Abstract
Background: Pelvic organ prolapse (POP) and stress incontinence (SUI) are very common medical conditions, affecting women’s quality of life worldwide. Current surgical and conservative therapies often yield variable outcomes and carry risks of complications or recurrence. Platelet-rich plasma (PRP) has emerged as a promising regenerative approach in various medical disciplines. Its application in urogynecology remains relatively new and emerging. The objective of this study was to review and consolidate existing evidence regarding the application of PRP injections for treating POP and/or SUI. Methods: This scoping review was conducted in accordance with the Preferred Reporting Items for Scoping Reviews (PRISMA-ScR). The search strategy included MEDLINE (PubMed), Web of Science, and Scopus databases, covering articles published up to February 2025, with no restrictions on publication date. Results: We included in our review a total of 13 manuscripts and 320 patients at the end of the screening process. A total of ten SUI studies, comprising 273 patients, and three POP studies, involving 47 patients, satisfied all the review criteria. Both clinical entities reported high subjective improvement following PRP treatment. Moreover, PRP appeared to have no significant adverse effects. Conclusions: Our scoping review suggests that PRP may have potential benefits in the treatment of POP and SUI. Nevertheless, the current evidence on its application in this area remains limited. Therefore, well-designed, large-scale randomized controlled trials (RCTs) with extended follow-up periods are urgently needed, in the era of personalized medicine.
1. Introduction
Pelvic organ prolapse (POP) is a very common medical condition, affecting women’s quality of life worldwide [1,2]. Approximately 19% of women will require surgery for POP at least once in their lifetime. Numerous techniques haven been employed for POP treatment [3]. Unfortunately, relapse is common, with 29% undergoing at least one additional procedure [4] and recurrence of anterior compartment prolapse being the most common [5]. In recent years, vaginal meshes have been widely utilized; however, due to their high complication rates, the Food and Drug Administration (FDA) issued reports informing both patients and physicians about the associated risks [6].
Stress urinary incontinence (SUI) is also a prevalent condition that significantly impacts women’s daily lives [7]. According to the International Continence Society (ICS), SUI is the unintentional loss of urine triggered by activities that elevate abdominal pressure, such as coughing, sneezing, or physical exertion, and it appears to be the most common type of urinary incontinence (UI) [8,9]. The therapeutic options for SUI are both conservative and surgical. If conservative treatments (lifestyle adjustments, pelvic floor muscle training-PFMT-, pessaries, and pharmacological treatment) are ineffective, surgical options for managing incontinence include a range of procedures such as synthetic mid-urethral slings, colposuspension, autologous fascial slings, and urethral bulking agents [10,11,12]. Nevertheless, surgical treatment for SUI carries potential risks and complications, while bulking agents can be costly and may not be accessible in many countries [13,14].
Due to the high prevalence of these clinical entities and their strong impact on women’s quality of life, multiple innovative alternative methods have been suggested to enhance the use of minimally invasive therapies lately. The most popular one is platelet-rich plasma (PRP). The term PRP was initially introduced in the 1970s to refer to plasma that contained a higher concentration of platelets compared to peripheral blood. In 1974, Kohler and Lipton, while researching fibroblast physiology, discovered that platelets could play a crucial role as growth stimulants [15]. PRP is shown to be rich in growth factors and able to promote angiogenesis, neuroprotection, neural regeneration, inflammation regulation, and wound healing, all of which contribute to improved organ function [16,17]. In the market, there are many different kits, such as the RegenKit® A-PRP, or TruPRP®, available for the preparation of a PRP solution with varying platelet concentrations and additional ingredient compositions [18]. PRP seems to be a safe therapeutic option due to its autologous nature which eliminates the risk of immune reactions and the potential transmission of microorganisms from external donors [19]. Moreover, it can be cost-effective due to its quick and straightforward preparation, requiring minimal costs [20]. However, PRP is contraindicated for certain patient groups, including those with coagulation disorders, cancer, pregnant women, or individuals with infectious diseases [21]. Although PRP has been extensively utilized across various medical specialties, including orthopedics, urology, ophthalmology, aesthetic medicine, and dermatology, its application in urogynecology remains relatively new and emerging. The objective of this study was to review and consolidate existing evidence regarding the application of PRP injections for treating POP and/or SUI.
2. Materials and Methods
2.1. Search Strategy
This scoping review was performed following the Preferred Reporting Items for Systematic reviews and Meta-Analyses extension for Scoping Reviews (Supplementary Table S1: PRISMA-ScR) guidelines [22]. Two researchers (L.V. and A.P.) conducted a comprehensive search of the Web of Science, Scopus, and MEDLINE (PubMed) databases, including studies published up to February 2025, without applying any historical restrictions. The search strategy involved various combinations of the following MeSH terms: Platelet-Rich Plasma, Stress Urinary Incontinence, Pelvic Organ Prolapse, Urogynaecology. For the articles’ selection, we included articles that focused on PRP treatment for UI and/or POP. The authors also searched for literature on PRP treatment of urgency alone, but by the target date, no articles were found. The PRISMA flow diagram of the selection process is provided in Figure 1.
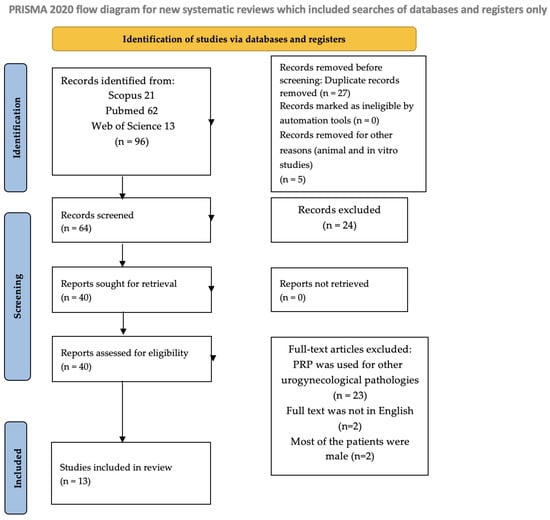
Figure 1.
Flow diagram.
2.2. Eligibility Criteria
The following study designs were deemed eligible for inclusion: case reports, randomized controlled trials, prospective controlled studies, prospective cohort studies, retrospective studies, and case series. Only full-text articles published in English were considered. In contrast, systematic reviews, meta-analyses, letters to the editor, and conference abstracts were excluded from the analysis. However, the reference lists of relevant reviews were screened to identify any additional eligible studies. Research with unclear, incomplete, poor-quality data, or outcomes that could not be quantified was also excluded. Articles whose full text was not written in English as well as studies in which patients were treated for other gynaecological and urogynaecological conditions were excluded. Furthermore, two case series were excluded due to a predominantly male patient population and the lack of clearly differentiated demographic data and outcomes between male and female participants. Therefore, the data quality was poor [23,24]. In vitro and animal studies were excluded [25,26,27,28].
2.3. Data Acquisition and Risk of Bias
All records identified through database searches were screened for publication year, citation, title, authors, abstract, and full text. Duplicate entries were manually detected and removed by two independent reviewers (L.V. and A.P.). During the eligibility assessment, the same two reviewers independently screened the titles and abstracts of the remaining records, excluding those not relevant to the research question. Full texts of potentially eligible studies were then independently assessed for inclusion. Any discrepancies between the reviewers were resolved through discussion and consensus. The methodological quality of the included studies was appraised using the JBI Critical Appraisal Checklists for Case Reports and Clinical Trials (Supplementary Table S2).
2.4. Data Synthesis and Statistical Analysis
We included in our review a total of 13 manuscripts and 320 patients at the end of the screening process (Supplementary Tables S3 and S4 (demographic table) Tables S1 and S2 (data table)). Ten studies investigating SUI (4 Randomized Control Clinical Trial and 6 Prospective studies) and 273 patients and three studies investigating POP (1 Randomized Control Clinical Trial and 2 Prospective studies) and 47 patients meet all the review requirements. We examined in our review the patients’ demography, their obstetrical history, inclusion and exclusion criteria, the PRP kit used, injection protocol, considered scores, results, and follow-up time from the baseline (first PRP injection). Heterogeneity of study type (RCT and observational study) and the high number of different outcomes considered prohibited systematic analysis; thus, a scoping review was performed. However, demographic and participant characteristics were comparable. A mean was used to summarize continuous variables and a percentile was used for dichotomous variables. Because it is a Scopus review and not a systematic review, this review was not registered.
3. Results
3.1. Pelvic Organ Prolapse
3.1.1. Demographics and Participant Characteristics
The mean age of the patients was 57.3 years, with a mean Body Mass Index (BMI) of 25.8 kg/m2 and a mean parity of 2.9. Seventy-four percent of the patients were post-menopausal. In the study by Atilgan et al., patients who underwent cystocele repair for other prolapse compartments or concomitant surgery for SUI were excluded [29]. In contrast, the studies by Einarsson et al. and Gorlero et al. included patients who underwent concomitant prolapse or SUI surgery, with 77.8% of the patients in the former and 100% in the latter receiving such procedures [30,31].
Gorlero et al. reported that 60% of the patients had a Pelvic Organ Prolapse Quantification (POP-Q) stage II prolapse, while 40% had a stage II prolapse [31]. Atilgan et al. reported Aa and Ba points of +3 and +3.6, respectively [29].
In the studies by Einarsson et al. and Atilgan et al., a history of prolapse surgery was an exclusion criterion [29,30]. Conversely, Gorlero et al. included patients with a history of prolapse surgery and those at high risk of recurrence [31].
Regarding platelet preparation, the specific kit used in the study by Einarsson to prepare autologous platelet gel (APG) was not specified [30]. Gorlero et al. employed a Vivostat system to prepare platelet-rich fibrin (PRF), which was then sprayed directly onto the surgical site [31]. Atilgan et al. utilized a Vacutainer Kit to PRP [29]. In all studies, platelet preparation was applied during surgery, and cystocele or other prolapse repairs followed standard surgical techniques. No adverse events were observed during follow-up in 100% of the patients. The mean follow-up duration across studies was 37.5 months.
3.1.2. Scores and Results
In Atilgan et al.’s RCT-single blind, 28 cases were treated with PRP injection into the pubocervical fascia and colporrhaphy and 28 controls only with colporrhaphy. The Aa and Ba points’ means were significantly lower in cases than in controls at 48 months follow-up (p = 0.001 and p = 0.002, respectively). Symptomatic (evaluated by Pelvic Floor Distress Inventory-PFDI), anatomic recurrence (POP-Q > 1), and reoperation rate were significantly lower in cases than in controls (p = 0.008, p = 0.001 and p = 0.001, respectively). Only 3.8% of the patients in the cases’ group had a symptomatic anatomic cystocele recurrence and were reoperated. Subjective success (evaluated by Patient’s Global Impression of Improvement-PGI-I) was significantly higher in cases, with a rate of 89% (p = 0.012) [29].
In their study involving a cohort of nine patients, Einarsson et al. collected punch biopsy specimens both before surgery and during follow-up to assess tissue weight and collagen content. No statistically significant difference in collagen content was observed at follow-up (p = 0.63); however, tissue samples collected during surgery were significantly heavier than those obtained at follow-up (p = 0.004). The POP-Q assessment demonstrated statistically significant improvements at 3 months postoperatively at points Aa and Ba. At the 20-month follow-up, only point Aa showed a statistically significant difference compared to baseline. In patients who completed the follow-up (77.8%), at 20 months, the subjective failure rate was 12.5% and the objective failure rate was 66.7%. The reoperation rate was 12.5% for a recurrent and symptomatic stage II cystocele and enterocele [30].
Furthermore, Gorlero et al. conducted a prospective observational study enrolling ten patients. The overall efficacy rate in terms of anatomical reconstruction was 80% for POP-Q stage 0 and 20% for Pop-Q stage I based on the Prolapse Quality of Life (P-QoL) questionnaire; repair of the vaginal wall descent led to a 20% increase in the number of patients engaging in sexual activity, with no cases of dyspareunia reported postoperatively. Urinary and bowel symptoms showed complete resolution by 24 months. At follow-up, all patients presented with normal scarring, as assessed by the Vancouver Scar Scale (Table 1) [31].

Table 1.
Characteristics and data extracted of included studies about PRP in POP treatment.
3.2. Stress Urinary Incontinence
3.2.1. Demographics and Participants Characteristics
In the included clinical trials, both groups (case and control) had similar demographics and participant characteristics. For our statistics, we considered only the case group.
The mean age was 53.1 years. BMI was reported in seven studies and the mean was 28.4 kg/m2 [32,33,34,35,36,37,38]. Behnia-Willson et al. reported only the percentage of obese patients [39]. Saraluck et al. excluded obese patients from their study.
The mean parity was reported in six studies and was 2.5 [32,33,35,36,38,40]. Two studies reported only that the patients had at least one vaginal delivery [34,39]. Menopausal status was reported in six studies, with 47.3% of patients across these studies being post-menopausal [32,34,35,36,38,39]. Five studies documented the mean VAS pain score during PRP injection, with an average of 2.6 [32,33,34,35,37].
In three studies, prior surgery for POP was listed as an exclusion criterion [32,39,40]. Specifically, Grigoriadis et al. explicitly stated that hysterectomy for POP was an exclusion criterion [32]. In contrast, Saraluck et al. and Ashton et al. reported the percentage of patients with a history of hysterectomy in their study populations [34,37]. Current POP was an exclusion criterion in seven studies [32,33,34,36,39,40,41]. In the study by Chiang et al., a prior suburethral sling procedure was an inclusion criterion [41]; however, previous surgery SUI was an exclusion criterion in five studies [32,33,34,36,40]. Additionally, detrusor overactivity or urgency symptoms identified on urodynamic testing were exclusion criteria in four studies [32,33,34,40].
Regarding the kit used for PRP preparation, four studies utilized the RegenKit system [33,34,35,39]. Ashton et al. employed the Arthrex Autologous Conditioned Plasma Double Syringe System [37], Grigoriadis et al. used the OMNIPLEX Gyno kit [32], and Tahoon et al. utilized the Golden VAC kit [38]. In contrast, three studies did not specify the PRP kit used, providing only a description of the preparation protocol [36,40,41].
Behnia-Willson et al. administered a combination therapy, employing the MonaLisa Touch SmartXide2 V2LR laser as an adjuvant to PRP injection [39].
Regarding the injection protocol, in three studies, patients received a single PRP injection [35,37,38]. In another three studies, two injections were administered per patient [32,33,34]. Ural et al. and Behnia-Willison et al. applied three PRP injections [36,39], while Chiang et al. administered four [41]. Furthermore, in the study conducted by Daneshpajooh et al., following the initial treatment, some patients chose to receive a second and, in some cases, a third injection [40]. In all of these studies, subsequent injections were administered within a 4–6-week interval after the previous treatment.
The mean follow-up duration was 8 months. Adverse events were reported in nine studies; however, no severe adverse events were documented following their completion.
3.2.2. Scores and Results
In the double-blind, RCT by Grigoriadis et al., two groups were evaluated. The case group was treated with PRP, while the control group received a sham treatment (0.9% sodium chloride). Significant improvements were observed in the case group at both 3 and 6 months in terms of the Incontinence Questionnaire—Female Lower Urinary Tract Symptoms (ICIQ-FLUTS), incontinence, total ICIQ-FLUTS mean score, PGI-I, and the 1 h pad test, whereas no such improvements were noted in the control group (p < 0.001). At the 6-month follow-up, the subjective cure rate was significantly higher in the PRP group (32%) compared to the sham group (4%) (p < 0.01). However, no patients were objectively cured during the 6-month follow-up [32].
In the single-blind, RCT conducted by Ashton et al., two groups were evaluated. The case group was treated with PRP, while the control group received a sham treatment (0.9% sodium chloride). No statistically significant differences were observed between the two groups in the primary outcomes, including PGI-I improvement and provocative cough stress test (CST). Additionally, there were no significant differences in Female Sexual Function Index (FSFI), Incontinence Quality of Life (I-QoL), and Questionnaire for Urinary Incontinence Diagnosis (QUID) scores at any follow-up interval [37].
In the single-blind RCT performed by Saraluck et al., the case group received treatment with PRP in conjunction with pelvic floor muscle training (PFMT), while the control group was treated only with PFMT. After a 5-month follow-up, a statistically significant improvement in the 1 h pad test was observed between the two groups (p < 0.05). Furthermore, the case group demonstrated significantly greater improvements in I-QoL scores, as well as in items 11a and 11b of the ICIQ-FLUTS, compared to the control group (p < 0.001). PGI-I scores also showed a significant advantage in the case group (p < 0.05). Additionally, 90% of patients in the case group reported a subjective improvement of at least 50% in their symptoms of SUI [34].
In the prospective RCT by Daneshpajooh et al., the case group received PRP treatment, while the control group underwent mid-urethral sling surgery. At the 1-month follow-up, the CST yielded negative results for 70% of patients in the PRP group, compared to 80% in the control group. However, no statistically significant differences were observed between the groups at both the 1-month and 3-month follow-ups. Results of the ICIQ, I-QoL, and Urinary Distress Inventory (UDI-6) were significantly improved before and after treatment in both groups. Nevertheless, the response to the mid-urethral sling procedure was superior, with a statistically significant difference between the two groups [40].
In the prospective observational pilot study conducted by Athanasiou et al., significant reductions were observed in the 11a question score, filling score, incontinence score, and total mean score of the ICIQ-FLUTS questionnaire (p < 0.001). At the 6-month follow-up, 10% of patients reported feeling subjectively cured, while 80% of patients experienced subjective improvement. The PGI-I demonstrated significant improvement in 80% of the patients throughout the follow-up period. A mean reduction of 50.2% in urine loss was observed in the 1 h pad test at the 6-month follow-up, with 10% of participants considered objectively cured [33].
In the study by Chiang et al., the patients were treated with four PRP injections. Following the PRP treatment, 80.8% of patients reported a positive response in alleviating SUI, with 50% achieving a GRA ≥ 2, which was considered a successful outcome. At 3 months, 46.2% of patients achieved complete continence and were pad-free (dry rate), while 26.9% maintained this outcome at 15 months. Additionally, 53.8% of patients remained mildly incontinent but with an acceptable outcome. The VAS score for SUI showed significant improvement immediately after the first PRP injection, with further consolidation following subsequent injections. This therapeutic effect was sustained throughout the 1-year follow-up period (p < 0.001). At 3 months, both UDI-6 and Incontinence Impact Questionnaire (IIQ-7) scores showed significant reductions (p < 0.001). However, no significant differences were observed in the videourodynamic study VUDS) parameters between baseline and post-treatment, with the exception of abdominal leak point pressure (ALPP) [41].
The prospective observational pilot study conducted by Behnia-Willson et al. assessed the Australian Pelvic Floor Questionnaire (APFQ), specifically question 6. At baseline, 0% of patients reported occasional or no stress urinary symptoms, while 100% reported frequent or daily symptoms. At the 3-month and 12–24-month follow-ups, 66.2% and 62.1% of patients, respectively, reported occasional or no symptoms. All symptoms assessed by the APFQ showed significant improvement from baseline to the end of follow-up [39].
In the observational study by Long et al., significant improvements in incontinence were observed at both 1 month and 6 months post-treatment, as measured by the Incontinence Questionnaire-Short Form (ICIQ-SF), UDI-6, and IIQ-7 in the 20 patients enrolled. Urodynamic studies revealed a significant increase in both residual urine and bladder volume at the first sensation to void [35].
In the observational study conducted by Ural et al., on the ICIQ-SF questionnaire, the rate of clinical improvement was 79.4% at 1 month and 64.7% at 6 months post-treatment. Scores on the ICIQ-SF, UDI-6, IIQ-7, and POPDI-6 at both 1 and 6 months demonstrated statistically significant improvement compared to baseline (p < 0.001) [36].
Tahoon et al. observed a significant increase in residual urine volume and bladder volume at the first sensation to void. Following PRP treatment, the average 1 h pad test result was 2.55 g, representing a reduction of 2.8 g from baseline. Significant improvements in incontinence were observed at both 1- and 3-months post-treatment, as measured by the ICIQ-SF, UDI-6, IIQ-7, OABSS, and FSFI. According to the ICIQ-SF, 54% of patients experienced symptom improvement or cure (Table 2) [38].

Table 2.
Characteristics and data extracted of included studies about PRP in SUI treatment.
4. Discussion
Our scoping review suggests that PRP therapy may lead to symptomatic improvement in POP and female SUI. However, it is important to emphasize that, due to significant clinical heterogeneity, definitive conclusions regarding the efficacy of PRP cannot yet be established.
POP has been linked to a marked reduction in collagen content within the pelvic connective tissues when compared to healthy individuals. This observation has provided the rationale for exploring PRP as a therapeutic option, given its known ability to enhance collagen synthesis and facilitate tissue repair through the release of growth factors involved in wound healing and extracellular matrix remodeling [42,43]. Despite its theoretical benefits, the current body of evidence is limited to small-scale studies and case series, with considerable heterogeneity in methodology, patient selection, and outcome measures. In all included studies, PRP was administered via injection into the pubocervical fascia, with findings indicating higher anatomical success rates and lower reoperation rates compared to control groups [29,30,31]. Due to the high heterogeneity regarding the validation questionnaires or scales used, it seems rather problematic to make any concrete conclusions about the impact of PRP in patients’ quality of life. However, a subjective success rate of 89%, as measured by the PGI-I questionnaire, was reported [29], while the VAS scale indicated a subjective failure rate of 12.5% in a pilot study by Einarsson et al. [30]. Furthermore, a 20% increase in sexual activity evaluated by P-QoL was demonstrated by Gorlero et al. [31].
Regarding SUI, all studies included in our scoping review utilized peri-urethral PRP injections, targeting the anatomical deficits of the urethra. This approach was based on PRP’s regenerative potential, including its capacity to enhance tissue repair, promote angiogenesis, and provide neuroprotective effects [44]. As observed in the POP trials, the use of heterogeneous questionnaires in the SUI studies similarly complicates outcome evaluation and restricts the ability to draw definitive conclusions. Nevertheless, each study reported a statistically significant improvement based on the specific questionnaire utilized. Daneshpajoch et al., however, demonstrated significantly greater improvement in outcomes with the mid-urethral sling procedure [40]. Regarding subjective cure rates, Grigoriadis et al. reported a significantly higher rate in the PRP group (32%) compared to the control group (4%) (p < 0.01) [32]. Comparable findings were reported by Saraluck et al., with 90% of patients in the intervention group experiencing a subjective improvement of at least 50% in their SUI symptoms [34]. Athanasiou et al. reported a 10% subjective cure rate, while 80% of patients experienced a perceived improvement in their symptoms [33].
PRP injections have been widely studied across diverse medical fields—including wound care, orthopedics, urology, dental surgery, and cosmetic procedures—and have consistently demonstrated a favorable safety profile, with no reports of serious adverse events such as infections, bleeding, or nerve damage [45,46]. Consistent with these findings, our scoping review revealed that none of the patients receiving PRP injections experienced serious complications. Only a small proportion—less than 1% (3 out of 317 patients)—reported minor adverse events, including vaginal spotting, discomfort, and urinary straining. These issues were self-limiting and resolved following a brief period of intermittent self-catheterization [37,41].
It is noteworthy that there are limitations that need to be addressed. First of all, as already mentioned, the considerable variability across the included studies—in PRP preparation methods, dosage, injection sites, and treatment duration—makes a systematic review unfeasible. Furthermore, the small number of enrolled patients and the short follow-up limit the generalizability of the results. Finally, it should be acknowledged that not all studies reported adverse effects following the application of PRP, representing a noteworthy limitation in the evidence base of the included trials.
5. Conclusions
According to our scoping review, PRP may contribute to the treatment of POP and SUI. However, evidence on PRP utilization in this field is scarce, and there is still no consensus about the ideal number or frequency of treatment sessions. Thus, the need for larger-scale RCTs with longer follow-up is of great importance.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/jpm15060214/s1, Table S1: PRISMA-ScR; Table S2: JBI Critical Appraisal Checklist; Table S3: Demographic table-POP; Table S4: Demographic table-SUI.
Author Contributions
Conceptualization, G.K.N., A.P., and L.V.; methodology, A.P. and L.V.; formal analysis, A.P. and G.K.N.; investigation, L.V. and A.P.; resources, A.P. and L.V.; data curation, L.V. and S.B.; software, L.V., S.B.; validation S.B., S.S., and G.K.N.; writing—original draft preparation, review and editing A.P. and L.V.; project administration, A.P. and L.V.; visualization, G.K.N., S.B. and S.S.; supervision, G.K.N. and S.S. All authors have read and agreed to the published version of the manuscript.
Funding
The authors received no financial support for the research, authorship, and/or publication of this article.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| POP | Pelvic Organ Prolapse |
| SUI | Stress Urinary Incontinence |
| PRP | Platelet-rich plasma |
| RCT | Randomized Controlled Trial |
| FDA | Food and Drug Administration |
| ICS | International Continence Society |
| UI | Urinary Incontinence |
| PFMT | Pelvic Floor Muscle Training |
| BMI | Body Mass Index |
| POP-Q | Pelvic Organ Prolapse Quantification |
| APG | Autologous Platelet Gel |
| PGI-I | Patient’s Global Impression of Improvement |
| VAS | Visual Analogue Scale |
| P-QoL | Prolapse Quality of Life |
| ICIQ-FLUTS | Incontinence Questionnaire—Female Lower Urinary Tract Symptoms |
| KHQ | King’s Health Questionnaire |
| CST | Cough Stress Test |
| FSFI | Female Sexual Function Index |
| QUID | Questionnaire for Urinary Incontinence Diagnosis |
| I-QoL | Incontinence Quality of Life |
| PFMT | Pelvic Floor Muscle Training |
| UDI-6 | Urinary Distress Inventory |
| GRA | Global Response Assessment |
| IIQ-7 | Incontinence Impact Questionnaire |
| VUDS | Videourodynamic Study |
| ALPP | Abdominal Leak Point Pressure |
| APFQ | Australian Pelvic Floor Questionnaire |
| ICIQ-SF | Incontinence Questionnaire-Short Form |
| OABSS | Overactive Bladder Symptom Scores |
| POPDI-6 | Pelvic Organ Prolapse Distress Inventory 6 |
| FSFI | Female Sexual Function Index |
References
- Haylen, B.T.; Maher, C.F.; Barber, M.D.; Camargo, S.; Dandolu, V.; Digesu, A.; Goldman, H.B.; Huser, M.; Milani, A.L.; Moran, P.A.; et al. An International Urogynecological Association (IUGA)/International Continence Society (ICS) Joint Report on the Terminology for Female Pelvic Organ Prolapse (POP). Int. Urogynecol. J. 2016, 27, 165–194. [Google Scholar] [CrossRef] [PubMed]
- Weintraub, A.Y.; Glinter, H.; Marcus-Braun, N. Narrative Review of the Epidemiology, Diagnosis and Pathophysiology of Pelvic Organ Prolapse. Int. Braz. J. Urol. 2020, 46, 5–14. [Google Scholar] [CrossRef]
- Nüssler, E.; Granåsen, G.; Bixo, M.; Löfgren, M. Long-Term Outcome after Routine Surgery for Pelvic Organ Prolapse—A National Register-Based Cohort Study. Int. Urogynecol. J. 2022, 33, 1863–1873. [Google Scholar] [CrossRef]
- Salvatore, S.; Siesto, G.; Serati, M. Risk Factors for Recurrence of Genital Prolapse. Curr. Opin. Obstet. Gynecol. 2010, 22, 420–424. [Google Scholar] [CrossRef] [PubMed]
- Slack, M. Management of Prolapse of the Anterior Compartment. BJOG 2004, 111, 67–72. [Google Scholar] [CrossRef][Green Version]
- Taylor, D.W.; Petrera, M.; Hendry, M.; Theodoropoulos, J.S. A Systematic Review of the Use of Platelet-Rich Plasma in Sports Medicine as a New Treatment for Tendon and Ligament Injuries. Clin. J. Sport. Med. 2011, 21, 344–352. [Google Scholar] [CrossRef] [PubMed]
- Chow, P.-M.; Chuang, Y.-C.; Hsu, K.C.P.; Shen, Y.-C.; Liu, S.-P. Impact of Female Stress Urinary Incontinence on Quality of Life, Mental Health, Work Limitation, and Healthcare Seeking in China, Taiwan, and South Korea (LUTS Asia): Results from a Cross-Sectional, Population-Based Study. Int. J. Womens Health 2022, 14, 1871–1880. [Google Scholar] [CrossRef]
- Vitale, S.G.; La Rosa, V.L.; Rapisarda, A.M.C.; Laganà, A.S. Sexual Life in Women with Stress Urinary Incontinence. Oman Med. J. 2017, 32, 174–175. [Google Scholar] [CrossRef]
- Haylen, B.T.; de Ridder, D.; Freeman, R.M.; Swift, S.E.; Berghmans, B.; Lee, J.; Monga, A.; Petri, E.; Rizk, D.E.; Sand, P.K.; et al. An International Urogynecological Association (IUGA)/International Continence Society (ICS) Joint Report on the Terminology for Female Pelvic Floor Dysfunction. Neurourol. Urodyn. 2010, 29, 4–20. [Google Scholar] [CrossRef]
- Ostrzenski, A. The New Etiology and Surgical Therapy of Stress Urinary Incontinence in Women. Eur. J. Obstet. Gynecol. Reprod. Biol. 2020, 245, 26–34. [Google Scholar] [CrossRef]
- Kowalik, C.G.; Dmochowski, R.R.; De, E.J.B. Surgery for Female SUI: The ICI Algorithm. Neurourol. Urodyn. 2019, 38, S21–S27. [Google Scholar] [CrossRef]
- Capobianco, G.; Madonia, M.; Morelli, S.; Dessole, F.; De Vita, D.; Cherchi, P.L.; Dessole, S. Management of Female Stress Urinary Incontinence: A Care Pathway and Update. Maturitas 2018, 109, 32–38. [Google Scholar] [CrossRef]
- Kobashi, K.C.; Vasavada, S.; Bloschichak, A.; Hermanson, L.; Kaczmarek, J.; Kim, S.K.; Kirkby, E.; Malik, R. Updates to Surgical Treatment of Female Stress Urinary Incontinence (SUI): AUA/SUFU Guideline (2023). J. Urol. 2023, 209, 1091–1098. [Google Scholar] [CrossRef] [PubMed]
- Nambiar, A.K.; Arlandis, S.; Bø, K.; Cobussen-Boekhorst, H.; Costantini, E.; de Heide, M.; Farag, F.; Groen, J.; Karavitakis, M.; Lapitan, M.C.; et al. European Association of Urology Guidelines on the Diagnosis and Management of Female Non-Neurogenic Lower Urinary Tract Symptoms. Part 1: Diagnostics, Overactive Bladder, Stress Urinary Incontinence, and Mixed Urinary Incontinence. Eur. Urol. 2022, 82, 49–59. [Google Scholar] [CrossRef] [PubMed]
- Kohler, N.; Lipton, A. Platelets as a Source of Fibroblast Growth-Promoting Activity. Exp. Cell Res. 1974, 87, 297–301. [Google Scholar] [CrossRef] [PubMed]
- Tian, J.; Cheng, L.; Cui, X.; Lei, X.; Tang, J.; Cheng, B. Application of Standardized Platelet-rich Plasma in Elderly Patients with Complex Wounds. Wound Repair. Regen. 2019, 27, 268–276. [Google Scholar] [CrossRef]
- Eppley, B.L.; Pietrzak, W.S.; Blanton, M. Platelet-Rich Plasma: A Review of Biology and Applications in Plastic Surgery. Plast. Reconstr. Surg. 2006, 118, 147e–159e. [Google Scholar] [CrossRef]
- Streit-Ciećkiewicz, D.; Kołodyńska, A.; Futyma-Gąbka, K.; Grzybowska, M.; Gołacki, J.; Futyma, K. Platelet Rich Plasma in Gynecology—Discovering Undiscovered—Review. Int. J. Environ. Res. Public Health 2022, 19, 5284. [Google Scholar] [CrossRef]
- Mehta, S.; Watson, J.T. Platelet Rich Concentrate: Basic Science and Current Clinical Applications. J. Orthop. Trauma. 2008, 22, 432–438. [Google Scholar] [CrossRef]
- Rutkowski, J.L.; Thomas, J.M.; Bering, C.L.; Speicher, J.L.; Radio, N.M.; Smith, D.M.; Johnson, D.A. Analysis of a Rapid, Simple, and Inexpensive Technique Used to Obtain Platelet-Rich Plasma for Use in Clinical Practice. J. Oral Implant. 2008, 34, 25–33. [Google Scholar] [CrossRef]
- Sampson, S.; Gerhardt, M.; Mandelbaum, B. Platelet Rich Plasma Injection Grafts for Musculoskeletal Injuries: A Review. Curr. Rev. Musculoskelet. Med. 2008, 1, 165–174. [Google Scholar] [CrossRef]
- Tricco, A.C.; Lillie, E.; Zarin, W.; O’Brien, K.K.; Colquhoun, H.; Levac, D.; Moher, D.; Peters, M.D.J.; Horsley, T.; Weeks, L.; et al. PRISMA Extension for Scoping Reviews (PRISMA-ScR): Checklist and Explanation. Ann. Intern. Med. 2018, 169, 467–473. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.-H.; Lee, P.-J.; Kuo, H.-C. Therapeutic Efficacy of Urethral Sphincter Injections of Platelet-Rich Plasma for the Treatment of Stress Urinary Incontinence Due to Intrinsic Sphincter Deficiency: A Proof-of-Concept Clinical Trial. Int. Neurourol. J. 2021, 25, 51–58. [Google Scholar] [CrossRef]
- Matz, E.L.; Pearlman, A.M.; Terlecki, R.P. Safety and Feasibility of Platelet Rich Fibrin Matrix Injections for Treatment of Common Urologic Conditions. Investig. Clin. Urol. 2018, 59, 61. [Google Scholar] [CrossRef] [PubMed]
- Medel, S.; Alarab, M.; Kufaishi, H.; Drutz, H.; Shynlova, O. Attachment of Primary Vaginal Fibroblasts to Absorbable and Nonabsorbable Implant Materials Coated With Platelet-Rich Plasma. Female Pelvic Med. Reconstr. Surg. 2015, 21, 190–197. [Google Scholar] [CrossRef] [PubMed]
- Chrysanthopoulou, E.L.; Pergialiotis, V.; Perrea, D.; Κourkoulis, S.; Verikokos, C.; Doumouchtsis, S.K. Platelet Rich Plasma as a Minimally Invasive Approach to Uterine Prolapse. Med. Hypotheses 2017, 104, 97–100. [Google Scholar] [CrossRef]
- Nikolopoulos, K.I.; Chrysanthopoulou, E.; Pergialiotis, V.; Korrou, L.M.; Perrea, D.N.; Dimitroulis, D.; Doumouchtsis, S.K. An Animal Experimental Study on Pubourethral Ligament Restoration with Platelet Rich Plasma for the Treatment of Stress Urinary Incontinence. Cent. Eur. J. Urol. 2019, 72, 134–141. [Google Scholar] [CrossRef]
- Gerullis, H.; Georgas, E.; Eimer, C.; Arndt, C.; Barski, D.; Lammers, B.; Klosterhalfen, B.; Borós, M.; Otto, T. Coating with Autologous Plasma Improves Biocompatibility of Mesh Grafts In Vitro: Development Stage of a Surgical Innovation. Biomed. Res. Int. 2013, 2013, 536814. [Google Scholar] [CrossRef][Green Version]
- Atılgan, A.E.; Aydın, A. Cystocele Repair with Platelet-Rich Plasma. Indian J. Surg. 2021, 83, 726–730. [Google Scholar] [CrossRef]
- Einarsson, J.I.; Jonsdottir, K.; Mandle, R. Use of Autologous Platelet Gel in Female Pelvic Organ Prolapse Surgery: A Feasibility Study. J. Minim. Invasive Gynecol. 2009, 16, 204–207. [Google Scholar] [CrossRef]
- Gorlero, F.; Glorio, M.; Lorenzi, P.; Bruno-Franco, M.; Mazzei, C. New Approach in Vaginal Prolapse Repair: Mini-Invasive Surgery Associated with Application of Platelet-Rich Fibrin. Int. Urogynecol. J. 2012, 23, 715–722. [Google Scholar] [CrossRef]
- Grigoriadis, T.; Kalantzis, C.; Zacharakis, D.; Kathopoulis, N.; Prodromidou, A.; Xadzilia, S.; Athanasiou, S. Platelet-Rich Plasma for the Treatment of Stress Urinary Incontinence—A Randomized Trial. Urogynecology 2023, 30, 42–49. [Google Scholar] [CrossRef]
- Athanasiou, S.; Kalantzis, C.; Zacharakis, D.; Kathopoulis, N.; Pontikaki, A.; Grigoriadis, T. The Use of Platelet-Rich Plasma as a Novel Nonsurgical Treatment of the Female Stress Urinary Incontinence: A Prospective Pilot Study. Female Pelvic Med. Reconstr. Surg. 2021, 27, e668–e672. [Google Scholar] [CrossRef]
- Saraluck, A.; Chinthakanan, O.; Kijmanawat, A.; Aimjirakul, K.; Wattanayingcharoenchai, R.; Manonai, J. Autologous Platelet Rich Plasma (A-PRP) Combined with Pelvic Floor Muscle Training for the Treatment of Female Stress Urinary Incontinence (SUI): A Randomized Control Clinical Trial. Neurourol. Urodyn. 2024, 43, 342–353. [Google Scholar] [CrossRef] [PubMed]
- Long, C.-Y.; Lin, K.-L.; Shen, C.-R.; Ker, C.-R.; Liu, Y.-Y.; Loo, Z.-X.; Hsiao, H.-H.; Lee, Y.-C. A Pilot Study: Effectiveness of Local Injection of Autologous Platelet-Rich Plasma in Treating Women with Stress Urinary Incontinence. Sci. Rep. 2021, 11, 1584. [Google Scholar] [CrossRef] [PubMed]
- Ural, Ü.M. The Effect of Injectable Platelet Rich Fibrin as a Nonsurgical Treatment of the Female Stress Urinary Incontinence. Arch. Gynecol. Obstet. 2024, 309, 2229–2236. [Google Scholar] [CrossRef]
- Ashton, L.; Nakatsuka, H.; Johnson, C.M.; Kenne, K.; Kreder, K.J.; Kruse, R.; Wendt, L.; Takacs, E.B.; Vollstedt, A.J. A Single Injection of Platelet-Rich Plasma Injection for the Treatment of Stress Urinary Incontinence in Females: A Randomized Placebo-Controlled Trial. Urology 2024, 193, 63–68. [Google Scholar] [CrossRef] [PubMed]
- Samy Tahoon, A.; El-Din Hussein Salem, H.; Anwar Abdo Mousa, A. The Role of Platelet Rich Plasma Injections in Cases of Stress Incontinence. 2022. Available online: https://www.qeios.com/read/KG77ZQ.2 (accessed on 10 May 2022).
- Behnia-Willison, F.; Nguyen, T.T.T.; Norbury, A.J.; Mohamadi, B.; Salvatore, S.; Lam, A. Promising Impact of Platelet Rich Plasma and Carbon Dioxide Laser for Stress Urinary Incontinence. Eur. J. Obstet. Gynecol. Reprod. Biol. X 2020, 5, 100099. [Google Scholar] [CrossRef]
- Daneshpajooh, A.; Mirzaei, M.; Farsinejad, A.; Naghibzadeh-Tahami, A.; Eslami, A. The Effect of Periurethral Injection of Pure Platelet-Rich Plasma in the Treatment of Urinary Incontinence in Female Patients: A Randomized Clinical Trial. J. Kerman Univ. Med. Sci. 2021, 28, 330–337. [Google Scholar] [CrossRef]
- Chiang, C.-H.; Kuo, H.-C. The Efficacy and Mid-Term Durability of Urethral Sphincter Injections of Platelet-Rich Plasma in Treatment of Female Stress Urinary Incontinence. Front. Pharmacol. 2022, 13, 847520. [Google Scholar] [CrossRef]
- Söderberg, M.W.; Falconer, C.; Byström, B.; Malmström, A.; Ekman, G. Young Women with Genital Prolapse Have a Low Collagen Concentration. Acta Obstet. Gynecol. Scand. 2004, 83, 1193–1198. [Google Scholar] [CrossRef] [PubMed]
- Jackson, S.R.; Eckford, S.D.; Abrams, P.; Avery, N.C.; Tarlton, J.F.; Bailey, A.J. Changes in Metabolism of Collagen in Genitourinary Prolapse. Lancet 1996, 347, 1658–1661. [Google Scholar] [CrossRef] [PubMed]
- Hart, M.L.; Izeta, A.; Herrera-Imbroda, B.; Amend, B.; Brinchmann, J.E. Cell Therapy for Stress Urinary Incontinence. Tissue Eng. Part. B Rev. 2015, 21, 365–376. [Google Scholar] [CrossRef] [PubMed]
- Poulios, E.; Mykoniatis, I.; Pyrgidis, N.; Kalyvianakis, D.; Hatzichristou, D. Platelet-Rich Plasma for the Treatment of Erectile Dysfunction: A Systematic Review of Preclinical and Clinical Studies. Sex. Med. Rev. 2023, 11, 359–368. [Google Scholar] [CrossRef]
- Sukgen, G.; Ellibeş Kaya, A.; Karagün, E.; Çalışkan, E. Platelet-Rich Plasma Administration to the Lower Anterior Vaginal Wall to Improve Female Sexuality Satisfaction. J. Turk. Soc. Obstet. Gynecol. 2020, 16, 228–234. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).