado, E.; Fernández Gorgojo, A.; García-Galán, R.; Badesa, F.J.; Vizan-Idoipe, A. Quasi-Uniform Density Non-Solid Infill Strategy for Axisymmetric Non-Planar Additive Manufacturing. Appl. Sci. 2025, 15, 5899. [Google Scholar] [CrossRef]
- Franco-Martínez, F.; Solórzano-Requejo, W.; de Blas-de Miguel, A.; Vostatek, M.; Grasl, C.; Bonora, M.; Moscato, F.; Lantada, A.D. Design and Manufacturing of Microtextured Patient-Specific Coronary Stent. In Proceedings of the 16th International Joint Conference on Biomedical Engineering Systems and Technologies (BIOSTEC 2023)—Volume 1: BIODEVICES; SCITEPRESS—Science and Technology Publications: Setúbal, Portugal, 2023; pp. 142–149. [Google Scholar]
- Cendrero, A.M.; Fortunato, G.M.; Munoz-Guijosa, J.M.; De Maria, C.; Díaz Lantada, A. Benefits of non-planar printing strategies towards eco-efficient 3d printing. Sustainability 2021, 13, 1599. [Google Scholar] [CrossRef]
- Gil-Villacastin, L.; Guzman-Bautista, A.; Lopez-Arrabal, A.; Chacon-Tanarro, E.; Sancho-Arellano, A.; Lerin-Alonso, M. Design for Robot-Based Non-Planar Additive Manufacturing Case Study: Soft Robotics Gripper. In Proceedings of the 2025 9th International Conference on Mechanical Engineering and Robotics Research (ICMERR), Barcelona, Spain, 15–17 January 2025; pp. 5–9. [Google Scholar]
- López-Arrabal, A.; Guzmán-Bautista, Á.; Solórzano-Requejo, W.; Franco-Martínez, F.; Villaverde, M. Axisymmetric non-planar slicing and path planning strategy for robot-based additive manufacturing. Mater. Des. 2024, 241, 112915. [Google Scholar] [CrossRef]
- López-Arrabal, A.; Guzmán-Bautista, Á.; Solórzano-Requejo, W.; Sancho-Arellano, A.; Franco-Martínez, F.; Lantada, A.D. Path planning design for robot based non-planar additive manufacturing case study: Coronary stent. In Proceedings of the 2025 9th International Conference on Mechanical Engineering and Robotics Research (ICMERR), Barcelona, Spain, 15–17 January 2025; pp. 17–21. [Google Scholar]
- Kainz, M.; Perak, S.; Stubauer, G.; Kopp, S.; Kauscheder, S.; Hemetzberger, J.; Martínez Cendrero, A.; Díaz Lantada, A.; Tupe, D.; Major, Z.; et al. Additive and lithographic manufacturing of biomedical scaffold structures using a versatile thiol-ene photocurable resin. Polymers 2024, 16, 655. [Google Scholar] [CrossRef]
- Lantada, A.D.; Jouda, M.; Solórzano-Requejo, W.; Mager, D.; Islam, M.; Korvink, J.G. Combining μ-MRI with cellular automata simulation for an improved insight into cell growth within scaffolds. Cell Rep. Phys. Sci. 2025, 6, 102629. [Google Scholar] [CrossRef]
- Solórzano-Requejo, W.; Martinez Cendrero, A.; Altun, A.A.; Nohut, S.; Ojeda, C.; Garcia Molleja, J.; Molina-Aldareguia, J.; Schwentenwein, M.; Diaz Lantada, A. Topology optimisation and lithography-based ceramic manufacturing of short-stem hip prostheses with enhanced biomechanical and mechanobiological performance. Virtual Phys. Prototyp. 2024, 19, e2387280. [Google Scholar] [CrossRef]


















| Characteristics and Manufacturing Parameters | Fused Deposition Modelling (FDM) |
|---|---|
| Company and model | Creality CR-X Pro (2019 Updated) |
| Maximum build envelope | 300 × 300 × 400 mm3 |
| Nozzle diameter | mm |
| Positioning resolution | 1.25 m/1.25 m/1 m |
| Selected layer thickness | mm |
| Printed filament line width | mm |
| Characteristics | PLA (FDM) | PMMA | Resin PolyJet | PEEK |
|---|---|---|---|---|
| Polymer | Thermoplastic | Thermoplastic | Photopolymer | Thermoplastic |
| Manufacturer | Creality HP | Veracril, New Stetic S.A. | Stratasys | Evonik Corporation |
| Commercial | HP-PLA | PMMA | MED610 | Vestakeep PEEK |
| Color | White (Bone) | Transparent | Transparent | Light gray |
| Density | 1.23 kg m−3 | 1.15–1.19 kg m−3 | 1.17–1.18 kg m−3 | 1300 kg m−3 |
| Tensile strength | 52 MPa | 30–50 MPa | 50–65 MPa | 90–100 MPa |
| Print/molding temperature | 190–220 °C (±5 °C) | 60–80 °C | 45–50 °C | 260–300 °C |
| Filament diameter | 1.75 mm | - | - | 1.75 mm |
| Printed diameter | 0.55 mm | - | - | 0.55 mm |
| Variable/Case | Case 1 | Case 2 | Case 3 | Case 4 | Case 5 | Case 6 | Case 7 |
|---|---|---|---|---|---|---|---|
| Age | 2 years old | 10 years old | 11 years old | 55 years old | 70 years old | 55 years old | 9 months old |
| Sex | Female | Male | Male | Female | Female | Female | Female |
| Diagnosis | Sequela of craniocerebral trauma | Post-trauma cranial collapse | Osteofibrous dysplasia | Sternoclavicular osteosarcoma | Posterior thoracic tumour | Sternoclavicular tumour | Metopic craniosynostosis and trigonocephaly |
| Anatomical region | Left temporoparietal and orbital | Left temporoparietal-occipital | Right frontal and orbital roof | Right sternoclavicular joint | Right posterior chest wall | Right sternum and clavicle | Frontal bone and metopic region |
| Type of intervention | Osteotomy, resection, reconstruction | Cranioplasty | Tumour resection, craniofacial reconstruction | En bloc resection, prosthesis | En bloc rib resection, thoracic reconstruction | Resection, sternoclavicular reconstruction | Cranial remodelling assisted with surgical guide |
| Medical device | Orbital prosthesis | Custom prosthesis | Reconstructed bone complex | Sternoclavicular implant | Custom rib implant | Sternoclavicular implant | Custom cutting guide |
| Implant material | Own bone | PMMA | Medical PEEK | PMMA | PMMA | PMMA | Not applicable |
| Surgical guide (yes/no) | Yes (3D model for planning) | Yes (3D model for planning) | Yes (3D model for planning) | Yes (3D model for planning) | Yes (3D model for planning) | Yes (3D model for planning) | Yes (3D model for planning and surgery) |
| Approximate surgery time | 2.2 h | 1.15 h | 1.45 h | 2.3 h | 1.55 h | 2.3 h | 1.5 h |
| Aesthetic result | Improvement of proptosis and cranial symmetry | Cranial contour restoration | Resolution of frontal and orbital deformity | Adequate anatomical reconstruction | Thoracic reconstruction | Restored sternoclavicular symmetry | Corrección morfológica craneofacial |
| Postoperative functionality | Adequate | Adequate | Adequate | Improved mobility of the right arm | Thoracic and respiratory stability | Preserved joint functionality | Prevention of intracranial hypertension |
| Medical follow-up time | Not specified (immediate control by TAC) | Not specified (immediate control by TAC) | Not specified (immediate control by TAC) | Not specified | Not specified | Not specified | Regular pediatric follow-up (ongoing) |
| Additional comments | Good clinical and aesthetic result | Good clinical and aesthetic result | Complete reconstruction of orbit and skull | Use of personalised cutting guide for resection | Resection with negative margins | Use of biocompatible resin in surgical guide | Effective technique in childhood with good prognosis |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moncayo-Matute, F.P.; Vázquez-Albornoz, J.H.; Vázquez-Silva, E.; Hidalgo-Bravo, A.J.; Torres-Jara, P.B.; Moya-Loaiza, D.P. 3D Printing and Virtual Surgical Planning in Craniofacial and Thoracic Surgery: Applications to Personalised Medicine. J. Pers. Med. 2025, 15, 397. https://doi.org/10.3390/jpm15090397
Moncayo-Matute FP, Vázquez-Albornoz JH, Vázquez-Silva E, Hidalgo-Bravo AJ, Torres-Jara PB, Moya-Loaiza DP. 3D Printing and Virtual Surgical Planning in Craniofacial and Thoracic Surgery: Applications to Personalised Medicine. Journal of Personalized Medicine. 2025; 15(9):397. https://doi.org/10.3390/jpm15090397
Chicago/Turabian StyleMoncayo-Matute, Freddy Patricio, Jhonatan Heriberto Vázquez-Albornoz, Efrén Vázquez-Silva, Ana Julia Hidalgo-Bravo, Paúl Bolívar Torres-Jara, and Diana Patricia Moya-Loaiza. 2025. "3D Printing and Virtual Surgical Planning in Craniofacial and Thoracic Surgery: Applications to Personalised Medicine" Journal of Personalized Medicine 15, no. 9: 397. https://doi.org/10.3390/jpm15090397
APA StyleMoncayo-Matute, F. P., Vázquez-Albornoz, J. H., Vázquez-Silva, E., Hidalgo-Bravo, A. J., Torres-Jara, P. B., & Moya-Loaiza, D. P. (2025). 3D Printing and Virtual Surgical Planning in Craniofacial and Thoracic Surgery: Applications to Personalised Medicine. Journal of Personalized Medicine, 15(9), 397. https://doi.org/10.3390/jpm15090397







