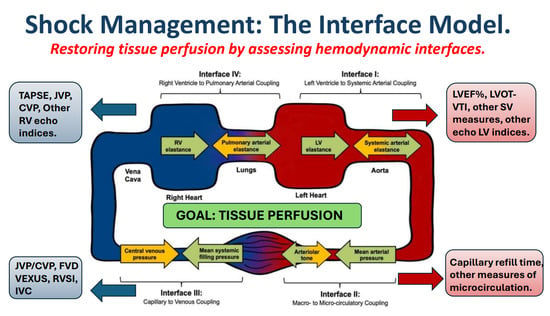
Point of View: A Holistic Four-Interface Conceptual Model for Personalizing Shock Resuscitation
Abstract
1. Background
2. Conceptual Purpose
3. Personalizing Resuscitation to Patient Pathophysiology
4. Circulatory Coupling
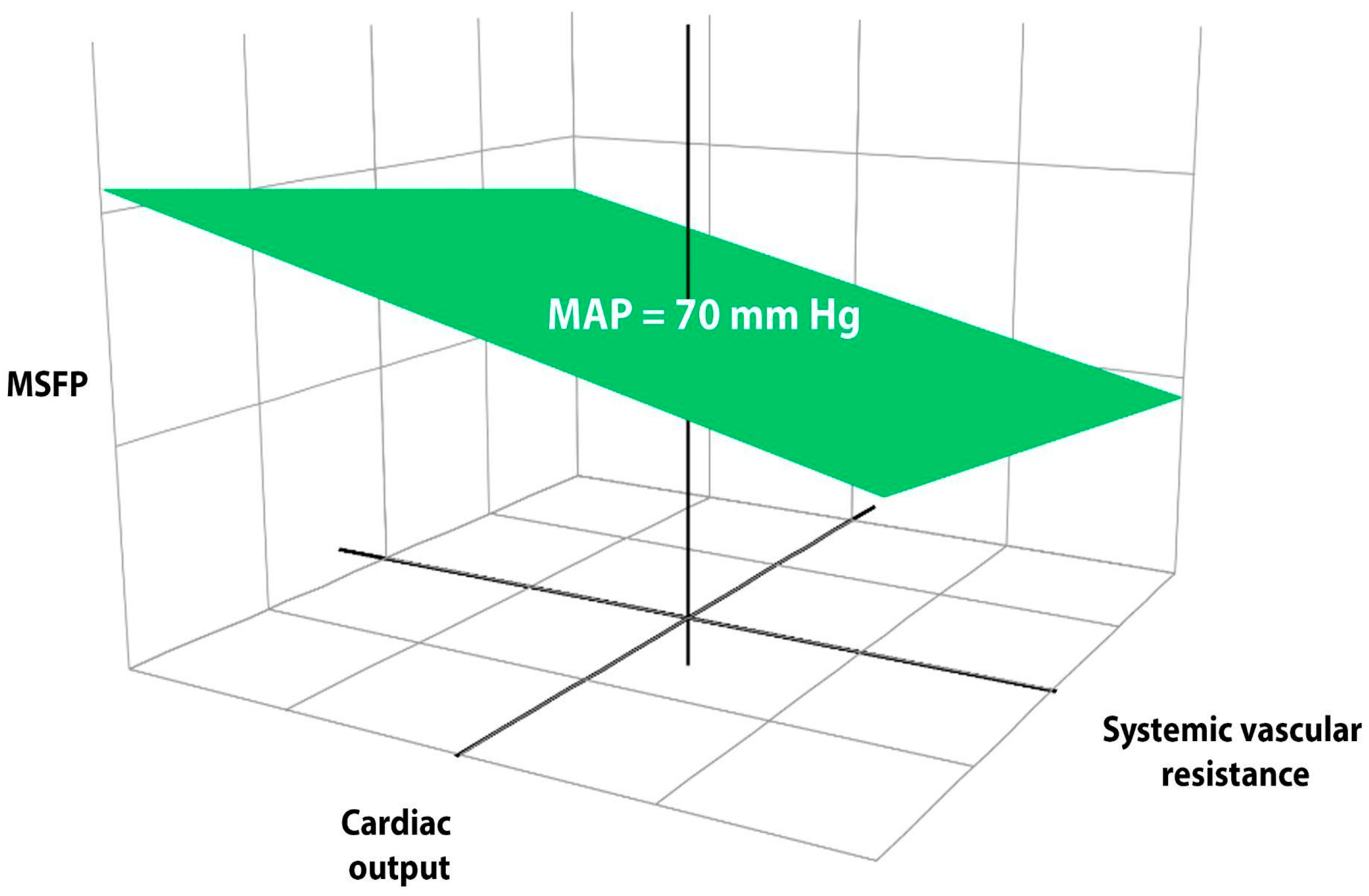
5. The Concept of Mean Systemic Filling Pressure (MSFP)
6. The Relevance of Microhemodynamic Variables
6.1. Interface I: Left Ventriculo-Arterial Coupling—End-Systolic Elastance and Systemic Arterial Elastance
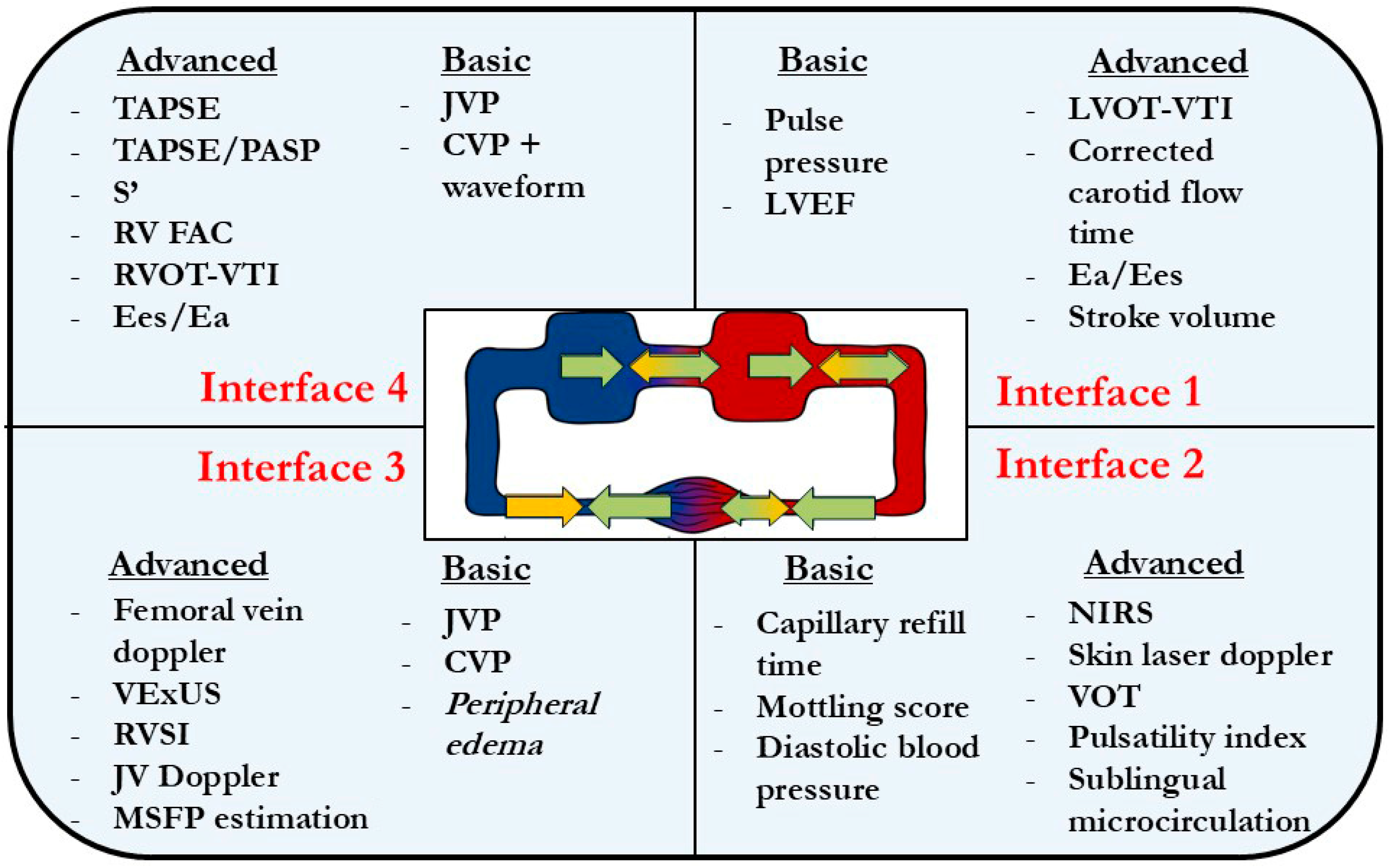
Interface I at the Bedside
6.2. Interface II: Arterioles to Capillary
Interface II at the Bedside
6.3. Interface III: Distal Capillary to Venular
Interface III at the Bedside
6.4. Interface IV—Right Ventricular (RV) to Pulmonary Arterial (RV-PA)
Interface IV at the Bedside
7. Integrating the Circuit
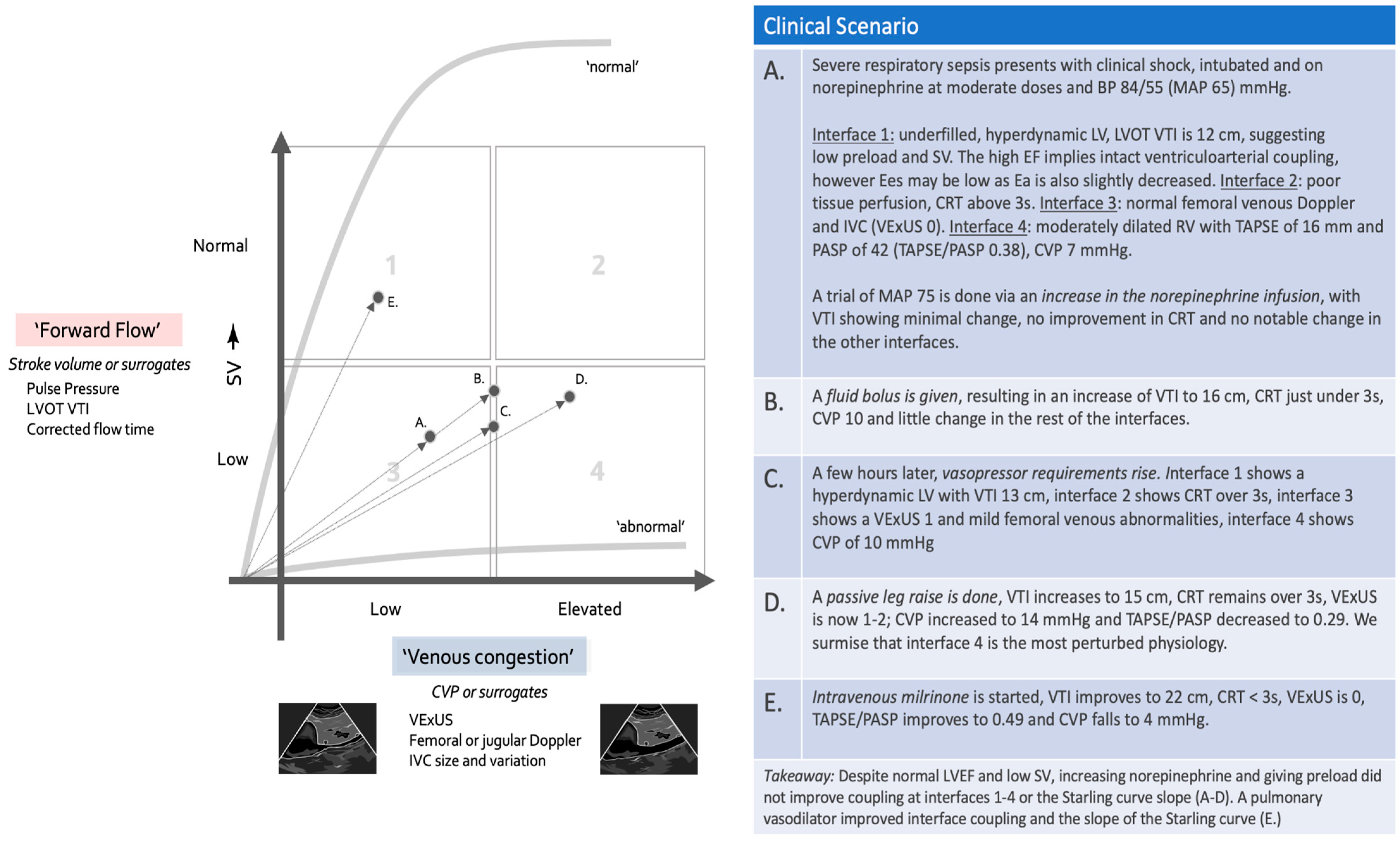
8. Concepts of Clinical Management and Using the Forrester–Kenny Diagram
8.1. Step 1: Placing the Patient in the Forrester–Kenny Diagram
8.2. Step 2: Assessing the Interfaces
8.3. Step 3: Tracking the Progress of Resuscitation on the Forrester–Kenny Diagram
9. Further Development
10. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Bakker, J.; Kattan, E.; Annane, D.; Castro, R.; Cecconi, M.; de Backer, D.; Dubin, A.; Evans, L.; Gong, M.N.; Hamzaoui, O.; et al. Current practice and evolving concepts in septic shock resuscitation. Intensive Care Med. 2022, 48, 148–163. [Google Scholar] [CrossRef] [PubMed]
- De Backer, D.; Cecconi, M.; Chew, M.S.; Hajjar, L.; Monnet, X.; Ospina-Tascón, G.A.; Ostermann, M.; Pinsky, M.R.; Vincent, J.-L. A plea for personalization of the hemodynamic management of septic shock. Crit. Care 2022, 26, 372. [Google Scholar] [CrossRef] [PubMed]
- Kattan, E.; Castro, R.; Miralles-Aguiar, F.; Hernandez, G.R.P. The emerging concept of Fluid Tolerance: A position paper. J. Crit. Care 2022, 71, 154070. [Google Scholar] [CrossRef]
- Malbrain, M.L.N.G.; Van Regenmortel, N.; Saugel, B.; De Tavernier, B.; Van Gaal, P.J.; Joannes-Boyau, O.; Teboul, J.L.; Rice, T.W.; Mythen, M.; Monnet, X. Principles of fluid management and stewardship in septic shock: It is time to consider the four D’s and the four phases of fluid therapy. Ann. Intensive Care 2018, 8, 66. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Evans, L.; Rhodes, A.; Alhazzani, W.; Antonelli, M.; Coopersmith, C.M.; French, C.; Machado, F.R.; Mcintyre, L.; Ostermann, M.; Prescott, H.C.; et al. Surviving sepsis campaign: International guidelines for management of sepsis and septic shock 2021. Intensive Care Med. 2021, 47, 1181–1247. [Google Scholar] [CrossRef]
- Monnet, X.; Shi, R.; Teboul, J.-L. Prediction of fluid responsiveness. What’s new? Ann. Intensive Care 2022, 12, 46. [Google Scholar] [CrossRef] [PubMed]
- Salinas, P.; Sohn, J.; Díaz-Gómez, J.L. Critical Care Echocardiography—A Driven Approach to Undifferentiated Shock. Texas Heart Inst. J. 2023, 50, e228075. [Google Scholar] [CrossRef]
- Hernández, G.; Valenzuela, E.D.; Kattan, E.; Castro, R.; Guzmán, C.; Kraemer, A.E.; Sarzosa, N.; Alegría, L.; Contreras, R.; Oviedo, V.; et al. Capillary refill time response to a fluid challenge or a vasopressor test: An observational, proof-of-concept study. Ann. Intensive Care 2024, 14, 49. [Google Scholar] [CrossRef]
- Hernandez, G.; Ospina-Tascon, G.; Damiani, L.P.; Estenssoro, E.; Dubin, A.; Hurtado, J.; Friedman, G.; Castro, R.; Alegría, L.; Teboul, J.-L.; et al. Effect of a Resuscitation Strategy Targeting Peripheral Perfusion Status vs Serum Lactate Levels on 28-Day Mortality Among Patients With Septic Shock. The ANDROMEDA-SHOCK Randomized Clinical Trial. JAMA 2019, 321, 654–664. [Google Scholar] [CrossRef]
- Hernandez, G.; Bruhn, A.; Castro, R.; Regueira, T. The holistic view on perfusion monitoring in septic shock. Curr. Opin. Crit. Care 2012, 18, 280–286. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, G.; Bellomo, R.; Bakker, J. The ten pitfalls of lactate clearance in sepsis. Intensive Care Med. 2019, 45, 82–85. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Messina, A.; Albini, M.; Samuelli, N.; Brunati, A.; Costantini, E.; Lionetti, G.; Lubian, M.; Greco, M.; Matronola, G.M.; Piccirillo, F.; et al. Fluid boluses and infusions in the early phase of resuscitation from septic shock and sepsis-induced hypotension: A retrospective report and outcome analysis from a tertiary hospital. Ann. Intensive Care 2024, 14, 123. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.Y.; Zhou, Y.; Wang, P.; Qi, E.Y.; Gu, W.J. Elevated central venous pressure is associated with increased mortality and acute kidney injury in critically ill patients: A meta-analysis. Crit. Care 2020, 24, 80. [Google Scholar] [CrossRef]
- Winton, F.R. The influence of venous pressure on the isolated mammalian kidney. J. Physiol. 1931, 72, 49. [Google Scholar] [CrossRef]
- Melchers, M.; de Smet, V.; Rooijakkers, C.; Huising, J.; Vermeulen, W.; Köktaş, B.N.N.; van de Vusse, K.J.; Sabzewar, K.M.; Mishra, S.B.; Bethlehem, C.; et al. Hemodynamic effects of adjunct arginine vasopressin to norepinephrine in septic shock: Insights from a prospective multicenter registry study. Ann. Intensive Care 2025, 15, 59. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tongyoo, S.; Chobngam, S.; Yolsiriwat, N.; Jiranakorn, C. Effects of adjunctive milrinone versus placebo on hemodynamics in patients with septic shock: A randomized controlled trial. Ann. Med. 2025, 57, 2484464. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ince, C. Hemodynamic coherence and the rationale for monitoring the microcirculation. Crit. Care 2015, 19, S8. [Google Scholar] [CrossRef]
- Vellinga, N.A.R.; Ince, C.; Boerma, E.C. Elevated central venous pressure is associated with impairment of microcirculatory blood flow in sepsis: A hypothesis generating post hoc analysis. BMC Anesthesiol. 2013, 13, 17. [Google Scholar] [CrossRef]
- Dugar, S.; Siuba, M.T.; Sacha, G.L.; Sato, R.; Moghekar, A.; Collier, P.; Grimm, R.A.; Vachharajani, V.; Bauer, S.R. Echocardiographic profiles and hemodynamic response after vasopressin initiation in septic shock: A cross-sectional study. J. Crit. Care 2023, 76, 154298. [Google Scholar] [CrossRef]
- Guarracino, F.; Ferro, B.; Morelli, A.; Bertini, P.; Baldassarri, R.; Pinsky, M.R. Ventriculoarterial decoupling in human septic shock. Crit. Care 2014, 18, R80. [Google Scholar] [CrossRef]
- Guinot, P.G.; Longrois, D.; Kamel, S.; Lorne, E.; Dupont, H. Ventriculo-Arterial Coupling Analysis Predicts the Hemodynamic Response to Norepinephrine in Hypotensive Postoperative Patients: A Prospective Observational Study. Crit. Care Med. 2018, 46, e17–e25. [Google Scholar] [CrossRef]
- Guyton, A.C.; Polizo, D.; Armstrong, G.G. Mean Circulatory Filling Pressure Measured Immediately After Cessation of Heart Pumping. Am. J. Phyisiol. 1954, 179, 261–267. [Google Scholar] [CrossRef]
- Guyton, A.C.; Lindsey, A.W.; Abernathy, B.; Richardson, T. Venous return at various right atrial pressures and the normal venous return curve. Am. J. Physiol. 1957, 189, 609–615. [Google Scholar] [CrossRef]
- Persichini, R.; Lai, C.; Teboul, J.L.; Adda, I.; Guérin, L.; Monnet, X. Venous return and mean systemic filling pressure: Physiology and clinical applications. Crit. Care 2022, 26, 150. [Google Scholar] [CrossRef]
- Magder, S.A. The Highs and Lows of Blood Pressure: Toward Meaningful Clinical Targets in Patients With Shock. Crit. Care Med. 2014, 42, 1241–1251. [Google Scholar] [CrossRef]
- Davis, M.J. Control of bat wing capillary pressure and blood flow during reduced perfusion pressure. Am. J. Physiol. 1988, 255, H1114–H1129. [Google Scholar] [CrossRef]
- Girling, F. Critical closing pressure and venous pressure. Am. J. Physiol. 1952, 171, 204–207. [Google Scholar] [CrossRef]
- Sylvester, J.T.; Gilbert, R.D.; Traystman, R.J.; Permutt, S. Effects of hypoxia on the closing pressure of the canine systemic arterial circulation. Circ. Res. 1981, 49, 980–987. [Google Scholar] [CrossRef]
- Wijnberge, M.; Schuurmans, J.; De Wilde, R.B.P.; Kerstens, M.K.; Vlaar, A.P.; Hollmann, M.W.; Veelo, D.P.; Pinsky, M.R.; Jansen, J.R.C.; Geerts, B.F. Defining human mean circulatory filling pressure in the intensive care unit. J. Appl. Physiol. 2020, 129, 311–316. [Google Scholar] [CrossRef]
- Permutt, S.; Riley, R.L. Hemodynamics of collapsible vessels with tone: The vascular waterfall. J. Appl. Physiol. 1963, 18, 924–932. [Google Scholar] [CrossRef]
- Magder, S. Starling resistor versus compliance. Which explains the zero-flow pressure of a dynamic arterial pressure-flow relation? Circ. Res. 1990, 67, 209–220. [Google Scholar] [CrossRef] [PubMed]
- Maas, J.J.; de Wilde, R.B.; Aarts, L.P.; Pinsky, M.R.; Jansen, J.R. Determination of vascular waterfall phenomenon by bedside measurement of mean systemic filling pressure and critical closing pressure in the intensive care unit. Anesth. Analg. 2012, 114, 803–810. [Google Scholar] [CrossRef] [PubMed]
- Andrei, S.; Bar, S.; Nguyen, M.; Bouhemad, B.; Guinot, P.G. Effect of norepinephrine on the vascular waterfall and tissue perfusion in vasoplegic hypotensive patients: A prospective, observational, applied physiology study in cardiac surgery. Intensive Care Med. Exp. 2023, 11, 52. [Google Scholar] [CrossRef]
- Chandrasekhar, A.; Padrós-Valls, R.; Pallarès-López, R.; Palanques-Tost, E.; Houstis, N.; Sundt, T.M.; Lee, H.-S.; Sodini, C.G.; Aguirre, A.D. Tissue perfusion pressure enables continuous hemodynamic evaluation and risk prediction in the intensive care unit. Nat. Med. 2023, 29, 1998–2006. [Google Scholar] [CrossRef]
- Warriner, D.R.; Brown, A.G.; Varma, S.; Sheridan, P.J.; Lawford, P.; Hose, D.R.; Al-Mohammad, A.; Shi, Y. Closing the loop: Modelling of heart failure progression from health to end-stage using a meta-analysis of left ventricular pressure-volume loops. PLoS ONE 2014, 9, e114153. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bronicki, R.A.; Tume, S.; Gomez, H.; Dezfulian, C.; Penny, D.J.; Pinsky, M.R.; Burkhoff, D. Application of Cardiovascular Physiology to the Critically Ill Patient. Crit. Care Med. 2024, 52, 821–832. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.H.; Fetics, B.; Nevo, E.; Rochitte, C.E.; Chiou, K.R.; Ding, P.Y.A.; Kawaguchi, M.; Kass, D.A. Noninvasive single-beat determination of left ventricular end-systolic elastance in humans. J. Am. Coll. Cardiol. 2001, 38, 2028–2034. [Google Scholar] [CrossRef]
- Monge García, M.I.; Jian, Z.; Hatib, F.; Settels, J.J.; Cecconi, M.; Pinsky, M.R. Dynamic Arterial Elastance as a Ventriculo-Arterial Coupling Index: An Experimental Animal Study. Front. Physiol. 2020, 11, 284. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mihaileanu, S.; Antohi, E.L. Revisiting the relationship between left ventricular ejection fraction and ventricular-arterial coupling. ESC Heart Fail. 2020, 7, 2214–2222. [Google Scholar] [CrossRef]
- Pinsky, M.R.; Guarracino, F. Pathophysiological implications of ventriculoarterial coupling in septic shock. ICMx 2023, 11, 87. [Google Scholar] [CrossRef]
- Kerrebijn, I.; Atwi, S.; Horner, C.; Elfarnawany, M.; Eibl, A.M.; Eibl, J.K.; Taylor, J.L.; Kim, C.H.; Johnson, B.D.; Kenny, J.S. Correlation between changing carotid artery corrected flow time and ascending aortic Doppler flow velocity. Br. J. Anaesth. 2023, 131, e192–e195. [Google Scholar] [CrossRef] [PubMed]
- Kenny, J.S.; Barjaktarevic, I.; Mackenzie, D.C.; Elfarnawany, M.; Yang, Z.; Eibl, A.M.; Eibl, J.K.; Kim, C.H.; Johnson, B.D. Carotid Doppler ultrasonography correlates with stroke volume in a human model of hypovolaemia and resuscitation: Analysis of 48 570 cardiac cycles. Br. J. Anaesth. 2021, 127, e60–e63. [Google Scholar] [CrossRef] [PubMed]
- Kenny, J.S.; Prager, R.; Rola, P.; Haycock, K.; Gibbs, S.O.; Johnston, D.H.; Horner, C.; Eibl, J.K.; Lau, V.C.; Kemp, B.O. Simultaneous Venous-Arterial Doppler Ultrasound During Early Fluid Resuscitation to Characterize a Novel Doppler Starling Curve: A Prospective Observational Pilot Study. J. Intensive Care Med. 2024, 39, 628–635. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Boudoulas, K.D.; Boudoulas, H. Time and left ventricular function: The forgotten dynamic factor. Eur. J. Heart Fail. 2021, 23, 552–554. [Google Scholar] [CrossRef] [PubMed]
- Alhakak, A.S.; Teerlink, J.R.; Lindenfeld, J.; Böhm, M.; Rosano, G.M.C.; Biering-Sørensen, T. The significance of left ventricular ejection time in heart failure with reduced ejection fraction. Eur. J. Heart Fail. 2021, 23, 541–551. [Google Scholar] [CrossRef] [PubMed]
- Pinsky, M.R.; García, M.I.M.; Dubin, A. Significance of critical closing pressures (starling resistors) in arterial circulation. Crit. Care 2024, 28, 127. [Google Scholar] [CrossRef]
- Hamzaoui, O.; Teboul, J.L. Importance of diastolic arterial pressure in septic shock: PRO. J. Crit. Care 2019, 51, 238–240. [Google Scholar] [CrossRef]
- Ospina-Tascón, G.A.; Teboul, J.L.; Hernandez, G.; Alvarez, I.; Sánchez-Ortiz, A.I.; Calderón-Tapia, L.E.; Manzano-Nunez, R.; Quiñones, E.; Madriñan-Navia, H.J.; Ruiz, J.E.; et al. Diastolic shock index and clinical outcomes in patients with septic shock. Ann. Intensive Care 2020, 10, 41. [Google Scholar] [CrossRef]
- Lagrèze, J.; Care, C.; Lagrèze, M.J.; Pernollet, A.; Kattan, E.; Oufella, H.A.; Chesnel, D.; Ruste, M.; Schweizer, R.; Allaouchiche, B.; et al. Prognostic value of capillary refill time in adult patients: A systematic review with meta—Analysis. Crit. Care 2023, 27, 473. [Google Scholar] [CrossRef]
- Kattan, E.; Ibarra-Estrada, M.; Ospina-Tascón, G.; Hernández, G. Perspectives on peripheral perfusion assessment. Curr. Opin. Crit. Care 2023, 29, 208–214. [Google Scholar] [CrossRef]
- Zampieri, F.G.; Damiani, L.P.; Bakker, J.; Ospina-Tascón, G.A.; Castro, R.; Cavalcanti, A.B.; Hernandez, G. Effect of a Resuscitation Strategy Targeting Peripheral Perfusion Status vs Serum Lactate Levels on 28-Day Mortality Among Patients with Septic Shock: A Bayesian Reanalysis of the ANDROMEDA-SHOCK Trial. Am. J. Respir. Crit. Care Med. 2020, 201, 423–429. [Google Scholar] [CrossRef] [PubMed]
- Fage, N.; Moretto, F.; Rosalba, D.; Shi, R.; Lai, C.; Teboul, J.L.; Monnet, X. Effect on capillary refill time of volume expansion and increase of the norepinephrine dose in patients with septic shock. Crit. Care 2023, 27, 429. [Google Scholar] [CrossRef] [PubMed]
- Jacquet-Lagrèze, M.; Bouhamri, N.; Portran, P.; Schweizer, R.; Baudin, F.; Lilot, M.; Fornier, W.; Fellahi, J.-L. Capillary refill time variation induced by passive leg raising predicts capillary refill time response to volume expansion. Crit. Care 2019, 23, 281. [Google Scholar] [CrossRef]
- Dumas, G.; Lavillegrand, J.R.; Joffre, J.; Bigé, N.; De-Moura, E.B.; Baudel, J.L.; Chevret, S.; Guidet, B.; Maury, E.; Amorim, F.; et al. Mottling score is a strong predictor of 14-day mortality in septic patients whatever vasopressor doses and other tissue perfusion parameters. Crit. Care 2019, 23, 211. [Google Scholar] [CrossRef]
- Merdji, H.; Bataille, V.; Curtiaud, A.; Bonello, L.; Roubille, F.; Levy, B.; Lim, P.; Schneider, F.; Khachab, H.; Dib, J.-C.; et al. Mottling as a prognosis marker in cardiogenic shock. Ann. Intensive Care 2023, 13, 80. [Google Scholar] [CrossRef]
- Fage, N.; Demiselle, J.; Seegers, V.; Merdji, H.; Grelon, F.; Mégarbane, B.; Anguel, N.; Mira, J.-P.; Dequin, P.-F.; Gergaud, S.; et al. Effects of mean arterial pressure target on mottling and arterial lactate normalization in patients with septic shock: A post hoc analysis of the SEPSISPAM randomized trial. Ann. Intensive Care 2022, 12, 78. [Google Scholar] [CrossRef]
- Gavelli, F.; Teboul, J.L.; Monnet, X. How can CO2-derived indices guide resuscitation in critically ill patients? J. Thorac. Dis. 2019, 11, 1528–1537. [Google Scholar] [CrossRef]
- Beaubien-Souligny, W.; Rola, P.; Haycock, K.; Bouchard, J.; Lamarche, Y.; Spiegel, R.; Denault, A.Y. Quantifying systemic congestion with Point-Of-Care ultrasound: Development of the venous excess ultrasound grading system. Ultrasound J. 2020, 12, 16. [Google Scholar] [CrossRef]
- Andrei, S.; Bahr, P.A.; Nguyen, M.; Bouhemad, B.; Guinot, P.G. Prevalence of systemic venous congestion assessed by Venous Excess Ultrasound Grading System (VExUS) and association with acute kidney injury in a general ICU cohort: A prospective multicentric study. Crit. Care 2023, 27, 224. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Rola, P.; Miralles-Aguiar, F.; Argaiz, E.; Beaubien-Souligny, W.; Haycock, K.; Karimov, T.; Dinh, V.A.; Spiegel, R. Clinical applications of the venous excess ultrasound (VExUS) score: Conceptual review and case series. Ultrasound J. 2021, 13, 32. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Anastasiou, V.; Peteinidou, E.; Moysidis, D.V.; Daios, S.; Gogos, C.; Liatsos, A.C.; Didagelos, M.; Gossios, T.; Efthimiadis, G.K.; Karamitsos, T.; et al. Multiorgan Congestion Assessment by Venous Excess Ultrasound Score in Acute Heart Failure. J. Am. Soc. Echocardiogr. 2024, 37, 923–933. [Google Scholar] [CrossRef] [PubMed]
- Lipton, B. Estimation of central venous pressure by ultrasound of the internal jugular vein. Am. J. Emerg. Med. 2000, 18, 432–434. [Google Scholar] [CrossRef] [PubMed]
- Ranganathan, N.; Sivaciyan, V.; Pryszlak, M.; Freeman, M.R. Changes in jugular venous flow velocity after coronary artery bypass grafting. Am. J. Cardiol. 1989, 63, 725–729. [Google Scholar] [CrossRef]
- Ranganathan, N.; Sivaciyan, V. Abnormalities in jugular venous flow velocity in pulmonary hypertension. Am. J. Cardiol. 1989, 63, 719–724. [Google Scholar] [CrossRef] [PubMed]
- Matsuyama, W.; Ohkubo, R.; Michizono, K.; Abe, M.; Nakamura, Y.; Kawabata, M.; Osame, M. Usefulness of transcutaneous Doppler jugular venous echo to predict pulmonary hypertension in COPD patients. Eur. Respir. J. 2001, 17, 1128–1131. [Google Scholar] [CrossRef] [PubMed]
- Ranganathan, N.; Sivaciyan, V. Jugular Venous Pulse Descent Patterns: Recognition and Clinical Relevance. CJC Open 2023, 5, 200. [Google Scholar] [CrossRef]
- Bhardwaj, V.; Rola, P.; Denault, A.; Vikneswaran, G.; Spiegel, R. Femoral vein pulsatility: A simple tool for venous congestion assessment. Ultrasound J. 2023, 15, 24. Available online: https://pubmed.ncbi.nlm.nih.gov/37165284/ (accessed on 2 October 2024). [CrossRef]
- Husain-Syed, F.; Birk, H.W.; Ronco, C.; Schörmann, T.; Tello, K.; Richter, M.J.; Wilhelm, J.; Sommer, N.; Steyerberg, E.; Bauer, P.; et al. Doppler-Derived Renal Venous Stasis Index in the Prognosis of Right Heart Failure. J. Am. Heart Assoc. 2019, 8, e013584. [Google Scholar] [CrossRef]
- Legrand, M.; Dupuis, C.; Simon, C.; Gayat, E.; Mateo, J.; Lukaszewicz, A.C.; Payen, D. Association between systemic hemodynamics and septic acute kidney injury in critically ill patients: A retrospective observational study. Crit. Care 2013, 17, R278. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Herrler, T.; Tischer, A.; Meyer, A.; Feiler, S.; Guba, M.; Nowak, S.; Rentsch, M.; Bartenstein, P.; Hacker, M.; Jauch, K.W. The intrinsic renal compartment syndrome: New perspectives in kidney transplantation. Transplantation 2010, 89, 40–46. [Google Scholar] [CrossRef] [PubMed]
- Ostermann, M.; Hall, A.; Crichton, S. Low mean perfusion pressure is a risk factor for progression of acute kidney injury in critically ill patients—A retrospective analysis. BMC Nephrol. 2017, 18, 151. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ma, Q.; Ding, C.; Wei, W.; Su, C.; Li, B.; Zhou, Z.; Chen, C.; Liu, B.; Zhang, X.; Wu, J. The value of right ventricular pulmonary artery coupling in determining the prognosis of patients with sepsis. Sci. Rep. 2024, 14, 15283. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tampakakis, E.; Shah, S.J.; Borlaug, B.A.; Leary, P.J.; Patel, H.H.; Miller, W.L.; Kelemen, B.W.; Houston, B.A.; Kolb, T.M.; Damico, R.; et al. Pulmonary Effective Arterial Elastance as a Measure of Right Ventricular Afterload and Its Prognostic Value in Pulmonary Hypertension Due to Left Heart Disease. Circ. Heart Fail 2018, 11, e004436. Available online: https://pubmed.ncbi.nlm.nih.gov/29643065/ (accessed on 10 November 2024). [CrossRef] [PubMed]
- Magder, S. Central venous pressure monitoring. Curr. Opin. Crit. Care 2006, 12, 219–227. [Google Scholar] [CrossRef] [PubMed]
- Tello, K.; Wan, J.; Dalmer, A.; Vanderpool, R.; Ghofrani, H.A.; Naeije, R.; Roller, F.; Mohajerani, E.; Seeger, W.; Herberg, U.; et al. Validation of the Tricuspid Annular Plane Systolic Excursion/Systolic Pulmonary Artery Pressure Ratio for the Assessment of Right Ventricular-Arterial Coupling in Severe Pulmonary Hypertension. Circ. Cardiovasc. Imaging 2019, 12, e009047. Available online: https://pubmed.ncbi.nlm.nih.gov/31500448/ (accessed on 22 October 2024). [CrossRef]
- Forrester, J.S.; Diamond, G.A.; Swan, H.J. Correlative classification of clinical and hemodynamic function after acute myocardial infarction. Am. J. Cardiol. 1977, 39, 137–145. [Google Scholar] [CrossRef] [PubMed]
- Kenny, J.-É.S.; Prager, R.; Rola, P.; Haycock, K.; Basmaji, J.; Hernández, G. Unifying Fluid Responsiveness and Tolerance With Physiology: A Dynamic Interpretation of the Diamond–Forrester Classification. Crit. Care Explor. 2023, 5, e1022. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rola, P.; Kattan, E.; Siuba, M.T.; Haycock, K.; Crager, S.; Spiegel, R.; Hockstein, M.; Bhardwaj, V.; Miller, A.; Kenny, J.-E.; et al. Point of View: A Holistic Four-Interface Conceptual Model for Personalizing Shock Resuscitation. J. Pers. Med. 2025, 15, 207. https://doi.org/10.3390/jpm15050207
Rola P, Kattan E, Siuba MT, Haycock K, Crager S, Spiegel R, Hockstein M, Bhardwaj V, Miller A, Kenny J-E, et al. Point of View: A Holistic Four-Interface Conceptual Model for Personalizing Shock Resuscitation. Journal of Personalized Medicine. 2025; 15(5):207. https://doi.org/10.3390/jpm15050207
Chicago/Turabian StyleRola, Philippe, Eduardo Kattan, Matthew T. Siuba, Korbin Haycock, Sara Crager, Rory Spiegel, Max Hockstein, Vimal Bhardwaj, Ashley Miller, Jon-Emile Kenny, and et al. 2025. "Point of View: A Holistic Four-Interface Conceptual Model for Personalizing Shock Resuscitation" Journal of Personalized Medicine 15, no. 5: 207. https://doi.org/10.3390/jpm15050207
APA StyleRola, P., Kattan, E., Siuba, M. T., Haycock, K., Crager, S., Spiegel, R., Hockstein, M., Bhardwaj, V., Miller, A., Kenny, J.-E., Ospina-Tascón, G. A., & Hernandez, G. (2025). Point of View: A Holistic Four-Interface Conceptual Model for Personalizing Shock Resuscitation. Journal of Personalized Medicine, 15(5), 207. https://doi.org/10.3390/jpm15050207