Functional Mitral Regurgitation in the Transcatheter Era: Diagnostic and Therapeutic Pathways
Abstract
1. Introduction
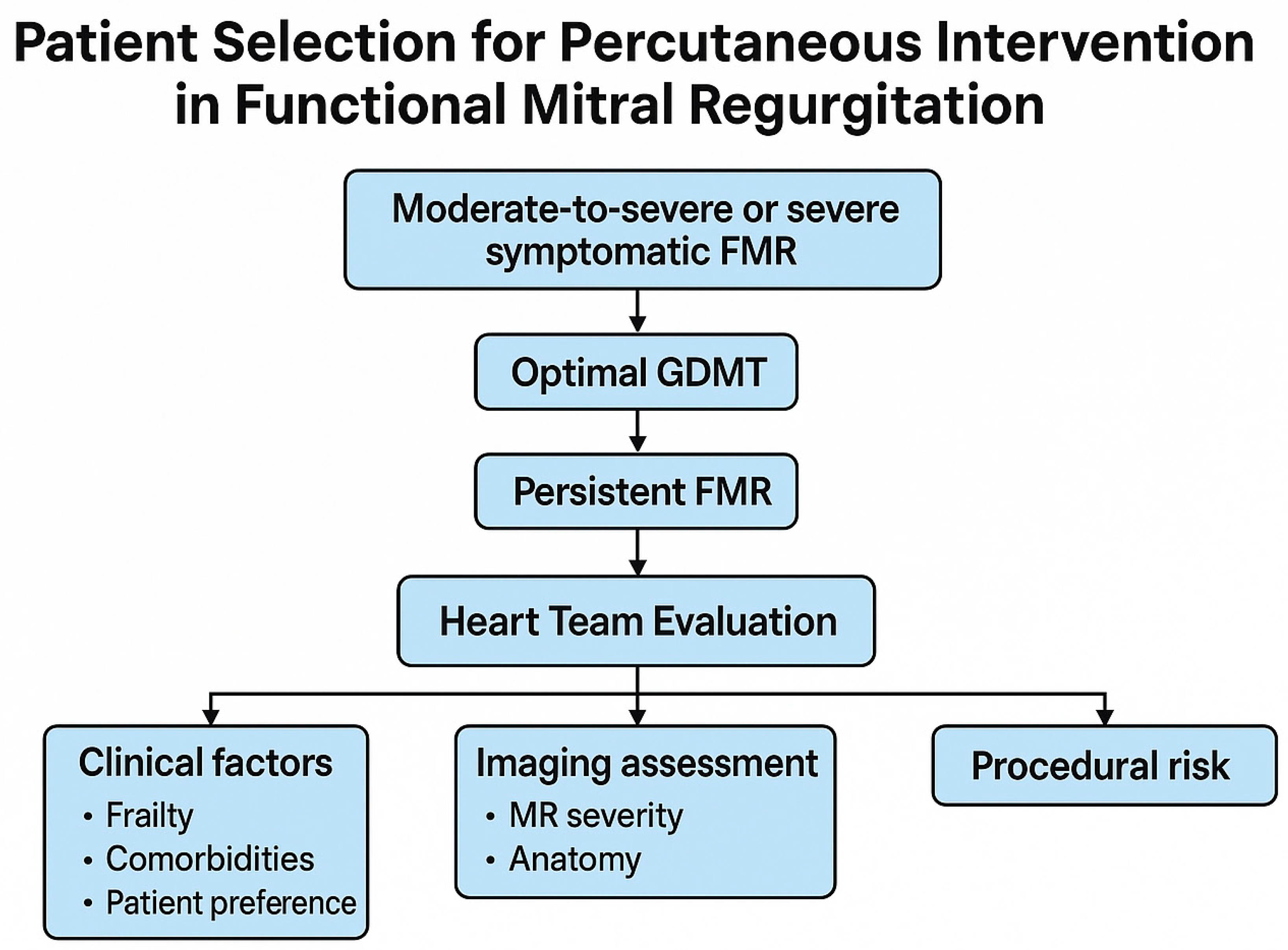
2. Identifying Patients Likely to Benefit from Mitral Percutaneous Intervention
2.1. Multidisciplinary Evaluation and the Heart Team Approach
2.2. The Role of Multimodal Imaging in Patient Selection
3. Device Selection and Procedural Steps
3.1. Transcatheter Mitral Valve Repair
3.1.1. Device Types
3.1.2. Evidence from Clinical Trials
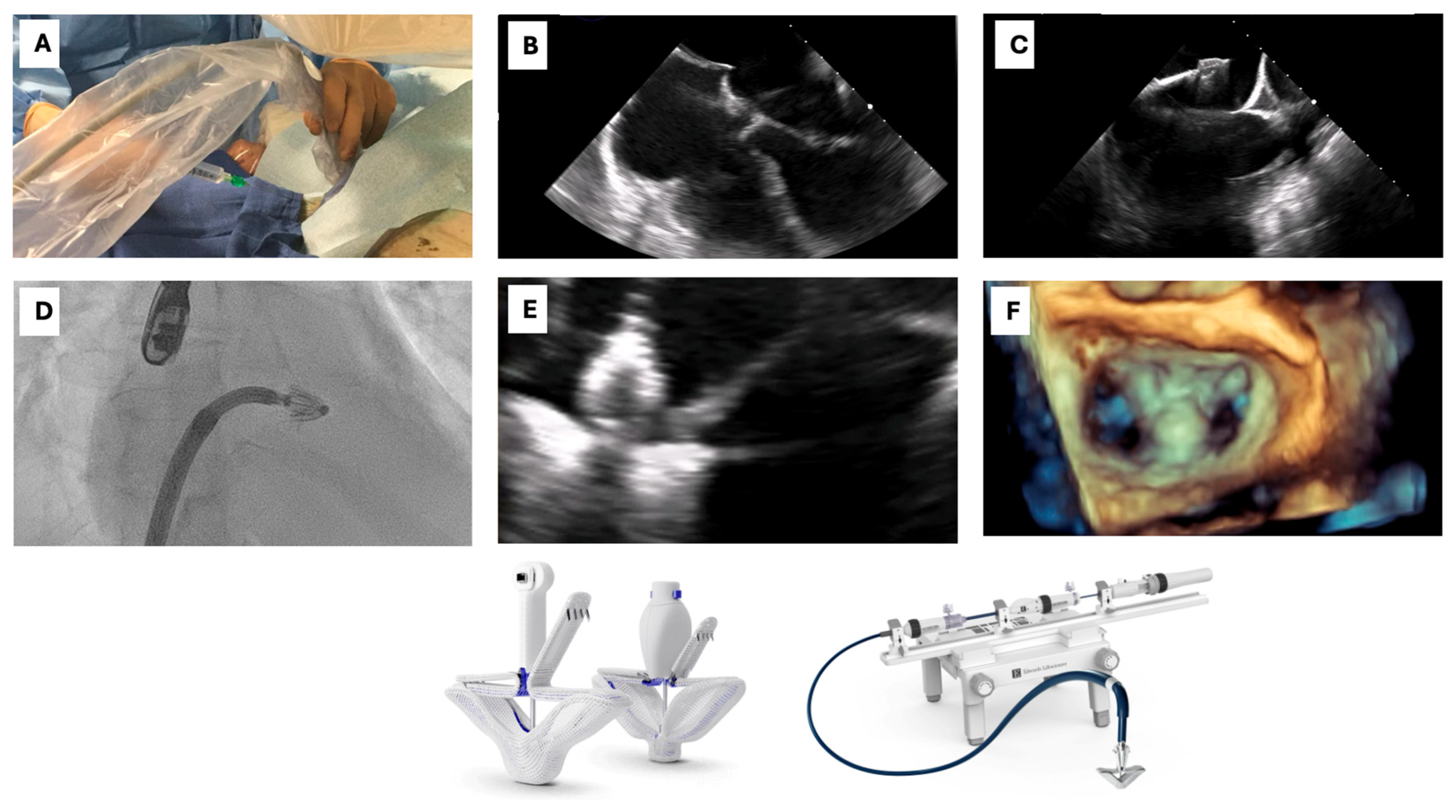
3.1.3. Procedural Steps
- (1)
- Vascular access
- (2)
- Transeptal puncture
- (3)
- Device advancement and implantation
- (4)
- Vascular access closure
- 2.
- Vascular access
- 2.
- Transeptal puncture
- 3.
- TEER Device Delivery and Implantation
- 4.
- Access closure
3.2. Transcatheter Mitral Valve Replacement
3.2.1. Current Landscape and Technical Evolution
3.2.2. Overview of Contemporary TMVR Devices
Tiara (Neovasc)
Tendyne (Abbott Structural)
Intrepid (Medtronic)
HighLife (HighLife SAS)
Sapien M3 (Edwards Lifesciences)
AltaValve (4C Medical Technologies)
Evoque Eos (Edwards Lifesciences)
Innovalve
Other Investigational Systems
3.3. TMVR in Complex Anatomies: Mitral Annular Calcification and Beyond
Which Patients Are Best Suited for TEER or TMVR?
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| Abbreviation | Full Term |
| AF | Atrial Fibrillation |
| ARNi | Angiotensin Receptor–Neprilysin Inhibitor |
| CAD | Coronary Artery Disease |
| CMR | Cardiac Magnetic Resonance |
| CRT | Cardiac Resynchronization Therapy |
| CT | Computed Tomography |
| EROA | Effective Regurgitant Orifice Area |
| FMR | Functional Mitral Regurgitation |
| GDMT | Guideline-Directed Medical Therapy |
| GLS | Global Longitudinal Strain |
| HF | Heart Failure |
| KCCQ-OS | Kansas City Cardiomyopathy Questionnaire–Overall Summary |
| LGE | Late Gadolinium Enhancement |
| LVEDVi | Left Ventricular End-Diastolic Volume Index |
| LVEF | Left Ventricular Ejection Fraction |
| LVOT | Left Ventricular Outflow Tract |
| MR | Mitral Regurgitation |
| NYHA | New York Heart Association |
| RASi | Renin–Angiotensin System Inhibitor |
| TAVR | Transcatheter Aortic Valve Replacement |
| TEER | Transcatheter Edge-to-Edge Repair |
| TEE | Transesophageal Echocardiography |
| TMVR | Transcatheter Mitral Valve Replacement |
References
- Ducas, R.A.; White, C.W.; Wassef, A.W.; Farag, A.; Bhagirath, K.M.; Freed, D.H.; Tam, J.W. Functional Mitral Regurgitation: Current Understanding and Approach to Management. Can. J. Cardiol. 2014, 30, 173–180. [Google Scholar] [CrossRef]
- Dwivedi, A.; Vainrib, A.; Saric, M. Functional mitral regurgitation in patients with heart failure and depressed ejection fraction. Curr. Opin. Cardiol. 2016, 31, 483–492. [Google Scholar] [CrossRef]
- Kumar, M.; Thompson, P.D.; Chen, K. New Perspective on Pathophysiology and Management of Functional Mitral Regurgitation. Trends Cardiovasc. Med. 2023, 33, 386–392. [Google Scholar] [CrossRef]
- Nkomo, V.T.; Gardin, J.M.; Skelton, T.N.; Gottdiener, J.S.; Scott, C.G.; Enriquez-Sarano, M. Burden of valvular heart diseases: A population-based study. Lancet 2006, 368, 1005–1011. [Google Scholar] [CrossRef] [PubMed]
- Enriquez-Sarano, M.; Akins, C.W.; Vahanian, A. Mitral regurgitation. Lancet 2009, 373, 1382–1394. [Google Scholar] [CrossRef]
- Rossi, A.; Dini, F.; Faggiano, P.; Agricola, E.; Cicoira, M.; Frattini, S.; Simioniuc, A.; Gullace, M.; Ghio, S.; Enriquez-Sarano, M.; et al. Independent prognostic value of functional mitral regurgitation in patients with heart failure. A quantitative analysis of 1256 patients with ischaemic and non-ischaemic dilated cardiomyopathy. Heart (Br. Card. Soc.) 2011, 97, 1675–1680. [Google Scholar] [CrossRef] [PubMed]
- Goliasch, G.; Bartko, P.E.; Pavo, N.; Neuhold, S.; Wurm, R.; Mascherbauer, J.; Lang, I.M.; Strunk, G.; Hülsmann, M. Refining the prognostic impact of functional mitral regurgitation in chronic heart failure. Eur. Heart J. 2018, 39, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Agricola, E.; Ielasi, A.; Oppizzi, M.; Faggiano, P.; Ferri, L.; Calabrese, A.; Vizzardi, E.; Alfieri, O.; Margonato, A. Long-term prognosis of medically treated patients with functional mitral regurgitation and left ventricular dysfunction. Eur. J. Heart Fail. 2009, 11, 581–587. [Google Scholar] [CrossRef] [PubMed]
- Ciardetti, N.; Ciatti, F.; Nardi, G.; Di Muro, F.M.; Demola, P.; Sottili, E.; Stolcova, M.; Ristalli, F.; Mattesini, A.; Meucci, F.; et al. Advancements in Transcatheter Aortic Valve Implantation: A Focused Update. Medicina 2021, 57, 711. [Google Scholar] [CrossRef]
- Di Muro, F.M.; Bellino, M.; Esposito, L.; Attisano, T.; Meucci, F.; Mattesini, A.; Galasso, G.; Vecchione, C.; Di Mario, C. Role of Mechanical Circulatory Support in Complex High-Risk and Indicated Percutaneous Coronary Intervention: Current Indications, Device Options, and Potential Complications. J. Clin. Med. 2024, 13, 4931. [Google Scholar] [CrossRef]
- Di Muro, F.M.; Crociani, M.F.; Nardi, G.; Ciardetti, N.; Biagiotti, L.; Bigi, E.; Meucci, F.; Stolcova, M.; Ristalli, F.; Cecchi, E.; et al. Coronary Plaque Characteristics Assessed by Optical Coherence Tomography and Plasma Lipoprotein(a) Levels in Patients With Acute Coronary Syndrome. Catheter. Cardiovasc. Interv. 2025, 106, 64–72. [Google Scholar] [CrossRef]
- Garot, P.; Iriart, X.; Aminian, A.; Kefer, J.; Freixa, X.; Cruz-Gonzalez, I.; Berti, S.; Rosseel, L.; Ibrahim, R.; Korsholm, K.; et al. Value of FEops HEARTguide patient-specific computational simulations in the planning of left atrial appendage closure with the Amplatzer Amulet closure device: Rationale and design of the PREDICT-LAA study. Open Heart 2020, 7, e001326. [Google Scholar] [CrossRef] [PubMed]
- Mirabel, M.; Iung, B.; Baron, G.; Messika-Zeitoun, D.; Détaint, D.; Vanoverschelde, J.-L.; Butchart, E.G.; Ravaud, P.; Vahanian, A. What are the characteristics of patients with severe, symptomatic, mitral regurgitation who are denied surgery? Eur. Heart J. 2007, 28, 1358–1365. [Google Scholar] [CrossRef]
- Stone, G.W.; Lindenfeld, J.; Abraham, W.T.; Kar, S.; Lim, D.S.; Mishell, J.M.; Whisenant, B.; Grayburn, P.A.; Rinaldi, M.; Kapadia, S.R.; et al. Transcatheter Mitral-Valve Repair in Patients with Heart Failure. N. Engl. J. Med. 2018, 379, 2307–2318. [Google Scholar] [CrossRef] [PubMed]
- Obadia, J.-F.; Messika-Zeitoun, D.; Leurent, G.; Iung, B.; Bonnet, G.; Piriou, N.; Lefèvre, T.; Piot, C.; Rouleau, F.; Carrié, D.; et al. Percutaneous Repair or Medical Treatment for Secondary Mitral Regurgitation. N. Engl. J. Med. 2018, 379, 2297–2306. [Google Scholar] [CrossRef] [PubMed]
- Anker, S.D.; Friede, T.; von Bardeleben, R.S.; Butler, J.; Khan, M.S.; Diek, M.; Heinrich, J.; Geyer, M.; Placzek, M.; Ferrari, R.; et al. Percutaneous repair of moderate-to-severe or severe functional mitral regurgitation in patients with symptomatic heart failure: Baseline characteristics of patients in the RESHAPE-HF2 trial and comparison to COAPT and MITRA-FR trials. Eur. J. Heart Fail. 2024, 26, 1608–1615. [Google Scholar] [CrossRef]
- von Stein, P.; Iliadis, C. Transcatheter edge-to-edge repair for mitral regurgitation. Trends Cardiovasc. Med. 2025, 35, 320–325. [Google Scholar] [CrossRef]
- Lancellotti, P.; Pibarot, P.; Chambers, J.; La Canna, G.; Pepi, M.; Dulgheru, R.; Dweck, M.; Delgado, V.; Garbi, M.; Vannan, M.A.; et al. Multi-modality imaging assessment of native valvular regurgitation: An EACVI and ESC council of valvular heart disease position paper. Eur. Heart J.—Cardiovasc. Imaging 2022, 23, e171–e232. [Google Scholar] [CrossRef]
- Namazi, F.; Vo, N.M.; Delgado, V. Imaging of the mitral valve: Role of echocardiography, cardiac magnetic resonance, and cardiac computed tomography. Curr. Opin. Cardiol. 2020, 35, 435–444. [Google Scholar] [CrossRef]
- Gheorghe, L.L.; Mobasseri, S.; Agricola, E.; Wang, D.D.; Milla, F.; Swaans, M.; Pandis, D.; Adams, D.H.; Yadav, P.; Sievert, H.; et al. Imaging for Native Mitral Valve Surgical and Transcatheter Interventions. JACC Cardiovasc. Imaging 2021, 14, 112–127. [Google Scholar] [CrossRef]
- Zoghbi, W.A.; Adams, D.; Bonow, R.O.; Enriquez-Sarano, M.; Foster, E.; Grayburn, P.A.; Hahn, R.T.; Han, Y.; Hung, J.; Lang, R.M.; et al. Recommendations for Noninvasive Evaluation of Native Valvular Regurgitation: A Report from the American Society of Echocardiography Developed in Collaboration with the Society for Cardiovascular Magnetic Resonance. J. Am. Soc. Echocardiogr. 2017, 30, 303–371. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.; Gonzales, H.; Tsai, S.; Lowenstern, A.; Lindenfeld, J. Functional Mitral Regurgitation and the Role of Transcatheter Repair. Struct. Heart 2024, 9, 100347. [Google Scholar] [CrossRef]
- Pagnesi, M.; Adamo, M.; Sama, I.E.; Anker, S.D.; Cleland, J.G.; Dickstein, K.; Filippatos, G.S.; Lang, C.C.; Ng, L.L.; Ponikowski, P.; et al. Impact of mitral regurgitation in patients with worsening heart failure: Insights from BIOSTAT-CHF. Eur. J. Heart Fail. 2021, 23, 1750–1758. [Google Scholar] [CrossRef] [PubMed]
- Di Muro, F.M.; Vogel, B.; Sartori, S.; Tchetche, D.; Feng, Y.; Petronio, A.S.; Mehilli, J.; Bay, B.; Gitto, M.; Lefevre, T.; et al. Impact of Baseline Left Ventricular Ejection Fraction on Midterm Outcomes in Women Undergoing Transcatheter Aortic Valve Implantation: Insight from the WIN-TAVI Registry. Am. J. Cardiol. 2025, 236, 56–63. [Google Scholar] [CrossRef]
- Vahanian, A.; Beyersdorf, F.; Praz, F.; Milojevic, M.; Baldus, S.; Bauersachs, J.; Capodanno, D.; Conradi, L.; De Bonis, M.; De Paulis, R.; et al. 2021 ESC/EACTS Guidelines for the management of valvular heart disease: Developed by the Task Force for the management of valvular heart disease of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS). Eur. Heart J. 2022, 43, 561–632. [Google Scholar] [CrossRef]
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Čelutkienė, J.; Chioncel, O.; et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: Developed by the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) With the special contribution of the Heart Failure Association (HFA) of the ESC. Eur. Heart J. 2021, 42, 3599–3726. [Google Scholar] [CrossRef]
- Spinka, G.; Bartko, P.E.; Heitzinger, G.; Prausmüller, S.; Winter, M.-P.; Arfsten, H.; Strunk, G.; Rosenhek, R.; Kastl, S.; Hengstenberg, C.; et al. Guideline directed medical therapy and reduction of secondary mitral regurgitation. Eur. Heart J.—Cardiovasc. Imaging 2022, 23, 755–764. [Google Scholar] [CrossRef]
- Kang, D.-H.; Park, S.-J.; Shin, S.-H.; Hwang, I.-C.; Yoon, Y.E.; Kim, H.-K.; Kim, M.; Kim, M.-S.; Yun, S.-C.; Song, J.-M.; et al. Ertugliflozin for Functional Mitral Regurgitation Associated With Heart Failure: EFFORT Trial. Circulation 2024, 149, 1865–1874. [Google Scholar] [CrossRef]
- Lin, Y.; Chen, C.; Shih, J.; Cheng, B.; Chang, C.; Lin, M.; Ho, C.; Chen, Z.; Fisch, S.; Chang, W. Dapagliflozin Improves Cardiac Hemodynamics and Mitigates Arrhythmogenesis in Mitral Regurgitation-Induced Myocardial Dysfunction. J. Am. Heart Assoc. 2021, 10, e019274. [Google Scholar] [CrossRef]
- Arnold, S.V. Frail Elderly, the Ideal Patients for MitraClip∗. JACC Cardiovasc. Interv. 2017, 10, 1930–1931. [Google Scholar] [CrossRef] [PubMed]
- Di Muro, F.M.; Cirillo, C.; Esposito, L.; Silverio, A.; Ferruzzi, G.J.; D’Elia, D.; Formisano, C.; Romei, S.; Vassallo, M.G.; Di Maio, M.; et al. Valve-in-Valve Transcatheter Aortic Valve Replacement: From Pre-Procedural Planning to Procedural Scenarios and Possible Complications. J. Clin. Med. 2024, 13, 341. [Google Scholar] [CrossRef]
- Guérin, P. Traitement percutané de l’insuffisance mitrale par Mitraclip chez le sujet âgé. Annales de Cardiologie et d’Angéiologie 2018, 67, 474–481. [Google Scholar] [CrossRef]
- Mischa, K.; Roberto, C.; Georg, N.; Silke, K.; David, H.; Christophe, W.; Ivano, R.; Felix, C.T.; Jeremy, K.; Nicolai, M.; et al. Heart team approach in treatment of mitral regurgitation: Patient selection and outcome. Open Heart 2020, 7, e001280. [Google Scholar] [CrossRef]
- Mitnitski, A.; Song, X.; Skoog, I.; Broe, G.; Cox, J.L.; Grunfeld, E.; Rockwood, K. Relative Fitness and Frailty of Elderly Men and Women in Developed Countries and Their Relationship with Mortality. J. Am. Geriatr. Soc. 2005, 53, 2184–2189. [Google Scholar] [CrossRef] [PubMed]
- Metze, C.; Matzik, A.-S.; Scherner, M.; Körber, M.I.; Michels, G.; Baldus, S.; Rudolph, V.; Pfister, R. Impact of Frailty on Outcomes in Patients Undergoing Percutaneous Mitral Valve Repair. JACC Cardiovasc. Interv. 2017, 10, 1920–1929. [Google Scholar] [CrossRef]
- Rios, S.; Li, W.; Mustehsan, M.H.; Hajra, A.; Takahashi, T.; Chengyue, J.; Wu, L.; Katamreddy, A.; Ghalib, N.; Scotti, A.; et al. Impact of Frailty on Outcomes After Transcatheter Edge-to-Edge Repair With MitraClip (from the National Inpatient Sample Database). Am. J. Cardiol. 2022, 179, 58–63. [Google Scholar] [CrossRef]
- Bricknell, R.A.T.; Schwarzman, L.S.; Taylor, J.; Soltes, T.; Vidovich, M.I. A Heart Team Approach to Assessing Frailty in the Cardiac Catheterization Laboratory. Cardiovasc. Revascularization Med. 2022, 43, 38–42. [Google Scholar] [CrossRef]
- Bax, J.J.; Debonnaire, P.; Lancellotti, P.; Ajmone Marsan, N.; Tops, L.F.; Min, J.K.; Piazza, N.; Leipsic, J.; Hahn, R.T.; Delgado, V. Transcatheter Interventions for Mitral Regurgitation: Multimodality Imaging for Patient Selection and Procedural Guidance. JACC Cardiovasc. Imaging 2019, 12, 2029–2048. [Google Scholar] [CrossRef]
- Benfari, G.; Cavalcante, J.L.; Enriquez-Sarano, M. Multimodality imaging in functional mitral regurgitation: Valvular disease and the chamber remodeling quantification. Int. J. Cardiol. 2022, 349, 106–108. [Google Scholar] [CrossRef] [PubMed]
- Robinson, S.; Ring, L.; Augustine, D.X.; Rekhraj, S.; Oxborough, D.; Harkness, A.; Lancellotti, P.; Rana, B. The assessment of mitral valve disease: A guideline from the British Society of Echocardiography. Echo Res. Pract. 2021, 8, G87–G136. [Google Scholar] [CrossRef]
- Galeazzi, M.; Rolando, M.; Berretta, P.; Bifulco, O.; Di Eusanio, M. Atrial Functional Mitral Regurgitation: From Echo to the surgical view. Can. J. Cardiol. 2025, in press. [Google Scholar] [CrossRef]
- Wunderlich, N.C.; Siegel, R.J. Peri-interventional echo assessment for the MitraClip procedure. Eur. Heart J.—Cardiovasc. Imaging 2013, 14, 935–949. [Google Scholar] [CrossRef] [PubMed]
- Cimino, S.; Agati, L.; Filomena, D.; Maestrini, V.; Monosilio, S.; Birtolo, L.I.; Mocci, M.; Mancone, M.; Sardella, G.; Grayburn, P.; et al. 3D Echo Characterization of Proportionate and Disproportionate Functional Mitral Regurgitation before and after Percutaneous Mitral Valve Repair. J. Clin. Med. 2022, 11, 645. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Palumbo, M.C.; Khalique, O.K.; Rong, L.Q.; Sultana, R.; Das, M.; Jantz, J.; Nagata, Y.; Devereux, R.B.; Wong, S.C.; et al. Transcatheter MitraClip repair alters mitral annular geometry—device induced annular remodeling on three-dimensional echocardiography predicts therapeutic response. Cardiovasc. Ultrasound 2019, 17, 31. [Google Scholar] [CrossRef] [PubMed]
- Hausleiter, J.; Stocker, T.J.; Adamo, M.; Karam, N.; Swaans, M.J.; Praz, F. Mitral valve transcatheter edge-to-edge repair. EuroIntervention 2023, 18, 957–976. [Google Scholar] [CrossRef]
- Samad, Z.; Shaw, L.K.; Phelan, M.; Glower, D.D.; Ersboll, M.; Toptine, J.H.; Alexander, J.H.; Kisslo, J.A.; Wang, A.; Mark, D.B.; et al. Long-term outcomes of mitral regurgitation by type and severity. Am. Heart J. 2018, 203, 39–48. [Google Scholar] [CrossRef]
- Lancellotti, P.; Moura, L.; Pierard, L.A.; Agricola, E.; Popescu, B.A.; Tribouilloy, C.; Hagendorff, A.; Monin, J.-L.; Badano, L.; Zamorano, J.L.; et al. European Association of Echocardiography recommendations for the assessment of valvular regurgitation. Part 2: Mitral and tricuspid regurgitation (native valve disease). Eur. J. Echocardiogr. 2010, 11, 307–332. [Google Scholar] [CrossRef]
- Patzelt, J.; Zhang, W.; Sauter, R.; Mezger, M.; Nording, H.; Ulrich, M.; Becker, A.; Patzelt, T.; Rudolph, V.; Eitel, I.; et al. Elevated Mitral Valve Pressure Gradient Is Predictive of Long-Term Outcome After Percutaneous Edge-to-Edge Mitral Valve Repair in Patients With Degenerative Mitral Regurgitation (MR), But Not in Functional MR. J. Am. Heart Assoc. 2019, 8, e011366. [Google Scholar] [CrossRef]
- Sharma, H.; Liu, B.; Mahmoud-Elsayed, H.; Myerson, S.G.; Steeds, R.P. Multimodality Imaging in Secondary Mitral Regurgitation. Front. Cardiovasc. Med. 2020, 7, 546279. [Google Scholar] [CrossRef]
- Velu, J.F.; Hirsch, A.; Boekholdt, S.M.; Koch, K.T.; Marije Vis, M.; Nils Planken, R.; Piek, J.J.; Baan, J., Jr.; Bouma, B.J. Myocardial fibrosis predicts adverse outcome after MitraClip implantation. Catheter. Cardiovasc. Interv. 2019, 93, 1146–1149. [Google Scholar] [CrossRef]
- Spieker, M.; Marpert, J.; Afzal, S.; Scheiber, D.; Bönner, F.; Horn, P.; Kelm, M.; Westenfeld, R. Extent and determinants of left ventricular reverse remodeling in patients with secondary mitral regurgitation undergoing MitraClip implantation. IJC Heart Vasc. 2021, 34, 100804. [Google Scholar] [CrossRef]
- Papapostolou, S.; Kearns, J.; Costello, B.T.; O’Brien, J.; Gutman, S.J.; Nanayakarra, S.; Kaye, D.; Walton, A.; Hare, J.; Stub, D.; et al. Comparison of Pressure vs Volume Overload Ventricular Wall Stress in Patients With Valvular Heart Disease. JACC 2024, 84, 635–644. [Google Scholar] [CrossRef]
- Reid, A.; Ben Zekry, S.; Turaga, M.; Tarazi, S.; Bax, J.J.; Wang, D.D.; Piazza, N.; Bapat, V.N.; Ihdayhid, A.R.; Cavalcante, J.L.; et al. Neo-LVOT and Transcatheter Mitral Valve Replacement: Expert Recommendations. JACC Cardiovasc. Imaging 2021, 14, 854–866. [Google Scholar] [CrossRef] [PubMed]
- Bapat, V.; Rajagopal, V.; Meduri, C.; Farivar, R.S.; Walton, A.; Duffy, S.J.; Gooley, R.; Almeida, A.; Reardon, M.J.; Kleiman, N.S.; et al. Early Experience With New Transcatheter Mitral Valve Replacement. J. Am. Coll. Cardiol. 2018, 71, 12–21. [Google Scholar] [CrossRef] [PubMed]
- Weir-McCall, J.R.; Blanke, P.; Naoum, C.; Delgado, V.; Bax, J.J.; Leipsic, J. Mitral Valve Imaging with CT: Relationship with Transcatheter Mitral Valve Interventions. Radiology 2018, 288, 638–655. [Google Scholar] [CrossRef]
- Bonow, R.O.; O’Gara, P.T.; Adams, D.H.; Badhwar, V.; Bavaria, J.E.; Elmariah, S.; Hung, J.W.; Lindenfeld, J.; Morris, A.; Satpathy, R.; et al. 2019 AATS/ACC/SCAI/STS Expert Consensus Systems of Care Document: Operator and Institutional Recommendations and Requirements for Transcatheter Mitral Valve Intervention. JACC 2020, 76, 96–117. [Google Scholar] [CrossRef]
- Maisano, F.; Alfieri, O.; Banai, S.; Buchbinder, M.; Colombo, A.; Falk, V.; Feldman, T.; Franzen, O.; Herrmann, H.; Kar, S.; et al. The future of transcatheter mitral valve interventions: Competitive or complementary role of repair vs. replacement? Eur. Heart J. 2015, 36, 1651–1659. [Google Scholar] [CrossRef]
- von Bardeleben, R.S.; Rogers, J.H.; Mahoney, P.; Price, M.J.; Denti, P.; Maisano, F.; Rinaldi, M.; Rollefson, W.A.; De Marco, F.; Chehab, B.; et al. Real-World Outcomes of Fourth-Generation Mitral Transcatheter Repair: 30-Day Results From EXPAND G4. JACC Cardiovasc. Interv. 2023, 16, 1463–1473. [Google Scholar] [CrossRef]
- Garcia-Sayan, E.; Raghunathan, D.; Li, F.M.; Dhoble, A.; Sheu, R.D.; Jelacic, S.; Reisman, M.; Smalling, R.W.; Mackensen, G.B. Initial experience with the fourth generation MitraClipTM: Outcomes, procedural aspects, and considerations for device selection. Catheter. Cardiovasc. Interv. 2021, 98, E626–E636. [Google Scholar] [CrossRef] [PubMed]
- Chakravarty, T.; Makar, M.; Patel, D.; Oakley, L.; Yoon, S.H.; Stegic, J.; Singh, S.; Skaf, S.; Nakamura, M.; Makkar, R.R. Transcatheter Edge-to-Edge Mitral Valve Repair With the MitraClip G4 System. JACC Cardiovasc. Interv. 2020, 13, 2402–2414. [Google Scholar] [CrossRef] [PubMed]
- Mauri, V.; Besler, C.; Riebisch, M.; Al-Hammadi, O.; Ruf, T.; Gerçek, M.; Horn, P.; Grothusen, C.; Mehr, M.; Becher, M.U.; et al. German Multicenter Experience With a New Leaflet-Based Transcatheter Mitral Valve Repair System for Mitral Regurgitation. JACC Cardiovasc. Interv. 2020, 13, 2769–2778. [Google Scholar] [CrossRef] [PubMed]
- Hellhammer, K.; Schindhelm, F.; Riebisch, M.; Janosi, R.A.; Lind, A.Y.; Totzeck, M.; Luedike, P.; Rassaf, T.; Mahabadi, A.A. Prospective Analysis of the Feasibility of the PASCAL System for Transcatheter Mitral Valve Repair (OneForAll-Registry). Catheter. Cardiovasc. Interv. 2025, 105, 1510–1515. [Google Scholar] [CrossRef]
- Corpataux, N.; Winkel, M.G.; Kassar, M.; Brugger, N.; Windecker, S.; Praz, F. The PASCAL Device—Early Experience with a Leaflet Approximation Device: What Are the Benefits/Limitations Compared with the MitraClip? Curr. Cardiol. Rep. 2020, 22, 74. [Google Scholar] [CrossRef]
- Stone, G.W.; Abraham, W.T.; Lindenfeld, J.; Kar, S.; Grayburn, P.A.; Lim, D.S.; Mishell, J.M.; Whisenant, B.; Rinaldi, M.; Kapadia, S.R.; et al. Five-Year Follow-up after Transcatheter Repair of Secondary Mitral Regurgitation. N. Engl. J. Med. 2023, 388, 2037–2048. [Google Scholar] [CrossRef]
- Anker, S.D.; Friede, T.; von Bardeleben, R.-S.; Butler, J.; Khan, M.-S.; Diek, M.; Heinrich, J.; Geyer, M.; Placzek, M.; Ferrari, R.; et al. Transcatheter Valve Repair in Heart Failure with Moderate to Severe Mitral Regurgitation. N. Engl. J. Med. 2024, 391, 1799–1809. [Google Scholar] [CrossRef]
- Prasad, P.; Chandrashekar, P.; Golwala, H.; Macon, C.J.; Steiner, J. Functional Mitral Regurgitation: Patient Selection and Optimization. Interv. Cardiol. Clin. 2024, 13, 167–182. [Google Scholar] [CrossRef]
- Grayburn, P.A.; Sannino, A.; Packer, M. Proportionate and Disproportionate Functional Mitral Regurgitation: A New Conceptual Framework That Reconciles the Results of the MITRA-FR and COAPT Trials. JACC Cardiovasc. Imaging 2019, 12, 353–362. [Google Scholar] [CrossRef] [PubMed]
- Keßler, M.; Seeger, J.; Muche, R.; Wöhrle, J.; Rottbauer, W.; Markovic, S. Predictors of rehospitalization after percutaneous edge-to-edge mitral valve repair by MitraClip implantation. Eur. J. Heart Fail. 2019, 21, 182–192. [Google Scholar] [CrossRef]
- Senni, M.; Adamo, M.; Metra, M.; Alfieri, O.; Vahanian, A. Treatment of functional mitral regurgitation in chronic heart failure: Can we get a ‘proof of concept’ from the MITRA-FR and COAPT trials? Eur. J. Heart Fail. 2019, 21, 852–861. [Google Scholar] [CrossRef]
- Kitamura, M.; Kaneko, H.; Schlüter, M.; Schewel, D.; Schmidt, T.; Alessandrini, H.; Kreidel, F.; Neuss, M.; Butter, C.; Kuck, K.-H.; et al. Predictors of mortality in ischaemic versus non-ischaemic functional mitral regurgitation after successful transcatheter mitral valve repair using MitraClip: Results from two high-volume centres. Clin. Res. Cardiol. 2019, 108, 264–272. [Google Scholar] [CrossRef]
- Cormican, D.S.; Drennen, Z.; Sonny, A.; Crowley, J.C.; Gil, I.J.N.; Ramakrishna, H. Functional Mitral Regurgitation in Heart Failure: Analysis of the ESC Multidisciplinary Heart-Team Position Statement and Review of Current Guidelines. J. Cardiothorac. Vasc. Anesth. 2022, 36, 3357–3364. [Google Scholar] [CrossRef]
- Cilingiroglu, M.; Marmagkiolis, K. Predictors of mortality after mitraclip therapy. Catheter. Cardiovasc. Interv. 2016, 87, 476–477. [Google Scholar] [CrossRef]
- Webb, J.G.; Hensey, M.; Szerlip, M.; Schäfer, U.; Cohen, G.N.; Kar, S.; Makkar, R.; Kipperman, R.M.; Spargias, K.; O’Neill, W.W.; et al. 1-Year Outcomes for Transcatheter Repair in Patients With Mitral Regurgitation From the CLASP Study. JACC Cardiovasc. Interv. 2020, 13, 2344–2357. [Google Scholar] [CrossRef] [PubMed]
- Szerlip, M.; Spargias, K.S.; Makkar, R.; Kar, S.; Kipperman, R.M.; O’Neill, W.W.; Ng, M.K.C.; Smith, R.L.; Fam, N.P.; Rinaldi Michael, J.; et al. 2-Year Outcomes for Transcatheter Repair in Patients With Mitral Regurgitation From the CLASP Study. JACC Cardiovasc. Interv. 2021, 14, 1538–1548. [Google Scholar] [CrossRef]
- Lim, D.S.; Smith, R.L.; Gillam, L.D.; Zahr, F.; Chadderdon, S.; Makkar, R.; Bardeleben, R.S.V.; Kipperman, R.M.; Rassi, A.N.; Szerlip, M.; et al. Randomized Comparison of Transcatheter Edge-to-Edge Repair for Degenerative Mitral Regurgitation in Prohibitive Surgical Risk Patients. JACC Cardiovasc. Interv. 2022, 15, 2523–2536. [Google Scholar] [CrossRef]
- Zahr, F.; Smith, R.L.; Gillam, L.D.; Chadderdon, S.; Makkar, R.; Bardeleben, R.S.V.; Ruf, T.F.; Kipperman, R.M.; Rassi, A.N.; Szerlip, M.; et al. 1-Year Outcomes From the CLASP IID Randomized Trial for Degenerative Mitral Regurgitation. JACC Cardiovasc. Interv. 2023, 16, 2803–2816. [Google Scholar] [CrossRef] [PubMed]
- Schneider, L.; Markovic, S.; Mueller, K.; Felbel, D.; Gerçek, M.; Friedrichs, K.; Stolz, L.; Rudolph, V.; Hausleiter, J.; Rottbauer, W.; et al. Mitral Valve Transcatheter Edge-to-Edge Repair Using MitraClip or PASCAL. JACC Cardiovasc. Interv. 2022, 15, 2554–2567. [Google Scholar] [CrossRef]
- Srinivasan, A.; Brown, J.; Ahmed, H.; Daniel, M. PASCAL repair system for patients with mitral regurgitation: A systematic review. Int. J. Cardiol. 2023, 376, 108–114. [Google Scholar] [CrossRef]
- Elbadawi, A.; Dang, A.T.; Hamed, M.; Ali, A.; Saad, M.; Jneid, H.; Chhatriwalla, A.K.; Goel, S.; Bhatt, A.; Mani, P.; et al. Transcatheter edge-to-edge repair for mitral regurgitation using PASCAL or MitraClip. Catheter. Cardiovasc. Interv. 2023, 102, 521–527. [Google Scholar] [CrossRef]
- von Stein, P.; Wienemann, H.; Stein, J.V.; Sugiura, A.; Tanaka, T.; Kavsur, R.; Öztürk, C.; Weber, M.; Haurand, J.M.; Horn, P.; et al. Early Outcomes of Two Large Mitral Valve Transcatheter Edge-to-Edge Repair Devices—A Propensity Score Matched Multicenter Comparison. J. Clin. Med. 2024, 13, 4187. [Google Scholar] [CrossRef]
- Whisenant, B.; Zahr, F. The PASCAL Transcatheter Valve Repair System: A User’s Guide. Struct. Heart 2023, 7, 100204. [Google Scholar] [CrossRef]
- Kadado, A.J.; Islam, A. Iatrogenic atrial septal defect following the MitraClip procedure: A state-of-the-art review. Catheter. Cardiovasc. Interv. 2021, 97, E1043–E1052. [Google Scholar] [CrossRef]
- Di Muro, F.M.; Vogel, B.; Oliva, A.; Bay, B.; Gitto, M.; Dangas, G.D.; Mehran, R. Pharmacology in Structural Intervention for Valvular Heart Disease: Current Practice and Future Perspectives. Struct. Heart 2024, 9, 100360. [Google Scholar] [CrossRef]
- Calabrò, P.; Gragnano, F.; Niccoli, G.; Marcucci, R.; Zimarino, M.; Spaccarotella, C.; Renda, G.; Patti, G.; Andò, G.; Moscarella, E.; et al. Antithrombotic Therapy in Patients Undergoing Transcatheter Interventions for Structural Heart Disease. Circulation 2021, 144, 1323–1343. [Google Scholar] [CrossRef]
- Guedeney, P.; Rodés-Cabau, J.; Ten Berg, J.M.; Windecker, S.; Angiolillo, D.J.; Montalescot, G.; Collet, J.-P. Antithrombotic therapy for transcatheter structural heart intervention. EuroIntervention 2024, 20, 972–986. [Google Scholar] [CrossRef]
- Alperi, A.; Granada, J.F.; Bernier, M.; Dagenais, F.; Rodés-Cabau, J. Current Status and Future Prospects of Transcatheter Mitral Valve Replacement: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2021, 77, 3058–3078. [Google Scholar] [CrossRef] [PubMed]
- Regueiro, A.; Granada, J.F.; Dagenais, F.; Rodés-Cabau, J. Transcatheter Mitral Valve Replacement: Insights From Early Clinical Experience and Future Challenges. J. Am. Coll. Cardiol. 2017, 69, 2175–2192. [Google Scholar] [CrossRef]
- Ben Ali, W.; Ludwig, S.; Duncan, A.; Weimann, J.; Nickenig, G.; Tanaka, T.; Coisne, A.; Vincentelli, A.; Makkar, R.; Webb, J.G.; et al. Characteristics and outcomes of patients screened for transcatheter mitral valve implantation: 1-year results from the CHOICE-MI registry. Eur. J. Heart Fail. 2022, 24, 887–898. [Google Scholar] [CrossRef]
- Ludwig, S.; Perrin, N.; Coisne, A.; Ben, A.W.; Weimann, J.; Adam, M.; Petronio, A.S.; Dumonteil, N.; Sondergaard, L.; Adamo, M.; et al. Clinical outcomes of transcatheter mitral valve replacement: Two-year results of the CHOICE-MI Registry. EuroIntervention 2023, 19, 512–525. [Google Scholar] [CrossRef]
- Cheung, A.; Stub, D.; Moss, R.; Boone, R.; Leipsic, J.A.; Verheye, S.; Banai, S.; Webb, J.G. Transcatheter mitral valve implantation with Tiara bioprosthesis. EuroIntervention 2014, 10, U115–U119. [Google Scholar] [CrossRef][Green Version]
- Cheung, A.; Webb, J.; Verheye, S.; Moss, R.; Boone, R.; Leipsic, J.; Ree, R.; Banai, S. Short-Term Results of Transapical Transcatheter Mitral Valve Implantation for Mitral Regurgitation. JACC 2014, 64, 1814–1819. [Google Scholar] [CrossRef] [PubMed]
- Banai, S.; Verheye, S.; Cheung, A.; Schwartz, M.; Marko, A.; Lane, R.; Jolicoeur, E.M.; Garceau, P.; Biner, S.; Tanguay, J.-F.; et al. Transapical Mitral Implantation of the Tiara Bioprosthesis. JACC Cardiovasc. Interv. 2014, 7, 154–162. [Google Scholar] [CrossRef][Green Version]
- Gössl, M.; Thourani, V.H.; Babaliaros, V.; Conradi, L.; Chehab, B.; Dumonteil, N.; Badhwar, V.; Rizik, D.; Sun, B.; Bae, R.; et al. Early outcomes of transcatheter mitral valve replacement with the Tendyne system in severe mitral annular calcification. EuroIntervention 2022, 17, 1523–1531. [Google Scholar] [CrossRef]
- Bapat, V.; Weiss, E.; Bajwa, T.; Thourani, V.H.; Yadav, P.; Thaden, J.J.; Lim, D.S.; Reardon, M.; Pinney, S.; Adams, D.H.; et al. 2-Year Clinical and Echocardiography Follow-Up of Transcatheter Mitral Valve Replacement With the Transapical Intrepid System. JACC Cardiovasc. Interv. 2024, 17, 1440–1451. [Google Scholar] [CrossRef]
- Zahr, F.; Song, H.K.; Chadderdon, S.M.; Gada, H.; Mumtaz, M.; Byrne, T.; Kirshner, M.; Bajwa, T.; Weiss, E.; Kodali, S.; et al. 30-Day Outcomes Following Transfemoral Transseptal Transcatheter Mitral Valve Replacement. JACC Cardiovasc. Interv. 2022, 15, 80–89. [Google Scholar] [CrossRef] [PubMed]
- Zahr, F.; Song, H.K.; Chadderdon, S.; Gada, H.; Mumtaz, M.; Byrne, T.; Kirshner, M.; Sharma, S.; Kodali, S.; George, I.; et al. 1-Year Outcomes Following Transfemoral Transseptal Transcatheter Mitral Valve Replacement. JACC Cardiovasc. Interv. 2023, 16, 2868–2879. [Google Scholar] [CrossRef]
- Schneider, L.-M.; Worthley, S.; Nickenig, G.; Huczek, Z.; Wojakowski, W.; Tchetche, D.; Dubois, C.; Nasr, M.; Verhees, L.; Rothman, M.; et al. 1-Year Outcomes Following Transfemoral Transseptal Transcatheter Mitral Valve Replacement. JACC Cardiovasc. Interv. 2023, 16, 2854–2865. [Google Scholar] [CrossRef]
- Barbanti, M.; Piazza, N.; Mangiafico, S.; Buithieu, J.; Bleiziffer, S.; Ronsivalle, G.; Scandura, S.; Giuffrida, A.; Popolo, R.A.; Mazzamuto, M.; et al. Transcatheter Mitral Valve Implantation Using the HighLife System. JACC Cardiovasc. Interv. 2017, 10, 1662–1670. [Google Scholar] [CrossRef]
- Penteris, M.; Lampropoulos, K. The SAPIEN M3 system for transcatheter mitral valve replacement: A new era begins. Cardiovasc. Revascularization Med. 2025, in press. [Google Scholar] [CrossRef]
- Alperi, A.; del Val, D.; Ferreira-Neto, A.N.; Bernier, M.; Freitas-Ferraz, A.B.; Dagenais, F.; Rodés-Cabau, J. Device profile of the AltaValve system for transcatheter mitral valve replacement: Overview of its safety and efficacy. Expert Rev. Med. Devices 2020, 17, 627–636. [Google Scholar] [CrossRef] [PubMed]
- Webb, J.; Hensey, M.; Fam, N.; Rodés-Cabau, J.; Daniels, D.; Smith, R.; Szeto, W.; Boone, R.; Ye, J.; Moss, R.; et al. Transcatheter Mitral Valve Replacement With the Transseptal EVOQUE System. JACC Cardiovasc. Interv. 2020, 13, 2418–2426. [Google Scholar] [CrossRef] [PubMed]
- Scorsin, M.; Andreas, M.; Corona, S.; Guta, A.C.; Aruta, P.; Badano, L.P. Novel Transcatheter Mitral Prosthesis Designed to Preserve Physiological Ventricular Flow Dynamics. Ann. Thorac. Surg. 2022, 113, 593–599. [Google Scholar] [CrossRef]
- Eleid, M.F.; Wang, D.D.; Pursnani, A.; Kodali, S.K.; George, I.; Palacios, I.; Russell, H.; Makkar, R.R.; Kar, S.; Satler, L.F.; et al. 2-Year Outcomes of Transcatheter Mitral Valve Replacement in Patients With Annular Calcification, Rings, and Bioprostheses. JACC 2022, 80, 2171–2183. [Google Scholar] [CrossRef]
- Yoon, S.-H.; Whisenant, B.K.; Bleiziffer, S.; Delgado, V.; Dhoble, A.; Schofer, N.; Eschenbach, L.; Bansal, E.; Murdoch, D.J.; Ancona, M.; et al. Outcomes of transcatheter mitral valve replacement for degenerated bioprostheses, failed annuloplasty rings, and mitral annular calcification. Eur. Heart J. 2019, 40, 441–451. [Google Scholar] [CrossRef] [PubMed]
- Tiwana, J.; Aldea, G.; Levin, D.B.; Johnson, K.; Don, C.W.; Dvir, D.; Mackensen, G.B.; Reisman, M.; McCabe, J.M. Contemporary Transcatheter Mitral Valve Replacement for Mitral Annular Calcification or Ring. JACC Cardiovasc. Interv. 2020, 13, 2388–2398. [Google Scholar] [CrossRef]
- Alvarez-Covarrubias, H.A.; Joner, M.; Lutz, M.; Xhepa, E.; Mayr, N.P.; Lachmann, M.; Cassese, S.; Rheude, T.; Pellegrini, C.; Kufner, S.; et al. Outcomes after transcatheter mitral valve implantation in valve-in-valve, valve-in-ring, and valve-in-mitral annular calcification. Catheter. Cardiovasc. Interv. 2024, 104, 837–852. [Google Scholar] [CrossRef]
- Crociani, M.F.; Di Mario, C.; Stolcova, M.; Ciardetti, N.; Mattesini, A.; Maiani, S.; Nardi, G.; Di Muro, F.M.; Mandarano, R.; Meucci, F. Transfemoral Transseptal Mitral Valve-in-Valve-in-Valve of a Degenerated Transcatheter in Surgical Bioprosthesis. JACC Case Rep. 2025, 30, 103398. [Google Scholar] [CrossRef]
- Guerrero, M.; Urena, M.; Himbert, D.; Wang, D.D.; Eleid, M.; Kodali, S.; George, I.; Chakravarty, T.; Mathur, M.; Holzhey, D.; et al. 1-Year Outcomes of Transcatheter Mitral Valve Replacement in Patients With Severe Mitral Annular Calcification. JACC 2018, 71, 1841–1853. [Google Scholar] [CrossRef] [PubMed]
- Yoon, S.-H.; Bleiziffer, S.; Latib, A.; Eschenbach, L.; Ancona, M.; Vincent, F.; Kim, W.-K.; Unbehaum, A.; Asami, M.; Dhoble, A.; et al. Predictors of Left Ventricular Outflow Tract Obstruction After Transcatheter Mitral Valve Replacement. JACC Cardiovasc. Interv. 2019, 12, 182–193. [Google Scholar] [CrossRef]
- Khan, J.M.; Babaliaros, V.C.; Greenbaum, A.B.; Foerst, J.R.; Yazdani, S.; McCabe, J.M.; Paone, G.; Eng, M.H.; Leshnower, B.G.; Gleason, P.T.; et al. Anterior Leaflet Laceration to Prevent Ventricular Outflow Tract Obstruction During Transcatheter Mitral Valve Replacement. JACC 2019, 73, 2521–2534. [Google Scholar] [CrossRef]
- Khan, J.M.; Babaliaros, V.C.; Greenbaum, A.B.; McCabe, J.M.; Rogers, T.; Eng, M.H.; Foerst, J.R.; Yazdani, S.; Paone, G.; Gleason, P.T.; et al. 5-Year Outcomes of Anterior Mitral Leaflet Laceration to Prevent Outflow Obstruction. JACC Cardiovasc. Interv. 2024, 17, 2157–2167. [Google Scholar] [CrossRef]
- Belfekih, A.; Masri, A.; Veugeois, A.; Diakov, C.; Mahmoudi, K.; Ribeyrolles, S.; Mami, Z.; Roig, C.; Amabile, N.; Caussin, C. Alcohol Septal Ablation for Left Ventricle Outflow Tract Obstruction Prevention Before Transcatheter Mitral Valve Replacement Procedure: Computed Tomography Analysis Series. Catheter. Cardiovasc. Interv. 2025, 105, 1241–1250. [Google Scholar] [CrossRef]
- Guerrero, M.; Wang, D.D.; Eleid, M.F.; Pursnani, A.; Salinger, M.; Russell, H.M.; Kodali, S.K.; George, I.; Bapat, V.N.; Dangas, G.D.; et al. Prospective Study of TMVR Using Balloon-Expandable Aortic Transcatheter Valves in MAC. JACC Cardiovasc. Interv. 2021, 14, 830–845. [Google Scholar] [CrossRef]

| Domain | Favorable Criteria | Unfavorable Criteria | Implications for Therapy |
|---|---|---|---|
| Symptoms | NYHA II–III, persistent despite GDMT | Asymptomatic or end-stage HF | Consider TEER if symptomatic |
| MR Severity | EROA ≥ 30 mm2, regurgitant volume ≥ 45 mL | EROA < 20 mm2, regurgitant volume < 30 mL | Severe MR more likely to benefit |
| LV Function | LVEF 25–50%, LVEDVi < 96 mL/m2 | LVEF < 20%, LV severely dilated | Severe dilation may indicate futility |
| Anatomy (TEE 3D) | Coaptation length ≥ 2 mm, depth < 11 mm, acceptable grasping | Severe tethering, calcification, leaflet fissures | Important for TEER feasibility |
| Comorbidities | Stable, limited burden | Frailty, malignancy, end-stage organ dysfunction | May shift focus to conservative approach |
| Myocardial Viability | Viable myocardium (no transmural LGE on CMR) | Extensive fibrosis, transmural infarct | Viable LV predicts better outcomes |
| Heart Team Consensus | Consensus with shared decision-making | No consensus, uncertain patient goals | Multidisciplinary assessment essential |
| TEE View | Angle | Purpose | What to Assess |
|---|---|---|---|
| Mid-esophageal 4-chamber (ME 4C) | 0–20° | General orientation; initial transseptal puncture guidance | Visualize interatrial septum and needle during transseptal puncture |
| Mid-esophageal bicaval view | 90–110° | Primary guidance for transseptal puncture | Ensure posterior and superior puncture site |
| Mid-esophageal 2-chamber (ME 2C) | 60–90° | Secondary guidance for puncture | Confirm adequate puncture height (ideal: 4–5 cm from mitral annulus) |
| Mid-esophageal mitral commissural view | 50–70° | Commissural visualization (A1–P1, A3–P3) | Identify location of MR jet and guide medial/lateral clip positioning |
| Mid-esophageal long axis (ME LAX) | 120–150° | Anterior–posterior alignment of the clip | Leaflet grasping (typically A2–P2), confirm tissue capture and coaptation |
| Transgastric short axis/2D-3D | 0–120° (probe advanced into stomach) | Ventricular perspective of the mitral valve | Assess clip arm orientation and residual MR jet direction |
| 3D en face (atrial surgeon’s view) | 0° with 3D | Global spatial orientation of mitral valve | Visualize mitral scallops (A1–A3, P1–P3), guide medial/lateral positioning |
| Color Doppler (all key views) | Any | MR jet visualization and quantification | Identify origin and severity of MR pre- and post-clip |
| PW/CW Doppler at mitral valve level | 0–150° | Hemodynamic assessment post-clip | Measure mean mitral gradient (concern for iatrogenic stenosis if >5 mmH |
| Complication | MITRA-FR (%) | COAPT (%) |
|---|---|---|
| Overall procedural complication | 14.6% | 8.5% |
| Pericardial tamponade | 1.4% | 0.7% |
| Cardiac perforation | 1.4% | 0.4% |
| Vascular access complications | 2.4% | 1.4% |
| Stroke/neurological events | 2.8% | 1.2% |
| Severe mitral stenosis post-implantation | Not reported | 7.6% |
| Conversion to surgical mitral repair | Not reported | 1.4% |
| Periprocedural mortality | 0.7% | 0.4% |
| Device Name | Description | Delivery System | Valve Sizes | Access | Available/Ongoing Studies | Approval Status |
|---|---|---|---|---|---|---|
| Tendyne | Symmetrical trileaflet porcine bioprosthetic valve with an outer and inner frame. The valve is anchored by a tether secured by an apical pad; repositionable and retrievable. | 34 or 36 Fr depending on valve size | The Tendyne valve is in 13 sizes, 8 standard profile (SP) and 5 low profile (LP). The size is calculated according to the anterior-posterior (AP) diameter, the inter-commisural diameter and the perimeter | Transapical | TENDER investigator-initiated, prospective, multicenter trial SUMMIT trial Randomization between Tendyne and Mitraclip | CE Mark approval in January 2020 |
| Tiara™ | Self-expanding nitinol frame with a trileaflet bovine pericardial valve. D-shaped configuration to conform to the MV annulus | 32 Fr for 35 mm valve 36 Fr for 40 mm valve | 35 mm and 40 mm | Transapical | TIARA-I feasibility study | N/A |
| Intrepid™ | Trileaflet bovine pericardial valve contained in a self-expanding nitinol frame which has a unique dual structure design consisting of a circular inner stent to house the valve and a conformable outer fixation ring to engage the mitral annular anatomy | 35 Fr transapical 35 Fr transseptal | Inner bioprosthetic valve is 27 mm in diameter. Outer stent available in two sizes (42 and 48 mm) | Transapical Transseptal | APOLLO trial | N/A |
| AltaValve™ | Self-expanding supra-annular nitinol sphere housing a 27 mm bovine pericardial valve | 29 Fr | Three annular ring sizes: 40 mm, 46 mm, and 54 mm | Transseptal | N/A | |
| EVOQUE™ | Self-expanding nitinol frame with bovine pericardial leaflets and a fabric sealing skirt to prevent PVL | 28 Fr | 44–48 mm | Transseptal | N/A | |
| HighLife™ | Two components consisting of a subannular ring implant delivered retrogradely via the femoral artery and aortic valve, and the valve component delivered transseptally | 30-F (Capsule) mitral valve delivery system with an 18-F shaft. | 28 mm valve and ring | Transseptal | N/A | |
| Sapien M3™ | Two components consisting of a subvalvular “dock”, which encases the balloon expandable Sapien valve (29 mm) | 20 Fr | 29 mm valve | Transseptal | ENCIRCLE Trial prospective, single arm, multicenter, pivotal, adaptive design study. | CE Mark approval in Europe |
| CardioValve™ | Two nitinol self-expanding frames: atrial and ventricular encasing bovine pericardial leaflets | 28 Fr | Three sizes available covering commissural diameters from 36 to 55 mm | Transseptal | N/A | |
| Cephea™ | Self-expanding nitinol double disc system connected via a central column that houses the bovine pericardial trileafet valve; repositionable and recapturable | 28 Fr | Three sizes (32, 36, and 40 mm) | Transseptal | N/A | |
| Innovalve | Self-expanding frame (26.5 mm inner diameter providing EOA of 2–2.2 cm2), which holds 3 leaflets made of bovine pericardial tissue. | 39 Fr | 28 or 31 mm | Transseptal | N/A | |
| Revalve (Palmetto) | Palmetto bovine bioprosthetic valve leverages a helical architecture integrating diverse wire thickness Dedicated valve-in-valveplatform (ReValvingSystem) | Not yet disclosed | Not yet disclosed | Transseptal | First-in-human procedure completed in November 2023 | N/A |
| Saturn | Low profile TMVR biopros thesis with a central valve which is mechanically connected to an annular ring | 39 Fr for transapical 29 Fr for transeptal | 28 and 31 mm | Transapical Transseptal | N/A |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Di Muro, F.M.; Spadafora, L.; Buonpane, A.; Leuzzi, F.; Nardi, G.; Bossone, E.; Biondi Zoccai, G.; Attisano, T.; Meucci, F.; Di Mario, C.; et al. Functional Mitral Regurgitation in the Transcatheter Era: Diagnostic and Therapeutic Pathways. J. Pers. Med. 2025, 15, 372. https://doi.org/10.3390/jpm15080372
Di Muro FM, Spadafora L, Buonpane A, Leuzzi F, Nardi G, Bossone E, Biondi Zoccai G, Attisano T, Meucci F, Di Mario C, et al. Functional Mitral Regurgitation in the Transcatheter Era: Diagnostic and Therapeutic Pathways. Journal of Personalized Medicine. 2025; 15(8):372. https://doi.org/10.3390/jpm15080372
Chicago/Turabian StyleDi Muro, Francesca Maria, Luigi Spadafora, Angela Buonpane, Francesco Leuzzi, Giulia Nardi, Eduardo Bossone, Giuseppe Biondi Zoccai, Tiziana Attisano, Francesco Meucci, Carlo Di Mario, and et al. 2025. "Functional Mitral Regurgitation in the Transcatheter Era: Diagnostic and Therapeutic Pathways" Journal of Personalized Medicine 15, no. 8: 372. https://doi.org/10.3390/jpm15080372
APA StyleDi Muro, F. M., Spadafora, L., Buonpane, A., Leuzzi, F., Nardi, G., Bossone, E., Biondi Zoccai, G., Attisano, T., Meucci, F., Di Mario, C., Vecchione, C., & Galasso, G. (2025). Functional Mitral Regurgitation in the Transcatheter Era: Diagnostic and Therapeutic Pathways. Journal of Personalized Medicine, 15(8), 372. https://doi.org/10.3390/jpm15080372









