Tissue Preservation and Access: Modern Innovation in Biobanking Moving Forwards a Personalized Treatment
Abstract
1. Introduction
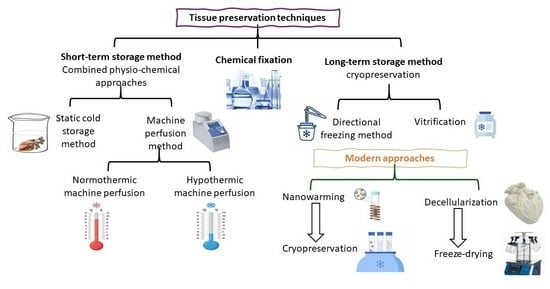
2. Tissue Preservation Techniques
2.1. Chemical Fixation
2.2. Combined Physical and Chemical Approaches for Graft Preservation
2.2.1. Static Cold Storage (SCS): Short-Term Graft Preservation
2.2.2. Machine Perfusion Method: Dynamic Approach for Graft Preservation
2.3. Cryopreservation: Classical Approach
2.3.1. Directional Freezing: Modified Cryopreservation Technique for Long-Term Graft Storage
2.3.2. Vitrification: Modified Cryopreservation for Long-Term Graft Storage
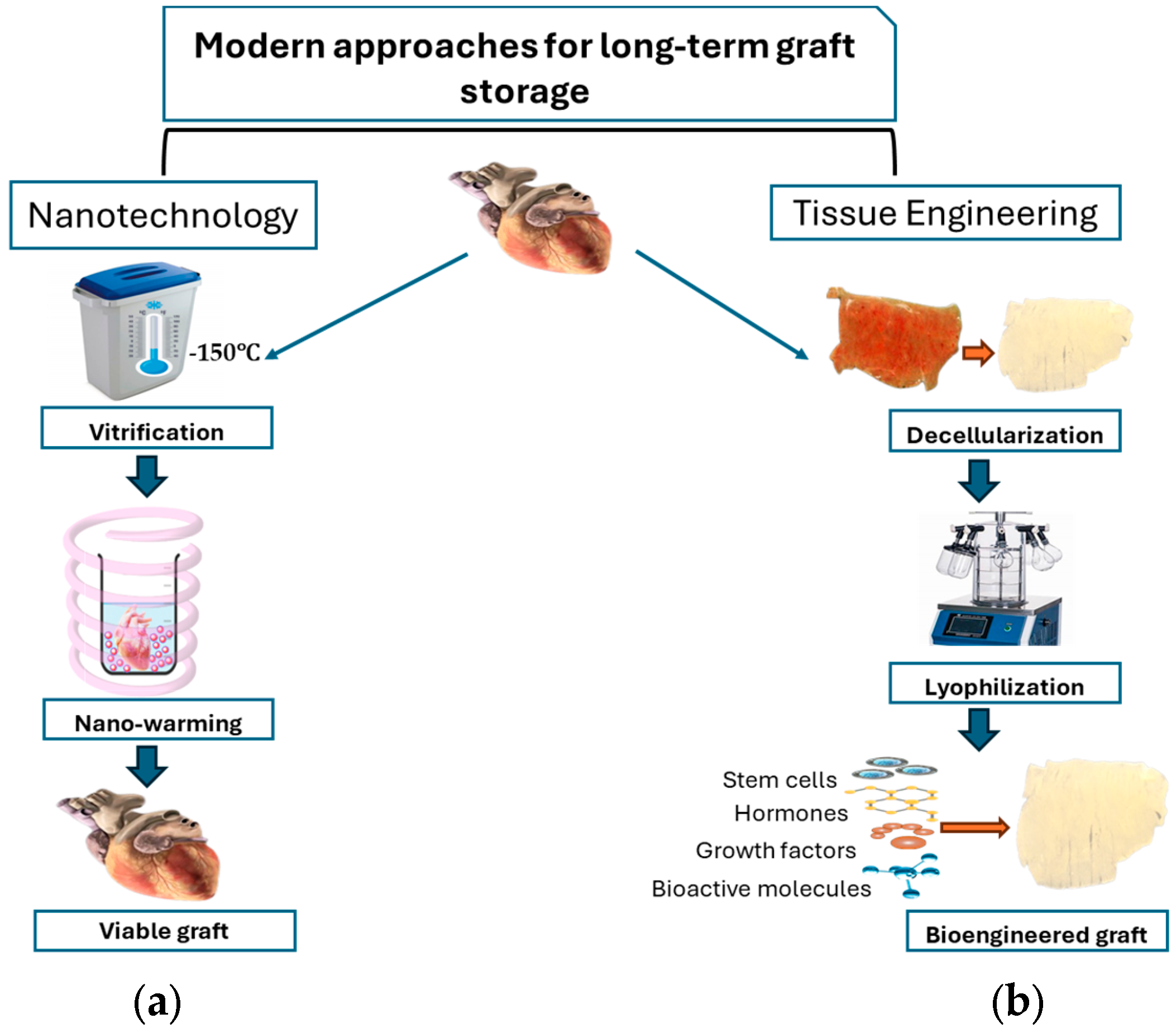
3. Modern Perspectives for Long-Term Tissue Storage
3.1. Nanotechnology in Graft Preservation
Nano-Warming
3.2. Tissue Engineering: A Revolutionary Approach in Graft Preservation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Vaduganathan, M.; Mensah, G.; Turco, J.; Fuster, V.; Roth, G.A. The Global Burden of Cardiovascular Diseases and Risk: A Compass for Future Health. J. Am. Coll. Cardiol. (JACC) 2022, 80, 2361–2371. [Google Scholar] [CrossRef] [PubMed]
- Manji, R.A.; Lee, W.; Cooper, D.K.C. Xenograft Bioprosthetic Heart Valves: Past, Present and Future. Int. J. Surg. 2015, 23, 280–284. [Google Scholar] [CrossRef] [PubMed]
- Saporito, W.F.; Pires, A.C.; Cardoso, S.H.; Correa, J.A.; Carlos De Abreu, L.; Valenti, V.E.; Miller, L.M.R.; Colombari, E. Bovine Pericardium Retail Preserved in Glutaraldehyde and Used as a Vascular Patch. BMC Surg. 2011, 11, 37. [Google Scholar] [CrossRef] [PubMed]
- Pires, A.C.; Saporito, W.F.; Cardoso, S.H.; Ramaciotti, O. Bovine Pericardium Used as a Cardiovascular Patch. Heart Surg. Forum 1999, 2, 60–69. [Google Scholar]
- Thiene, G.; Valente, M. Calcification of valve bioprostheses: The cardiac surgeon’s nightmare. Eur. J. Cardiothorac. Surg. 1994, 8, 476. [Google Scholar] [CrossRef]
- Buttany, J.; Nair, V.; Leong, S.W.; Soor, G.S.; Feindle, C. Carpentier-Edwards Perimount valves morphological findings in surgical explants. J. Card. Surg. 2007, 22, 7–22. [Google Scholar] [CrossRef]
- Ughetto, A.; Roubille, F.; Molina, A.; Battistella, P.; Gaudard, P.; Demaria, R.; Guihaire, J.; Lacampagne, A.; Delmas, C. Heart Graft Preservation Technics and Limits: An Update and Perspectives. Front. Cardiovasc. Med. 2023, 10, 1248606. [Google Scholar] [CrossRef]
- Herijgers, P.; Flameng, W.J. Graft Protection in Organ Transplantation. Best. Pract. Res. Clin. Anaesthesiol. 2008, 22, 225–239. [Google Scholar] [CrossRef]
- Chen, J.; Liu, X.; Hu, Y.; Chen, X.; Tan, S. Cryopreservation of Tissues and Organs: Present, Bottlenecks, and Future. Front. Vet. Sci. 2023, 10, 1201794. [Google Scholar] [CrossRef]
- Kotani, Y.; Ishino, K.; Osaki, S.; Honjo, O.; Suezawa, T.; Kanki, K.; Yutani, C.; Sano, S. Efficacy of MCI-186, a Free-Radical Scavenger and Antioxidant, for Resuscitation of Nonbeating Donor Hearts. J. Thorac. Cardiovasc. Surg. 2007, 133, 1626–1632. [Google Scholar] [CrossRef]
- Jing, L.; Yao, L.; Zhao, M.; Peng, L.P.; Liu, M. Organ Preservation: From the Past to the Future. Acta Pharmacol. Sin. 2018, 39, 845–857. [Google Scholar] [CrossRef]
- Lechiancole, A.; Sponga, S.; Benedetti, G.; Vendramin, I.; Maiani, M.; Spagna, E.; Guzzi, G.; Ferrara, V.; Livi, U. The tell-tale heart. Machine perfusion in heart transplantation. Eur. J. Transplant. 2022, 1, 13–20. [Google Scholar] [CrossRef]
- Ali, A.A.; White, P.; Xiang, B.; Lin, H.Y.; Tsui, S.S.; Ashley, E.; Lee, T.W.; Klein, J.R.H.; Kumar, K.; Arora, R.C.; et al. Hearts from DCD Donors Display Acceptable Biventricular Function after Heart Transplantation in Pigs. Am. J. Transplant. 2011, 11, 1621–1632. [Google Scholar] [CrossRef] [PubMed]
- Repse, S.; Pepe, S.; Anderson, J.; McLean, C.; Rosenfeldt, F.L. Cardiac Reanimation for Donor Heart Transplantation after Cardiocirculatory Death. J. Heart Lung Transplant. 2010, 29, 747–755. [Google Scholar] [CrossRef] [PubMed]
- Koerner, M.M.; Ghodsizad, A.; Schulz, U.; Banayosy, A.E.; Koerfer, R.; Tenderich, G. Normothermic Ex Vivo Allograft Blood Perfusion in Clinical Heart Transplantation. Heart Surg. Forum 2014, 17, E141–E145. [Google Scholar] [CrossRef]
- Nilsson, J.; Jernryd, V.; Qin, G.; Paskevicius, A.; Metzsch, C.; Sjöberg, T.; Steen, S. A Nonrandomized Open-Label Phase 2 Trial of Nonischemic Heart Preservation for Human Heart Transplantation. Nat. Commun. 2020, 11, 2976. [Google Scholar] [CrossRef]
- Polge, C.; Smith, A.U.; Parkes, A.S. Revival of Spermatozoa after Vitrification and Dehydration at Low Temperatures. Nature 1949, 164, 666. [Google Scholar] [CrossRef]
- Pegg, S.E. The History and Principles of Cryopreservation; Thieme Medical Publishers, Inc.: New York, NY, USA, 2002; Volume 20. [Google Scholar]
- LaSala, V.R.; Cordoves, E.M.; Kalfa, D.M. Adaptation of Cold Preservation Techniques to Partial Heart Transplant. J. Thorac. Cardiovasc. Surg. 2025, 169, 395–399. [Google Scholar] [CrossRef]
- de Vries, R.J.; Tessier, S.N.; Banik, P.D.; Nagpal, S.; Cronin, S.E.J.; Ozer, S.; Hafiz, E.O.A.; van Gulik, T.M.; Yarmush, M.L.; Markmann, J.F.; et al. Supercooling Extends Preservation Time of Human Livers. Nat. Biotechnol. 2019, 37, 1131–1136. [Google Scholar] [CrossRef]
- Bruinsma, B.G.; Berendsen, T.A.; Izamis, M.L.; Yeh, H.; Yarmush, M.L.; Uygun, K. Supercooling Preservation and Transplantation of the Rat Liver. Nat. Protoc. 2015, 10, 484–494. [Google Scholar] [CrossRef]
- Que, W.; Hu, X.; Fujino, M.; Terayama, H.; Sakabe, K.; Fukunishi, N.; Zhu, P.; Yi, S.Q.; Yamada, Y.; Zhong, L.; et al. Prolonged Cold Ischemia Time in Mouse Heart Transplantation Using Supercooling Preservation. Transplantation 2020, 104, 1879–1889. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, M.; Buff, S.; Desnos, H.; Loiseau, C.; Bruyère, P.; Joly, T.; Commin, L. Ice Nucleating Agents Allow Embryo Freezing without Manual Seeding. Theriogenology 2017, 104, 173–178. [Google Scholar] [CrossRef] [PubMed]
- Tessier, S.N.; de Vries, R.J.; Pendexter, C.A.; Cronin, S.E.J.; Ozer, S.; Hafiz, E.O.A.; Raigani, S.; Oliveira-Costa, J.P.; Wilks, B.T.; Lopera Higuita, M.; et al. Partial Freezing of Rat Livers Extends Preservation Time by 5-Fold. Nat. Commun. 2022, 13, 4008. [Google Scholar] [CrossRef] [PubMed]
- Năstase, G.; Botea, F.; Beșchea, G.A.; Câmpean, Ș.I.; Barcu, A.; Neacșu, I.; Herlea, V.; Popescu, I.; Chang, T.T.; Rubinsky, B.; et al. Isochoric Supercooling Organ Preservation System. Bioengineering 2023, 10, 934. [Google Scholar] [CrossRef]
- Wan, L.; Powell-Palm, M.J.; Lee, C.; Gupta, A.; Weegman, B.P.; Clemens, M.G.; Rubinsky, B. Preservation of Rat Hearts in Subfreezing Temperature Isochoric Conditions to –8 °C and 78 MPa. Biochem. Biophys. Res. Commun. 2018, 496, 852–857. [Google Scholar] [CrossRef]
- Maffei, S.; Pennarossa, G.; Brevini, T.A.L.; Arav, A.; Gandolfi, F. Beneficial Effect of Directional Freezing on in Vitro Viability of Cryopreserved Sheep Whole Ovaries and Ovarian Cortical Slices. Hum. Reprod. 2014, 29, 114–124. [Google Scholar] [CrossRef]
- Arav, A.; Gavish, Z.; Elami, A.; Natan, Y.; Revel, A.; Silber, S.; Gosden, R.G.; Patrizio, P. Ovarian Function 6 Years after Cryopreservation and Transplantation of Whole Sheep Ovaries. Reprod. Biomed. Online 2010, 20, 48–52. [Google Scholar] [CrossRef]
- Patrizio, P.; Gavish, Z.; Martel, M.; Azodi, M.; Silber, S.; Arav, A. Whole Human Ovaries Cryopreservation Using a Novel Multi-Gradient Freezing Device. Fertil. Steril. 2007, 88, S355. [Google Scholar] [CrossRef]
- Gavish, Z.; Ben-Haim, M.; Arav, A. Cryopreservation of Whole Murine and Porcine Livers. Rejuvenation Res. 2008, 11, 765–772. [Google Scholar] [CrossRef]
- Elami, A.; Gavish, Z.; Korach, A.; Houminer, E.; Schneider, A.; Schwalb, H.; Arav, A. Successful Restoration of Function of Frozen and Thawed Isolated Rat Hearts. J. Thorac. Cardiovasc. Surg. 2008, 135, 666–672.e1. [Google Scholar] [CrossRef]
- Dinh, T.; Son, W.-Y.; Demirtas, E.; Dahan, M.H. How Long Can Oocytes Be Frozen with Vitrification and Still Produce Competent Embryos? A Series of Six Cases. Obstet. Gynecol. Sci. 2022. Epub ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Arav, A. Cryopreservation by Directional Freezing and Vitrification Focusing on Large Tissues and Organs. Cells 2022, 11, 1072. [Google Scholar] [CrossRef] [PubMed]
- He, B.; Su, S.; Yuan, G.; Duan, J.; Zhu, Z.; Wang, Z. Clinical Guideline for Vascularized Composite Tissue Cryopreservation. J. Tissue Eng. Regen. Med. 2021, 15, 527–533. [Google Scholar] [CrossRef] [PubMed]
- Gonzales, F.; Luyet, B. Esumption of Heart-Beat in Chick Embryo Frozen in Liquid Nitrogen. Biodynamica 1950, 7, 126–128. [Google Scholar]
- Rubinsky, B.; Ikeda, M. A Cryomicroscope Using Directional Solidification for the Controlled Freezing of Biological Material. Cryobiology 1985, 22, 55–68. [Google Scholar] [CrossRef]
- Kuleshova, L.; Gianaroli, L.; Magli, C.; Ferraretti, A.; Trounson, A. Birth Following Vitrification of a Small Number of Human Oocytes: Case Report. Hum. Reprod. 1999, 14, 3077–3079. [Google Scholar] [CrossRef]
- Mukaida, T.; Wada, S.; Takahashi, K.; Pedro, P.B.; An, T.Z.; Kasai, M. Vitrification of Human Embryos Based on the Assessment of Suitable Conditions for 8-Cell Mouse Embryos. Hum. Reprod. 1998, 13, 2874–2879. [Google Scholar] [CrossRef]
- Kometas, M.; Christman, G.M.; Kramer, J.; Rhoton-Vlasak, A. Methods of Ovarian Tissue Cryopreservation: Is Vitrification Superior to Slow Freezing?—Ovarian Tissue Freezing Methods. Reprod. Sci. 2021, 28, 3291–3302. [Google Scholar] [CrossRef]
- Jiang, H.; Zhao, Q.; Ye, X. Application of Nanomaterials in Heart Transplantation: A Narrative Review. J. Thorac. Dis. 2024, 16, 3389–3405. [Google Scholar] [CrossRef]
- Ozgur, O.S.; Namsrai, B.-E.; Pruett, T.L.; Bischof, J.C.; Toner, M.; Finger, E.B.; Uygun, K. Current Practice and Novel Approaches in Organ Preservation. Front. Transplant. 2023, 2, 11568435. [Google Scholar] [CrossRef]
- Andrew, D.V.; Rebecca, S.; Christa, H.; Patrick, G.D.; Kristi, L.H.; Marc, H.; David, C.F.; Joseph, W.T.; Kelvin, G.M.B.; Taufiek, K.R. The Impact of Heart Valve and Partial Heart Transplant Models on the Development of Banking Methods for Tissues and Organs: A Concise Review. Cryobiology 2024, 115, 104880. [Google Scholar] [CrossRef]
- Manuchehrabadi, N.; Gao, Z.; Zhang, J.; Ring, H.L.; Shao, Q.; Liu, F.; McDermott, M.; Fok, A.; Rabin, Y.; Brockbank, K.G.M.; et al. Improved Tissue Cryopreservation Using Inductive Heating of Magnetic Nanoparticles. Sci. Transl. Med. 2017, 9, eaah4586. [Google Scholar] [CrossRef] [PubMed]
- Chiu-Lam, A.; Staples, E.; Pepine, C.J.; Rinaldi, C. Perfusion, Cryopreservation, and Nanowarming of Whole Hearts Using Colloidally Stable Magnetic Cryopreservation Agent Solutions. Sci. Adv. 2021, 7, eabe3005. [Google Scholar] [CrossRef]
- Sharma, A.; Rao, J.S.; Han, Z.; Gangwar, L.; Namsrai, B.; Gao, Z.; Ring, H.L.; Magnuson, E.; Etheridge, M.; Wowk, B.; et al. Vitrification and Nanowarming of Kidneys. Adv. Sci. 2021, 8, e2101691. [Google Scholar] [CrossRef] [PubMed]
- Gao, Z.; Namsrai, B.; Han, Z.; Joshi, P.; Rao, J.S.; Ravikumar, V.; Sharma, A.; Ring, H.L.; Idiyatullin, D.; Magnuson, E.C.; et al. Vitrification and Rewarming of Magnetic Nanoparticle-Loaded Rat Hearts. Adv. Mater. Technol. 2022, 7, 2100873. [Google Scholar] [CrossRef]
- Chen, P.; Wang, S.; Chen, Z.; Ren, P.; Hepfer, R.G.; Greene, E.D.; Campbell, L.H.; Helke, K.L.; Nie, X.; Jensen, J.H.; et al. Nanowarming and Ice-Free Cryopreservation of Large Sized, Intact Porcine Articular Cartilage. Commun. Biol. 2023, 6, 220. [Google Scholar] [CrossRef]
- Perota, A.; Lagutina, I.; Duchi, R.; Lazzari, G.; Judor, J.P.; Conchon, S.; Bach, J.M.; Bottio, T.; Gerosa, G.; Costa, C.; et al. Generation of cattle knockout for galactose-α1,3-galactose and N-glycolylneuraminic acid antigens. Xenotransplantation 2019, 26, e12524. [Google Scholar] [CrossRef]
- Moroni, F.; Mirabella, T. Review Article Decellularized Matrices for Cardiovascular Tissue Engineering. Am. J. Stem Cells 2014, 3, 1–20. [Google Scholar]
- Lee, E.J.; Kasper, F.K.; Mikos, A.G. Biomaterials for Tissue Engineering. Ann. Biomed. Eng. 2014, 42, 323–337. [Google Scholar] [CrossRef]
- Khanna, A.; Zamani, M.; Huang, N.F. Extracellular Matrix-Based Biomaterials for Cardiovascular Tissue Engineering. J. Cardiovasc. Dev. Dis. 2021, 8, 137. [Google Scholar] [CrossRef]
- Mendibil, U.; Ruiz-Hernandez, R.; Retegi-Carrion, S.; Garcia-Urquia, N.; Olalde-Graells, B.; Abarrategi, A. Tissue-Specific Decellularization Methods: Rationale and Strategies to Achieve Regenerative Compounds. Int. J. Mol. Sci. 2020, 21, 5447. [Google Scholar] [CrossRef] [PubMed]
- Hennessy, R.S.; Jana, S.; Tefft, B.J.; Helder, M.R.; Young, M.D.; Hennessy, R.R.; Stoyles, N.J.; Lerman, A. Supercritical Carbon Dioxide-Based Sterilization of Decellularized Heart Valves. JACC Basic Transl. Sci. 2017, 2, 71–84. [Google Scholar] [CrossRef]
- de Wit, R.J.J.; van Dis, D.J.; Bertrand, M.E.; Tiemessen, D.; Siddiqi, S.; Oosterwijk, E.; Verhagen, A.F.T.M. Scaffold-Based Tissue Engineering: Supercritical Carbon Dioxide as an Alternative Method for Decellularization and Sterilization of Dense Materials. Acta Biomater. 2023, 155, 323–332. [Google Scholar] [CrossRef]
- Zouhair, S.; Aguiari, P.; Iop, L.; Vásquez-Rivera, A.; Filippi, A.; Romanato, F.; Korossis, S.; Wolkers, W.F.; Gerosa, G. Preservation Strategies for Decellularized Pericardial Scaffolds for Off-the-Shelf Availability. Acta Biomater. 2019, 84, 208–221. [Google Scholar] [CrossRef]
- Golebiowska, A.A.; Intravaia, J.T.; Sathe, V.M.; Kumbar, S.G.; Nukavarapu, S.P. Decellularized Extracellular Matrix Biomaterials for Regenerative Therapies: Advances, Challenges and Clinical Prospects. Bioact. Mater. 2024, 32, 98–123. [Google Scholar] [CrossRef]
- Schleicher, M.; Wendel, H.P.; Fritze, O.; Stock, U.A. In vivo tissue engineering of heart valves: Evolution of a novel concept. Regen. Med. 2009, 4, 613–919. [Google Scholar] [CrossRef]
- Nimjee, S.M.; Rusconi, C.P.; Sullenger, B.A. Aptamaters: An emerging class of therapeutics. Annu. Rev. Med. 2005, 56, 555–583. [Google Scholar] [CrossRef]
- Waqanivavalagi, S.W.; Bhat, S.; Ground, M.B.; Milsom, P.F.; Cornish, J. Clinical performance of decellularized heart valves versus standard tissue conduits: A systematic review and meta-analysis. J. Cardiothorac. Surg. 2020, 15, 260. [Google Scholar] [CrossRef]
- Oripov, F.; Ramm, R.; Falk, C.; Goecke, T.; Ebken, J.; Jashari, R.; Böthig, D.; Horke, A.; Avsar, M.; Bobylev, D.; et al. Serial assessment of early antibody binding to decellularized valved allografts. Front. Cardiovasc. Med. 2022, 9, 895943. [Google Scholar] [CrossRef]
- Ramm, R.; Goecke, T.; Köhler, P.; Tudorache, I.; Cebotari, S.; Ciubotaru, A.; Sarikouch, S.; Höffler, K.; Bothe, F.; Petersen, B.; et al. Immunological and functional features of decellularized xenogeneic heart valves after transplantation into GGTA1-KO pigs. Regen. Biomater. 2021, 8, rbab036. [Google Scholar] [CrossRef]
| Name of Method | Storage Temperature | Storage Time | Storage Phase | Conditions | Reported Tissue Grafts | Advantages | Disadvantages | References |
|---|---|---|---|---|---|---|---|---|
| Supercooling | −4 °C to −8 °C | 2–6 days | Liquid | Cryoprotective agents (CPAs) | Liver and heart |
|
| [20,21,22] |
| Partial freezing | −10 °C to −15 °C | ~5 days | Partially frozen state | Cryoprotecting agents (CPAs) and ice-nucleators | Liver |
|
| [23,24] |
| Isochoric preservation | ~−8 °C | ~2 days | Liquid non-frozen state | Constant volume | Liver and heart |
|
| [25,26] |
| Directional freezing | −80 °C to −196 °C | For weeks | Partially frozen state | Progressive decrease in temperature | Ovaries, liver, and heart |
|
| [27,28,29,30,31] |
| Vitrification and nano-warming | −80 °C to −150 °C | 13 years | Glassy non-crystalline state | Combined use of CPAs with highly regulated rapid cooling system. | Embryos, Cardiac valves, arteries, and myocardia |
|
| [26,32] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tessari, C.; Imran, S.J.; Akbar, N.; Gerosa, G. Tissue Preservation and Access: Modern Innovation in Biobanking Moving Forwards a Personalized Treatment. J. Pers. Med. 2025, 15, 190. https://doi.org/10.3390/jpm15050190
Tessari C, Imran SJ, Akbar N, Gerosa G. Tissue Preservation and Access: Modern Innovation in Biobanking Moving Forwards a Personalized Treatment. Journal of Personalized Medicine. 2025; 15(5):190. https://doi.org/10.3390/jpm15050190
Chicago/Turabian StyleTessari, Chiara, Saima Jalil Imran, Nukhba Akbar, and Gino Gerosa. 2025. "Tissue Preservation and Access: Modern Innovation in Biobanking Moving Forwards a Personalized Treatment" Journal of Personalized Medicine 15, no. 5: 190. https://doi.org/10.3390/jpm15050190
APA StyleTessari, C., Imran, S. J., Akbar, N., & Gerosa, G. (2025). Tissue Preservation and Access: Modern Innovation in Biobanking Moving Forwards a Personalized Treatment. Journal of Personalized Medicine, 15(5), 190. https://doi.org/10.3390/jpm15050190