Clinicopathological Features of Non-Small Cell Lung Carcinoma with NRAS Mutation
Abstract
1. Introduction
2. Materials and Methods
2.1. Clinical Data
2.2. Pathological Data
2.3. Molecular Analysis
2.4. Immunohistochemistry
2.5. Statistical Analysis
3. Results
3.1. Clinical Features
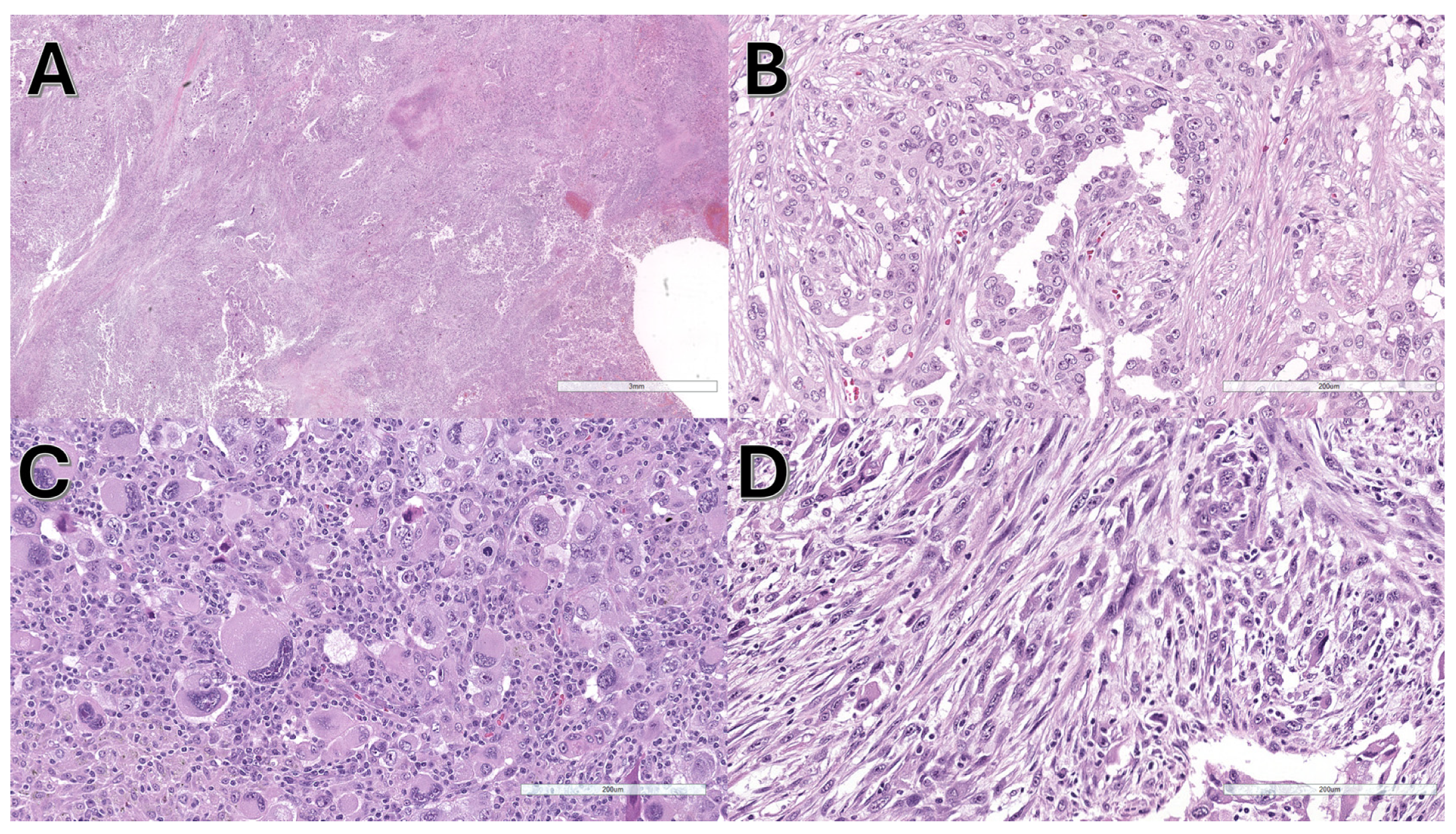
3.2. Pathological and Molecular Features
3.3. Statistical Analyses
4. Discussion
- NRAS mutations were frequently associated with sarcomatoid features.
- NRAS mutations in codon 61 were more frequent and displayed better prognosis than other NRAS mutations, being this difference statistically significant in OS analysis.
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Wagle, N.S.; Jemal, A. Cancer Statistics, 2023. CA Cancer J. Clin. 2023, 73, 17–48. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Vaccarella, S.; Morgan, E.; Li, M.; Etxeberria, J.; Chokunonga, E.; Manraj, S.S.; Kamate, B.; Omonisi, A.; Bray, F. Global Variations in Lung Cancer Incidence by Histological Subtype in 2020: A Population-Based Study. Lancet Oncol. 2023, 24, 1206–1218. [Google Scholar] [CrossRef] [PubMed]
- Ettinger, D.S.; Wood, D.E.; Aisner, D.L.; Akerley, W.; Bauman, J.R.; Bharat, A.; Bruno, D.S.; Chang, J.Y.; Chirieac, L.R.; D’Amico, T.A.; et al. Non–Small Cell Lung Cancer, Version 3.2022, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2022, 20, 497–530. [Google Scholar] [CrossRef] [PubMed]
- Nagl, L.; Pall, G.; Wolf, D.; Pircher, A.; Horvath, L. Molecular Profiling in Lung Cancer. Memo Mag. Eur. Med. Oncol. 2022, 15, 201–205. [Google Scholar] [CrossRef]
- Dinić, J.; Dragoj, M.; Jovanović Stojanov, S.; Stepanović, A.; Lupšić, E.; Pajović, M.; Mohr, T.; Glumac, S.; Marić, D.; Ercegovac, M.; et al. Multidrug-Resistant Profiles in Non-Small Cell Lung Carcinoma Patient-Derived Cells: Implications for Personalized Approaches with Tyrosine Kinase Inhibitors. Cancers 2024, 16, 1984. [Google Scholar] [CrossRef]
- Sholl, L.M. Biomarkers of Response to Checkpoint Inhibitors beyond PD-L1 in Lung Cancer. Mod. Pathol. 2022, 35, 66–74. [Google Scholar] [CrossRef]
- Friedlaender, A.; Perol, M.; Banna, G.L.; Parikh, K.; Addeo, A. Oncogenic Alterations in Advanced NSCLC: A Molecular Super-Highway. Biomark. Res. 2024, 12, 24. [Google Scholar] [CrossRef]
- Ninomiya, H.; Hiramatsu, M.; Inamura, K.; Nomura, K.; Okui, M.; Miyoshi, T.; Okumura, S.; Satoh, Y.; Nakagawa, K.; Nishio, M.; et al. Correlation between Morphology and EGFR Mutations in Lung Adenocarcinomas. Lung Cancer 2009, 63, 235–240. [Google Scholar] [CrossRef]
- Warth, A.; Penzel, R.; Lindenmaier, H.; Brandt, R.; Stenzinger, A.; Herpel, E.; Goeppert, B.; Thomas, M.; Herth, F.J.F.; Dienemann, H.; et al. EGFR, KRAS, BRAF and ALK Gene Alterations in Lung Adenocarcinomas: Patient Outcome, Interplay with Morphology and Immunophenotype. Eur. Respir. J. 2014, 43, 872–883. [Google Scholar] [CrossRef]
- Dernbach, G.; Kazdal, D.; Ruff, L.; Alber, M.; Romanovsky, E.; Schallenberg, S.; Christopoulos, P.; Weis, C.-A.; Muley, T.; Schneider, M.A.; et al. Dissecting AI-Based Mutation Prediction in Lung Adenocarcinoma: A Comprehensive Real-World Study. Eur. J. Cancer 2024, 211, 114292. [Google Scholar] [CrossRef]
- Pao, J.J.; Biggs, M.; Duncan, D.; Lin, D.I.; Davis, R.; Huang, R.S.P.; Ferguson, D.; Janovitz, T.; Hiemenz, M.C.; Eddy, N.R.; et al. Predicting EGFR Mutational Status from Pathology Images Using a Real-World Dataset. Sci. Rep. 2023, 13, 4404. [Google Scholar] [CrossRef] [PubMed]
- Nishino, M.; Klepeis, V.E.; Yeap, B.Y.; Bergethon, K.; Morales-Oyarvide, V.; Dias-Santagata, D.; Yagi, Y.; Mark, E.J.; Iafrate, A.J.; Mino-Kenudson, M. Histologic and Cytomorphologic Features of ALK-Rearranged Lung Adenocarcinomas. Mod. Pathol. 2012, 25, 1462–1472. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Chung, J.-H. Overview of Clinicopathologic Features of ALK-Rearranged Lung Adenocarcinoma and Current Diagnostic Testing for ALK Rearrangement. Transl. Lung Cancer Res. 2015, 4, 149–155. [Google Scholar] [PubMed]
- Park, E.; Choi, Y.-L.; Ahn, M.-J.; Han, J. Histopathologic Characteristics of Advanced-Stage ROS1-Rearranged Non-Small Cell Lung Cancers. Pathol. Res. Pract. 2019, 215, 152441. [Google Scholar] [CrossRef]
- Luo, J.; Ostrem, J.; Pellini, B.; Imbody, D.; Stern, Y.; Solanki, H.S.; Haura, E.B.; Villaruz, L.C. Overcoming KRAS-Mutant Lung Cancer. Am. Soc. Clin. Oncol. Educ. Book 2022, 42, 700–710. [Google Scholar] [CrossRef]
- Pirlog, R.; Piton, N.; Lamy, A.; Guisier, F.; Berindan-Neagoe, I.; Sabourin, J.-C.; Marguet, F. Morphological and Molecular Characterization of KRAS G12C-Mutated Lung Adenocarcinomas. Cancers 2022, 14, 1030. [Google Scholar] [CrossRef]
- Kinno, T.; Tsuta, K.; Shiraishi, K.; Mizukami, T.; Suzuki, M.; Yoshida, A.; Suzuki, K.; Asamura, H.; Furuta, K.; Kohno, T.; et al. Clinicopathological Features of Nonsmall Cell Lung Carcinomas with BRAF Mutations. Ann. Oncol. 2014, 25, 138–142. [Google Scholar] [CrossRef]
- Dehem, A.; Mazieres, J.; Chour, A.; Guisier, F.; Ferreira, M.; Boussageon, M.; Girard, N.; Moro-Sibilot, D.; Cadranel, J.; Zalcman, G.; et al. Characterization of 164 Patients with NRAS Mutated Non-Small Cell Lung Cancer (NSCLC). Lung Cancer 2023, 186, 107393. [Google Scholar] [CrossRef]
- Ohashi, K.; Sequist, L.V.; Arcila, M.E.; Lovly, C.M.; Chen, X.; Rudin, C.M.; Moran, T.; Camidge, D.R.; Vnencak-Jones, C.L.; Berry, L.; et al. Characteristics of Lung Cancers Harboring NRAS Mutations. Clin. Cancer Res. 2013, 19, 2584–2591. [Google Scholar] [CrossRef]
- Sasaki, H.; Okuda, K.; Kawano, O.; Endo, K.; Yukiue, H.; Yokoyama, T.; Yano, M.; Fujii, Y. Nras and Kras Mutation in Japanese Lung Cancer Patients: Genotyping Analysis Using LightCycler. Oncol. Rep. 2007, 18, 623–628. [Google Scholar] [CrossRef]
- Phadke, M.S.; Smalley, K.S.M. Targeting NRAS Mutations in Advanced Melanoma. J. Clin. Oncol. 2023, 41, 2661–2664. [Google Scholar] [CrossRef] [PubMed]
- Bahar, M.E.; Kim, H.J.; Kim, D.R. Targeting the RAS/RAF/MAPK Pathway for Cancer Therapy: From Mechanism to Clinical Studies. Signal. Transduct. Target. Ther. 2023, 8, 455. [Google Scholar] [CrossRef] [PubMed]
- Kun, E.; Tsang, Y.T.M.; Ng, C.W.; Gershenson, D.M.; Wong, K.K. MEK Inhibitor Resistance Mechanisms and Recent Developments in Combination Trials. Cancer Treat. Rev. 2021, 92, 102137. [Google Scholar] [CrossRef] [PubMed]
- Whaby, M.; Ketavarapu, G.; Koide, A.; Mazzei, M.; Mintoo, M.; Glasser, E.; Patel, U.; Nasarre, C.; Sale, M.J.; McCormick, F.; et al. Inhibition and Degradation of NRAS with a Pan-NRAS Monobody. Oncogene 2024, 43, 3489–3497. [Google Scholar] [CrossRef]
- Janku, F.; Kim, T.M.; Iyer, G.; Spreafico, A.; Elez, E.; De Jonge, M.; Yamamoto, N.; Van Der Wekken, A.J.; Ascierto, P.A.; Maur, M.; et al. First-in-Human Study of Naporafenib (LXH254) with or without Spartalizumab in Adult Patients with Advanced Solid Tumors Harboring MAPK Signaling Pathway Alterations. Eur. J. Cancer 2024, 196, 113458. [Google Scholar] [CrossRef]
- Ambrosini-Spaltro, A.; Rengucci, C.; Capelli, L.; Chiadini, E.; Calistri, D.; Bennati, C.; Cravero, P.; Limarzi, F.; Nosseir, S.; Panzacchi, R.; et al. Clinicopathological Features of Non-Small Cell Lung Carcinoma with BRAF Mutation. Curr. Oncol. 2023, 30, 10019–10032. [Google Scholar] [CrossRef]
- Ambrosini-Spaltro, A.; Dubini, A.; Pieri, F.; Ravaglia, C.; Delmonte, A.; Poletti, V. PD-L1 Expression in NSCLC: Role of Cell Blocks and Concordance between Samples. Diagn. Cytopathol. 2021, 49, 303–310. [Google Scholar] [CrossRef]
- Wong, D.W.-S.; Leung, E.L.-H.; So, K.K.-T.; Tam, I.Y.-S.; Sihoe, A.D.-L.; Cheng, L.-C.; Ho, K.-K.; Au, J.S.-K.; Chung, L.-P.; Pik Wong, M.; et al. The EML4-ALK Fusion Gene Is Involved in Various Histologic Types of Lung Cancers from Nonsmokers with Wild-Type EGFR and KRAS. Cancer 2009, 115, 1723–1733. [Google Scholar] [CrossRef]
- Jain, D.; Nambirajan, A.; Borczuk, A.; Chen, G.; Minami, Y.; Moreira, A.L.; Motoi, N.; Papotti, M.; Rekhtman, N.; Russell, P.A.; et al. Immunocytochemistry for Predictive Biomarker Testing in Lung Cancer Cytology. Cancer Cytopathol. 2019, 127, 325–339. [Google Scholar] [CrossRef]
- Herbst, R.S.; Baas, P.; Kim, D.-W.; Felip, E.; Pérez-Gracia, J.L.; Han, J.-Y.; Molina, J.; Kim, J.-H.; Arvis, C.D.; Ahn, M.-J.; et al. Pembrolizumab versus Docetaxel for Previously Treated, PD-L1-Positive, Advanced Non-Small-Cell Lung Cancer (KEYNOTE-010): A Randomised Controlled Trial. Lancet 2016, 387, 1540–1550. [Google Scholar] [CrossRef]
- Timar, J.; Kashofer, K. Molecular Epidemiology and Diagnostics of KRAS Mutations in Human Cancer. Cancer Metastasis Rev. 2020, 39, 1029–1038. [Google Scholar] [CrossRef] [PubMed]
- Hobbs, G.A.; Der, C.J.; Rossman, K.L. RAS Isoforms and Mutations in Cancer at a Glance. J. Cell Sci. 2016, 129, 1287–1292. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Zhang, H.; Huang, S.; Chu, Q. KRAS Mutations in Solid Tumors: Characteristics, Current Therapeutic Strategy, and Potential Treatment Exploration. J. Clin. Med. 2023, 12, 709. [Google Scholar] [CrossRef]
- Huang, L.; Guo, Z.; Wang, F.; Fu, L. KRAS Mutation: From Undruggable to Druggable in Cancer. Signal. Transduct. Target. Ther. 2021, 6, 386. [Google Scholar] [CrossRef]
- Simanshu, D.K.; Nissley, D.V.; McCormick, F. RAS Proteins and Their Regulators in Human Disease. Cell 2017, 170, 17–33. [Google Scholar] [CrossRef]
- Randic, T.; Kozar, I.; Margue, C.; Utikal, J.; Kreis, S. NRAS Mutant Melanoma: Towards Better Therapies. Cancer Treat. Rev. 2021, 99, 102238. [Google Scholar] [CrossRef]
- Johnson, D.B.; Puzanov, I. Treatment of NRAS-Mutant Melanoma. Curr. Treat. Options Oncol. 2015, 16, 15. [Google Scholar] [CrossRef]
- De Maglio, G.; Pasello, G.; Dono, M.; Fiorentino, M.; Follador, A.; Sciortino, M.; Malapelle, U.; Tiseo, M. The Storm of NGS in NSCLC Diagnostic-Therapeutic Pathway: How to Sun the Real Clinical Practice. Crit. Rev. Oncol./Hematol. 2022, 169, 103561. [Google Scholar] [CrossRef]
- Wei, Y.; Wang, L.; Jin, Z.; Jia, Q.; Brcic, L.; Akaba, T.; Chu, Q. Biological Characteristics and Clinical Treatment of Pulmonary Sarcomatoid Carcinoma: A Narrative Review. Transl. Lung Cancer Res. 2024, 13, 635–653. [Google Scholar] [CrossRef]
- Gong, C.; Xiong, H.; Qin, K.; Wang, J.; Cheng, Y.; Zhao, J.; Zhang, J. MET Alterations in Advanced Pulmonary Sarcomatoid Carcinoma. Front. Oncol. 2022, 12, 1017026. [Google Scholar] [CrossRef]
- Pécuchet, N.; Vieira, T.; Rabbe, N.; Antoine, M.; Blons, H.; Cadranel, J.; Laurent-Puig, P.; Wislez, M. Molecular Classification of Pulmonary Sarcomatoid Carcinomas Suggests New Therapeutic Opportunities. Ann. Oncol. 2017, 28, 1597–1604. [Google Scholar] [CrossRef] [PubMed]
- Vieira, T.; Antoine, M.; Hamard, C.; Fallet, V.; Duruisseaux, M.; Rabbe, N.; Rodenas, A.; Cadranel, J.; Wislez, M. Sarcomatoid Lung Carcinomas Show High Levels of Programmed Death Ligand-1 (PD-L1) and Strong Immune-Cell Infiltration by TCD3 Cells and Macrophages. Lung Cancer 2016, 98, 51–58. [Google Scholar] [CrossRef] [PubMed]
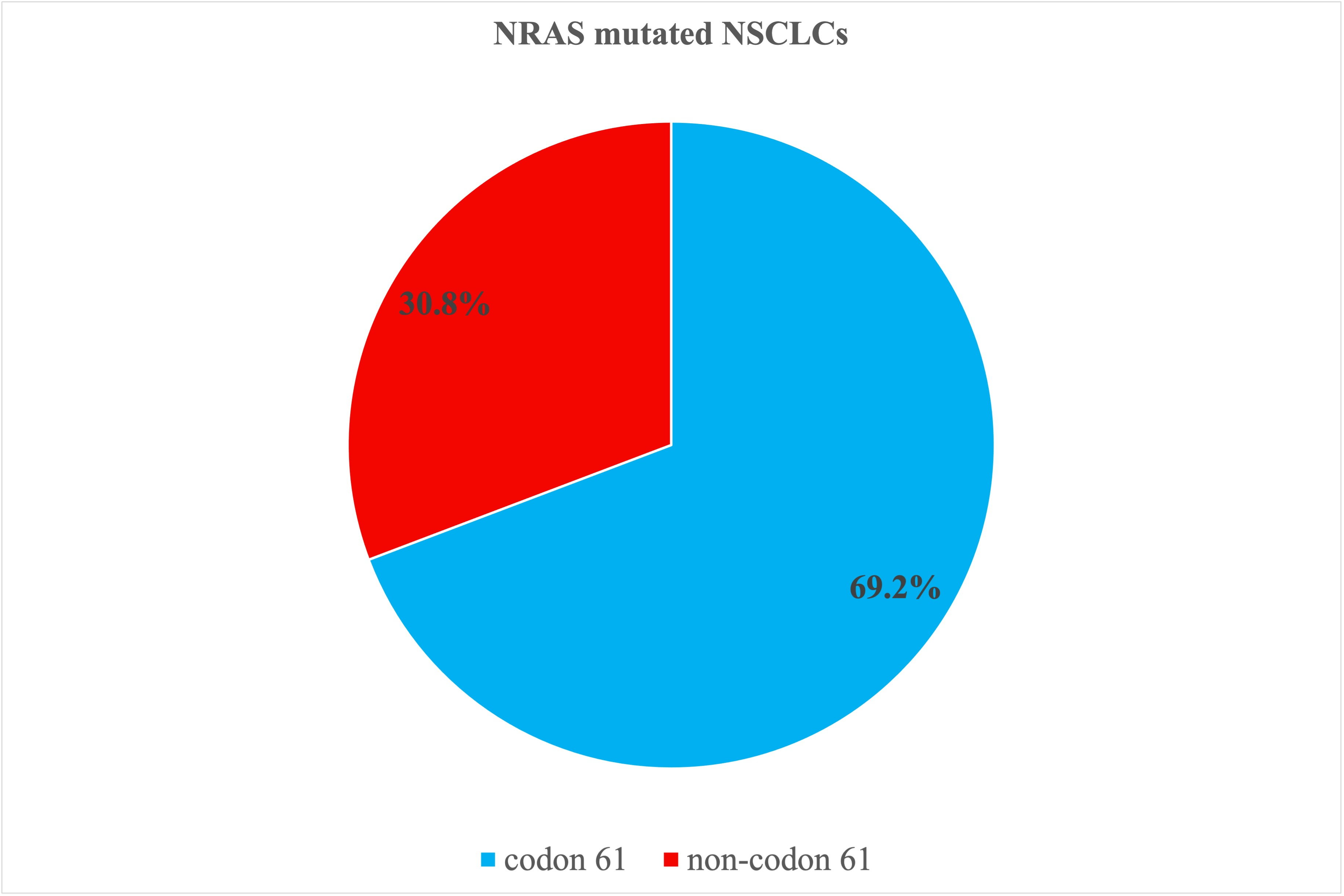

| Codon | N. (%) | Mutation | Exon | N. | |
|---|---|---|---|---|---|
| Codon 61 | 9 (69.2%) | ||||
| c.182A > T, p.(Gln61Leu) | 3 | 4 | |||
| c.181C > A, p.(Gln61Lys) | 3 | 3 | |||
| p.Q61L (c.182 A > T) | 3 | 1 | |||
| c.182A > G, p.(Gln61Arg), (Q61R) | 3 | 1 | |||
| Non-codon 61 | 4 (30.8%) | ||||
| codon 12 | 2 (15.4%) | c.35G > C, p.(Gly12Ala) | 2 | 1 | |
| c.425T > C, p.(Ile142Thr) | 4 | 1 | |||
| codon 13 | 1 (7.7%) | c.38G > A, p.(Gly13Asp) | 3 | 1 | |
| codon 142 | 1 (7.7%) | c.425T > C, p.(Ile142Thr) | 4 | 1 | |
| Total | 13 |
| Case N. | Sex | Age | Exon | Codon | Co-alterations | Sarcomatoid Features | PD-L1 | OS Status | OS Months |
|---|---|---|---|---|---|---|---|---|---|
| 1 | M | 63 | 3 | 61 | None | NA | 1 | 27 | |
| 2 | M | 58 | 3 | 13 | STK11, CDKN2A, NF1, FGFR2 | Y | NA | 1 | 14 |
| 3 | F | 72 | 3 | 61 | None | Low | 0 | 48 | |
| 4 | M | 63 | 2 | 12 | None | Low | 1 | 12 | |
| 5 | F | 66 | 3 | 61 | None | Neg | 0 | 24 | |
| 6 | F | 83 | 2 | 12 | MET, JAK2 | Low | 1 | 0 | |
| 7 | M | 46 | 3 | 61 | RET | High | 1 | 1 | |
| 8 | F | 81 | 4 | 142 | TP53, MET, ATR | Low | NA | ||
| 9 | F | 76 | 3 | 61 | None | Low | 0 | 35 | |
| 10 | M | 51 | 3 | 61 | None | Y | NA | 0 | 41 |
| 11 | M | 79 | 3 | 61 | KIT, ATR, RAC1, AR | Y | Neg | 0 | 4 |
| 12 | M | 65 | 3 | 61 | STK11, EGFR | Low | 1 | 3 | |
| 13 | M | 71 | 3 | 61 | STK11 | Low | NA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ambrosini-Spaltro, A.; Rengucci, C.; Capelli, L.; Chiadini, E.; Bennati, C.; Delmonte, A.; Vecchiarelli, S.; Limarzi, F.; Nosseir, S.; Gallo, G.; et al. Clinicopathological Features of Non-Small Cell Lung Carcinoma with NRAS Mutation. J. Pers. Med. 2025, 15, 199. https://doi.org/10.3390/jpm15050199
Ambrosini-Spaltro A, Rengucci C, Capelli L, Chiadini E, Bennati C, Delmonte A, Vecchiarelli S, Limarzi F, Nosseir S, Gallo G, et al. Clinicopathological Features of Non-Small Cell Lung Carcinoma with NRAS Mutation. Journal of Personalized Medicine. 2025; 15(5):199. https://doi.org/10.3390/jpm15050199
Chicago/Turabian StyleAmbrosini-Spaltro, Andrea, Claudia Rengucci, Laura Capelli, Elisa Chiadini, Chiara Bennati, Angelo Delmonte, Silvia Vecchiarelli, Francesco Limarzi, Sofia Nosseir, Graziana Gallo, and et al. 2025. "Clinicopathological Features of Non-Small Cell Lung Carcinoma with NRAS Mutation" Journal of Personalized Medicine 15, no. 5: 199. https://doi.org/10.3390/jpm15050199
APA StyleAmbrosini-Spaltro, A., Rengucci, C., Capelli, L., Chiadini, E., Bennati, C., Delmonte, A., Vecchiarelli, S., Limarzi, F., Nosseir, S., Gallo, G., Valli, M., Ulivi, P., & Calistri, D. (2025). Clinicopathological Features of Non-Small Cell Lung Carcinoma with NRAS Mutation. Journal of Personalized Medicine, 15(5), 199. https://doi.org/10.3390/jpm15050199









