Psychological Traits of Bariatric Surgery Candidates and Predictors of Outcomes
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Psychological Assessments
2.2.1. Beck Depression Inventory (BDI-II)
2.2.2. Binge Eating Scale (BES)
2.2.3. Body Uneasiness Test (BUT)
2.2.4. Short-Form Health Survey (SF-36)
2.3. Statistical Analysis
3. Results
3.1. Total Sample
Sex Differences
3.2. Follow-Up Sample
3.2.1. Sex Differences
3.2.2. Post- Versus Pre-Surgery in Males and Females Combined
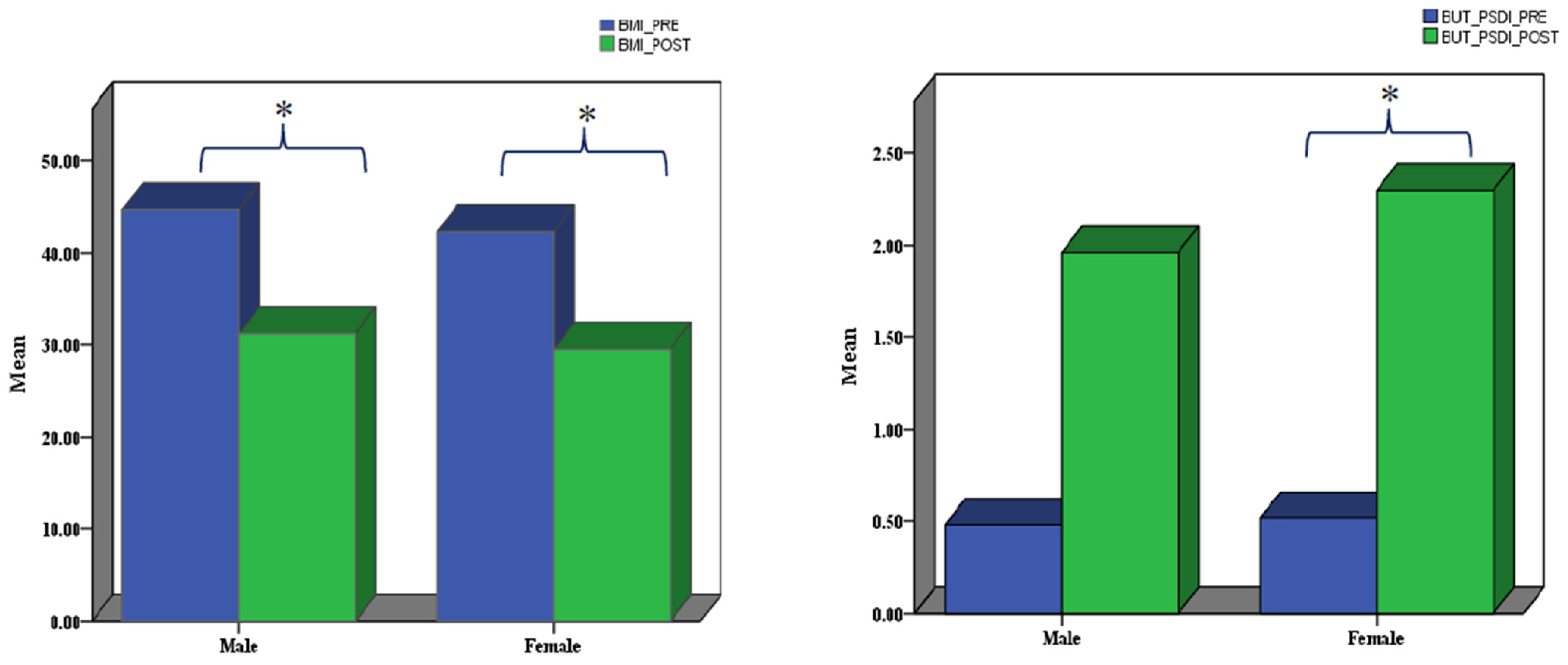
3.2.3. Post- Versus Pre-Assessments in Males and Females Separately
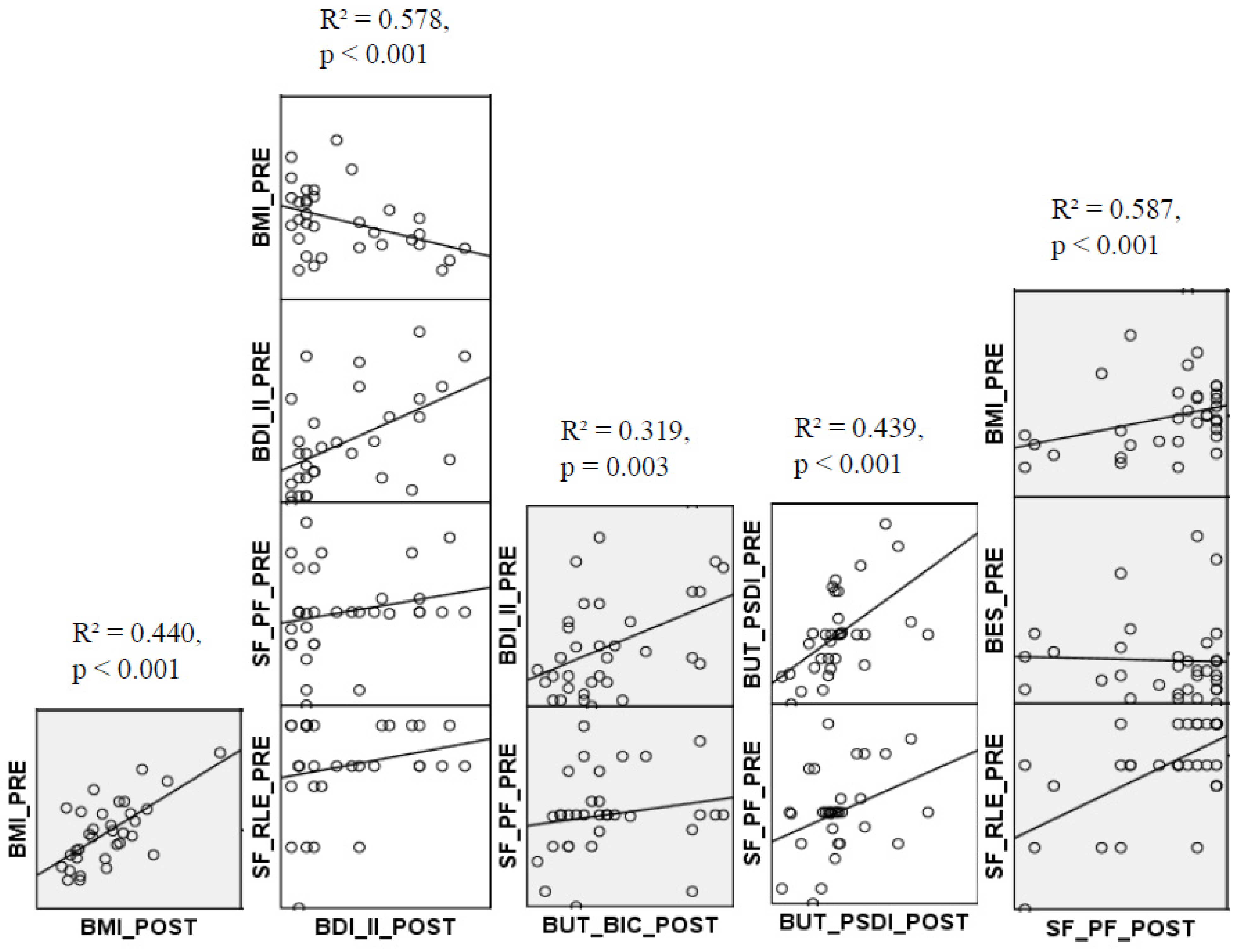
3.3. Regression Results
3.3.1. Prediction of Post-Surgery BMI
3.3.2. Prediction of Post-Surgery BDI-II
3.3.3. Prediction of Post-Surgery BUT_BIC
3.3.4. Prediction of Post-Surgery BUT_PSDI
3.3.5. Prediction of Post-Surgery SF_PF
4. Discussion
5. Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Berrington de Gonzalez, A.; Hartge, P.; Cerhan, J.R.; Flint, A.J.; Hannan, L.; MacInnis, R.J.; Moore, S.C.; Tobias, G.S.; Anton-Culver, H.; Freeman, L.B.; et al. Body-Mass Index and Mortality among 1.46 Million White Adults. N. Engl. J. Med. 2011, 365, 869. [Google Scholar] [CrossRef] [PubMed]
- Fontaine, K.R.; Redden, D.T.; Wang, C.; Westfall, A.O.; Allison, D.B. Years of Life Lost Due to Obesity. JAMA 2003, 289, 187–193. [Google Scholar] [CrossRef] [PubMed]
- Prospective Studies Collaboration. Body-mass index and cause-specific mortality in 900,000 adults: Collaborative analyses of 57 prospective studies. Lancet 2009, 373, 1083–1096. [Google Scholar] [CrossRef]
- Luppino, F.S.; de Wit, L.M.; Bouvy, P.F.; Stijnen, T.; Cuijpers, P.; Penninx, B.W.; Zitman, F.G. Overweight, obesity, and depression: A systematic review and meta-analysis of longitudinal studies. Arch. Gen. Psychiatry 2010, 67, 220–229. [Google Scholar] [CrossRef]
- Blüher, M. Obesity: Global epidemiology and pathogenesis. Nat. Rev. Endocrinol. 2019, 15, 288–298. [Google Scholar] [CrossRef]
- Lauby-Secretan, B.; Scoccianti, C.; Loomis, D.; Grosse, Y.; Bianchini, F.; Straif, K. Body fatness and cancer—Viewpoint of the IARC Working Group. N. Engl. J. Med. 2016, 375, 794–798. [Google Scholar] [CrossRef] [PubMed]
- Wadden, T.A.; Webb, V.L.; Moran, C.H.; Bailer, B.A. Lifestyle modification for obesity: New developments in diet, physical activity, and behavior therapy. Circulation 2012, 125, 1157–1170. [Google Scholar] [CrossRef]
- Bray, G.A.; Frühbeck, G.; Ryan, D.H.; Wilding, J.P. Management of obesity. Lancet 2016, 387, 1947–1956. [Google Scholar] [CrossRef]
- Chao, A.M.; Taylor, S.; Moore, M.; Amaro, A.; Wadden, T.A. Evolving approaches for pharmacological therapy of obesity. Annu. Rev. Pharmacol. Toxicol. 2024, 65, 169–189. [Google Scholar] [CrossRef]
- Van Strien, T. Causes of emotional eating and matched treatment of obesity. Curr. Diabetes Rep. 2018, 18, 35. [Google Scholar] [CrossRef]
- Fagundo, A.B.; de la Torre, R.; Jiménez-Murcia, S.; Agüera, Z.; Granero, R.; Tárrega, S.; Botella, C.; Baños, R.; Fernández-Real, J.M.; Rodríguez, R.; et al. Executive Functions Profile in Extreme Eating/Weight Conditions: From Anorexia Nervosa to Obesity. PLoS ONE 2012, 7, e43382. [Google Scholar] [CrossRef] [PubMed]
- Cournot, M.; Marquie, J.C.; Ansiau, D.; Martinaud, C.; Fonds, H.; Ferrieres, J.; Ruidavets, J.B. Relation between body mass index and cognitive function in healthy middle-aged men and women. Neurology 2006, 67, 1208–1214. [Google Scholar] [CrossRef] [PubMed]
- Dahl, A.K.; Hassing, L.B.; Fransson, E.I.; Gatz, M.; Reynolds, C.A.; Pedersen, N.L. Body mass index across midlife and cognitive change in late life. Int. J. Obes. 2013, 37, 296–302. [Google Scholar] [CrossRef] [PubMed]
- Sabia, S.; Kivimaki, M.; Shipley, M.J.; Marmot, M.G.; Singh-Manoux, A. Body mass index over the adult life course and cognition in late midlife: The Whitehall II Cohort Study. Am. J. Clin. Nutr. 2009, 89, 601–607. [Google Scholar] [CrossRef]
- Alosco, M.L.; Spitznagel, M.B.; Strain, G.; Devlin, M.; Cohen, R.; Paul, R.; Crosby, R.D.; Mitchell, J.E.; Gunstad, J. Improved memory function two years after bariatric surgery. Obesity 2014, 22, 32–38. [Google Scholar] [CrossRef]
- Brinkworth, G.D. Long-term Effects of a Very Low-Carbohydrate Diet and a Low-Fat Diet on Mood and Cognitive Function. Arch. Intern. Med. 2009, 169, 1873–1880. [Google Scholar] [CrossRef]
- Miller, L.A.; Crosby, R.D.; Galioto, R.; Strain, G.; Devlin, M.J.; Wing, R.; Cohen, R.A.; Paul, R.H.; Mitchell, J.E.; Gunstad, J. Bariatric Surgery Patients Exhibit Improved Memory Function 12 Months Postoperatively. Obes. Surg. 2013, 23, 1527–1535. [Google Scholar] [CrossRef]
- Siervo, M.; Arnold, R.; Wells, J.C.K.; Tagliabue, A.; Colantuoni, A.; Albanese, E.; Brayne, C.; Stephan, B.C.M. Intentional weight loss in overweight and obese individuals and cognitive function: A systematic review and meta-analysis. Obes. Rev. 2011, 12, 968–983. [Google Scholar] [CrossRef]
- Castanon, N.; Lasselin, J.; Capuron, L. Neuropsychiatric Comorbidity in Obesity: Role of Inflammatory Processes. Front. Endocrinol. 2014, 5, 74. [Google Scholar] [CrossRef]
- Expert Panel on the Identification, Treatment of Overweight, Obesity in Adults (US), National Heart, Lung, Blood Institute, National Institute of Diabetes, & Kidney Diseases (US). Clinical Guidelines on the Identification, Evaluation, and Treatment of Overweight and Obesity in Adults: The Evidence Report (Issue 98); National Institutes of Health, National Heart, Lung, and Blood Institute: Bethesda, MD, USA, 1998. [Google Scholar]
- Mingrone, G.; Panunzi, S.; De Gaetano, A.; Guidone, C.; Iaconelli, A.; Nanni, G.; Castagneto, M.; Bornstein, S.; Rubino, F. Bariatric-metabolic surgery versus conventional medical treatment in obese patients with type 2 diabetes: 5 year follow-up of an open-label, single-centre, randomised controlled trial. Lancet 2015, 386, 964–973. [Google Scholar] [CrossRef]
- Arterburn, D.E.; Telem, D.A.; Kushner, R.F.; Courcoulas, A.P. Benefits and Risks of Bariatric Surgery in Adults: A Review. JAMA 2020, 324, 879–887. [Google Scholar] [CrossRef]
- Colquitt, J.L.; Pickett, K.; Loveman, E.; Frampton, G.K. Surgery for weight loss in adults. Cochrane Database Syst. Rev. 2014, 8, CD003641. [Google Scholar] [CrossRef]
- Courcoulas, A.P.; King, W.C.; Belle, S.H.; Berk, P.D.; Flum, D.R.; Garcia, L.; Gourash, W.; Horlick, M.; Mitchell, J.E.; Pomp, A.; et al. Seven-year weight trajectories and health outcomes in the Longitudinal Assessment of Bariatric Surgery (LABS) Study. JAMA Surg. 2018, 153, 427–434. [Google Scholar] [CrossRef] [PubMed]
- Magro, D.O.; Geloneze, B.; Delfini, R.; Pareja, B.C.; Callejas, F.; Pareja, J.C. Long-term weight regain after gastric bypass: A 5-year prospective study. Obes. Surg. 2008, 18, 648–651. [Google Scholar] [CrossRef]
- Odom, J.; Zalesin, K.C.; Washington, T.L.; Miller, W.W.; Hakmeh, B.; Zaremba, D.L.; Altattan, M.; Balasubramaniam, M.; Gibbs, D.S.; Krause, K.R.; et al. Behavioral predictors of weight regain after bariatric surgery. Obes. Surg. 2010, 20, 349–356. [Google Scholar] [CrossRef]
- Beck, A.T.; Steer, R.A.; Ball, R.; Ranieri, W.F. Comparison of Beck Depression Inventories-IA and-II in Psychiatric Outpatients. J. Personal. Assess. 1996, 67, 588–597. [Google Scholar] [CrossRef] [PubMed]
- Sica, C.; Ghisi, M. The Italian versions of the Beck Anxiety Inventory and the Beck Depression Inventory-II: Psychometric properties and discriminant power. In Leading-Edge Psychological Tests and Testing Research; NOVA Science Publishers: Hauppauge, NY, USA, 2007; pp. 27–50. [Google Scholar]
- Gormally, J.; Black, S.; Daston, S.; Rardin, D. The assessment of binge eating severity among obese persons. Addict. Behav. 1982, 7, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Timmerman, G.M. Binge Eating Scale: Further Assessment of Validity and Reliability. J. Appl. Biobehav. Res. 1999, 4, 1–12. [Google Scholar] [CrossRef]
- Cuzzolaro, M.; Vetrone, G.; Marano, G.; Garfinkel, P.E. The Body Uneasiness Test (BUT): Development and validation of a new body image assessment scale. Eat. Weight Disord. 2006, 11, 1–13. [Google Scholar] [CrossRef]
- Tomczak, M.; Tomczak, E. The need to report effect size estimates revisited. An overview of some recommended measures of effect size. Trends Psychol. 2014, 22, 17–24. [Google Scholar]
- Thompson, C.G.; Kim, R.S.; Aloe, A.M.; Becker, B.J. Extracting the Variance Inflation Factor and Other Multicollinearity Diagnostics from Typical Regression Results. Basic Appl. Soc. Psychol. 2017, 39, 81–90. [Google Scholar] [CrossRef]
- Buchwald, H.; Avidor, Y.; Braunwald, E.; Jensen, M.D.; Pories, W.; Fahrbach, K.; Schoelles, K. Bariatric Surgery: A Systematic Review and Meta-analysis. JAMA 2004, 292, 1724–1737. [Google Scholar] [CrossRef] [PubMed]
- Jumbe, S.; Hamlet, C.; Meyrick, J. Psychological Aspects of Bariatric Surgery as a Treatment for Obesity. Curr. Obes. Rep. 2017, 6, 71–78. [Google Scholar] [CrossRef]
- Kubik, J.F.; Gill, R.S.; Laffin, M.; Karmali, S. The Impact of Bariatric Surgery on Psychological Health. J. Obes. 2013, 2013, 837989. [Google Scholar] [CrossRef]
- Van Hout, G.; Van Heck, G. Bariatric Psychology, Psychological Aspects of Weight Loss Surgery. Obes. Facts 2009, 2, 10–15. [Google Scholar] [CrossRef] [PubMed]
- Marano, G.; Cuzzolaro, M.; Vetrone, G.; Garfinkel, P.E.; Temperilli, F.; Spera, G.; Dalle Grave, R.; Calugi, S.; Marchesini, G. Validating the Body Uneasiness Test (BUT) in obese patients. Eat. Weight Disord. 2007, 12, 70–82. [Google Scholar] [CrossRef] [PubMed]
- Friedman, M.A.; Brownell, K.D. Psychological correlates of obesity: Moving to the next research generation. Psychol. Bull. 1995, 117, 3–20. [Google Scholar] [CrossRef]
- Pecori, L.; Cervetti, G.G.S.; Marinari, G.M.; Migliori, F.; Adami, G.F. Attitudes of Morbidly Obese Patients to Weight Los2s and Body Image following Bariatric Surgery and Body Contouring. Obes. Surg. 2007, 17, 68–73. [Google Scholar] [CrossRef]
- Rosta, M.L.; Porfiri, F.; Zaccaria, A.; Giannetti, G.; Scoppetta, M.; Giustacchini, P.; Iaconelli, A.; Chieffo, D.; Mingrone, G.; Raffaelli, M.; et al. Body Image in Bariatric surgery candidates. Eur. Psychiatry 2017, 41, S548. [Google Scholar] [CrossRef]
- Ivezaj, V.; Grilo, C.M. The complexity of body image following bariatric surgery: A systematic review of the literature. Obes. Rev. 2018, 19, 1116–1140. [Google Scholar] [CrossRef]
- Risi, R.; Rossini, G.; Tozzi, R.; Pieralice, S.; Monte, L.; Masi, D.; Castagneto-Gissey, L.; Gallo, I.F.; Strigari, L.; Casella, G.; et al. Sex difference in the safety and efficacy of bariatric procedures: A systematic review and meta-analysis. Surg. Obes. Relat. Dis. 2022, 18, 983–996. [Google Scholar] [CrossRef] [PubMed]
- Cullasi, S.; Rohrer, J.M.; Bahm, C. Body-Image Perceptions across Sex and Age Groups. Percept. Mot. Ski. 1998, 87, 839–847. [Google Scholar] [CrossRef]
- Feingold, A.; Mazzella, R. Gender Differences in Body Image Are Increasing. Psychol. Sci. 1998, 9, 190–195. [Google Scholar] [CrossRef]
- Bordignon, S.; Aparício, M.J.G.; Bertoletti, J.; Trentini, C.M. Personality characteristics and bariatric surgery outcomes: A systematic review. Trends Psychiatry Psychother. 2017, 39, 124–134. [Google Scholar] [CrossRef] [PubMed]
- Van Hout, G.C.M.; Verschure, S.K.M.; Van Heck, G.L. Psychosocial Predictors of Success following Bariatric Surgery. Obes. Surg. 2005, 15, 552–560. [Google Scholar] [CrossRef]
- Livhits, M.; Mercado, C.; Yermilov, I.; Parikh, J.A.; Dutson, E.; Mehran, A.; Ko, C.Y.; Gibbons, M.M. Preoperative Predictors of Weight Loss Following Bariatric Surgery: Systematic Review. Obes. Surg. 2012, 22, 70–89. [Google Scholar] [CrossRef]
- Herpertz, S.; Kielmann, R.; Wolf, A.M.; Hebebrand, J.; Senf, W. Do psychosocial variables predict weight loss or mental health after obesity surgery? A systematic review. Obes. Res. 2004, 12, 1554–1569. [Google Scholar] [CrossRef]
- Dawes, A.J.; Maggard-Gibbons, M.; Maher, A.R.; Booth, M.J.; Miake-Lye, I.; Beroes, J.M.; Shekelle, P.G. Mental health conditions among patients seeking and undergoing bariatric surgery: A meta-analysis. Jama 2016, 315, 150–163. [Google Scholar] [CrossRef]
- Mitchell, J.E.; King, W.C.; Chen, J.Y.; Devlin, M.J.; Flum, D.; Garcia, L.; Inabet, W.; Pender, J.R.; Kalarchian, M.A.; Khandelwal, S.; et al. Course of depressive symptoms and treatment in the longitudinal assessment of bariatric surgery (LABS-2) study. Obesity 2014, 22, 1799–1806. [Google Scholar] [CrossRef]
- Bosc, L.; Mathias, F.; Monsaingeon, M.; Gronnier, C.; Pupier, E.; Gatta-Cherifi, B. Long-term changes in body image after bariatric surgery: An observational cohort study. PLoS ONE 2022, 17, e0276167. [Google Scholar] [CrossRef]
| SEX | N | Mean | Std. Deviation | Std. Error Mean | |
|---|---|---|---|---|---|
| BMI | M | 25 | 43.0080 | 6.88076 | 1.37615 |
| F | 72 | 40.8107 | 5.52220 | 0.65080 | |
| Total | 97 | 41.3770 | 5.94330 | 0.6035 | |
| AGE | M | 25 | 41.6800 | 10.58584 | 2.11717 |
| F | 72 | 44.0556 | 11.59272 | 1.36622 | |
| Total | 97 | 43.4433 | 11.33576 | 1.151 | |
| EDUCATION | M | 25 | 10.4722 | 2.68483 | 0.53697 |
| F | 72 | 10.5918 | 3.40035 | 0.40074 | |
| Total | 97 | 10.5610 | 3.21810 | 0.3267 | |
| BDI_II | M | 25 | 7.4353 | 7.34159 | 1.46832 |
| F | 72 | 10.7329 | 7.55669 | 0.89056 | |
| Total | 97 | 9.8830 | 7.72492 | 0.7843 | |
| BES | M | 25 | 10.4611 | 10.01213 | 2.00243 |
| F | 72 | 10.1952 | 7.40435 | 0.87261 | |
| Total | 97 | 10.2637 | 8.36638 | 0.8495 | |
| BUT_GSI | M | 25 | 0.8953 | 1.00018 | 0.20004 |
| F | 72 | 1.4796 | 1.25404 | 0.14779 | |
| Total | 97 | 1.3290 | 1.21620 | 0.1235 | |
| BUT_WP | M | 25 | 1.0536 | 0.97950 | 0.19590 |
| F | 72 | 1.7760 | 1.56574 | 0.18452 | |
| Total | 97 | 1.5898 | 1.47531 | 0.1498 | |
| BUT_BIC | M | 25 | 1.2844 | 1.34374 | 0.26875 |
| F | 72 | 1.9198 | 1.56194 | 0.18408 | |
| Total | 97 | 1.7560 | 1.52766 | 0.1551 | |
| BUT_AV | M | 25 | 0.6133 | 0.95224 | 0.19045 |
| F | 72 | 1.1088 | 1.25975 | 0.14846 | |
| Total | 97 | 0.9811 | 1.20326 | 0.1222 | |
| BUT_CSM | M | 25 | 0.5200 | 0.61486 | 0.12297 |
| F | 72 | 0.9722 | 0.93782 | 0.11052 | |
| Total | 97 | 0.8557 | 0.88572 | 0.0899 | |
| BUT_DEP | M | 25 | 0.5520 | 1.04288 | 0.20858 |
| F | 72 | 1.1889 | 1.28124 | 0.15100 | |
| Total | 97 | 1.0247 | 1.25075 | 0.127 | |
| BUT_PST | M | 25 | 5.4400 | 6.54523 | 1.30905 |
| F | 72 | 10.1389 | 8.21250 | 0.96785 | |
| Total | 97 | 8.9278 | 8.05353 | 0.8177 | |
| BUT_PSDI | M | 25 | 0.4900 | 0.16991 | 0.03398 |
| F | 72 | 0.5561 | 0.16744 | 0.01973 | |
| Total | 97 | 0.5391 | 0.16969 | 0.0172 |
| N | Mean | Std. Deviation | |
|---|---|---|---|
| MAX_WEIGHT | 33 | 125.2727 | 22.76822 |
| BMI_PRE (**) | 33 | 42.8882 | 5.37253 |
| BMI_POST | 33 | 29.8901 | 5.62705 |
| BDI_II_PRE | 33 | 9.8125 | 7.79197 |
| BDI_II_POST | 33 | 7.0000 | 7.18505 |
| BES_PRE | 33 | 9.2188 | 8.44739 |
| BES_POST | 33 | 5.9697 | 4.59269 |
| BUT_GSI_PRE | 33 | 1.4875 | 1.08478 |
| BUT_GSI_POST | 33 | 1.2825 | 0.82448 |
| BUT_WP_PRE | 33 | 1.8164 | 1.37464 |
| BUT_WP_POST | 33 | 1.7917 | 0.96757 |
| BUT_BIC_PRE | 33 | 2.0337 | 1.39484 |
| BUT_BIC_POST | 33 | 1.4899 | 1.13188 |
| BUT_AV_PRE | 33 | 0.9949 | 1.06839 |
| BUT_AV_POST | 33 | 0.7323 | 0.90613 |
| BUT_CSM_PRE | 33 | 0.9293 | 0.76038 |
| BUT_CSM_POST | 33 | 0.8909 | 0.58329 |
| BUT_DEP_PRE | 33 | 0.9455 | 1.11722 |
| BUT_DEP_POST | 33 | 0.8838 | 0.82966 |
| BUT_PST_PRE | 33 | 10.1818 | 7.23902 |
| BUT_PST_POST | 33 | 13.7879 | 8.42525 |
| BUT_PSDI_PRE (*) | 33 | 0.5073 | 0.18500 |
| BUT_PSDI_POST | 33 | 2.2143 | 0.69847 |
| SF_PF_PRE | 33 | 60.5026 | 13.73800 |
| SF_PF_POST | 33 | 73.6364 | 31.55533 |
| SF_RLP_PRE | 33 | 66.6667 | 29.53635 |
| SF_RLP_POST | 33 | 81.8182 | 34.95126 |
| SF_RLE_PRE | 33 | 77.7773 | 25.45903 |
| SF_RLE_POST | 33 | 76.7677 | 38.62646 |
| SF_ENERGY_PRE | 33 | 60.4762 | 12.17738 |
| SF_ENERGY_POST | 33 | 66.5152 | 18.85325 |
| SF_WELL_PRE | 33 | 69.5619 | 11.62837 |
| SF_WELL_POST | 33 | 75.0303 | 17.94520 |
| SF_SF_PRE | 33 | 75.5952 | 18.08082 |
| SF_SF_POST | 33 | 68.0303 | 22.33934 |
| SF_PAIN_PRE | 33 | 64.8750 | 20.99549 |
| SF_PAIN_POST | 33 | 78.4848 | 26.10779 |
| SF_GH_PRE | 33 | 65.6548 | 13.28996 |
| SF_GH_POST | 33 | 67.1212 | 20.88025 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hadipour Lakmehsari, A.; Mento, C.; Scaramuzzino, C.; Arena, F.; Turiaco, F.; Muscatello, M.R.A.; Navarra, G.; Pandolfo, G.; Lombardo, C. Psychological Traits of Bariatric Surgery Candidates and Predictors of Outcomes. J. Pers. Med. 2025, 15, 215. https://doi.org/10.3390/jpm15060215
Hadipour Lakmehsari A, Mento C, Scaramuzzino C, Arena F, Turiaco F, Muscatello MRA, Navarra G, Pandolfo G, Lombardo C. Psychological Traits of Bariatric Surgery Candidates and Predictors of Outcomes. Journal of Personalized Medicine. 2025; 15(6):215. https://doi.org/10.3390/jpm15060215
Chicago/Turabian StyleHadipour Lakmehsari, Abed, Carmela Mento, Claudia Scaramuzzino, Federica Arena, Fabrizio Turiaco, Maria Rosaria Anna Muscatello, Giuseppe Navarra, Gianluca Pandolfo, and Clara Lombardo. 2025. "Psychological Traits of Bariatric Surgery Candidates and Predictors of Outcomes" Journal of Personalized Medicine 15, no. 6: 215. https://doi.org/10.3390/jpm15060215
APA StyleHadipour Lakmehsari, A., Mento, C., Scaramuzzino, C., Arena, F., Turiaco, F., Muscatello, M. R. A., Navarra, G., Pandolfo, G., & Lombardo, C. (2025). Psychological Traits of Bariatric Surgery Candidates and Predictors of Outcomes. Journal of Personalized Medicine, 15(6), 215. https://doi.org/10.3390/jpm15060215









