The Gut Microbiome in Early-Onset Colorectal Cancer: Distinct Signatures, Targeted Prevention and Therapeutic Strategies
Abstract
1. Introduction
2. Methods
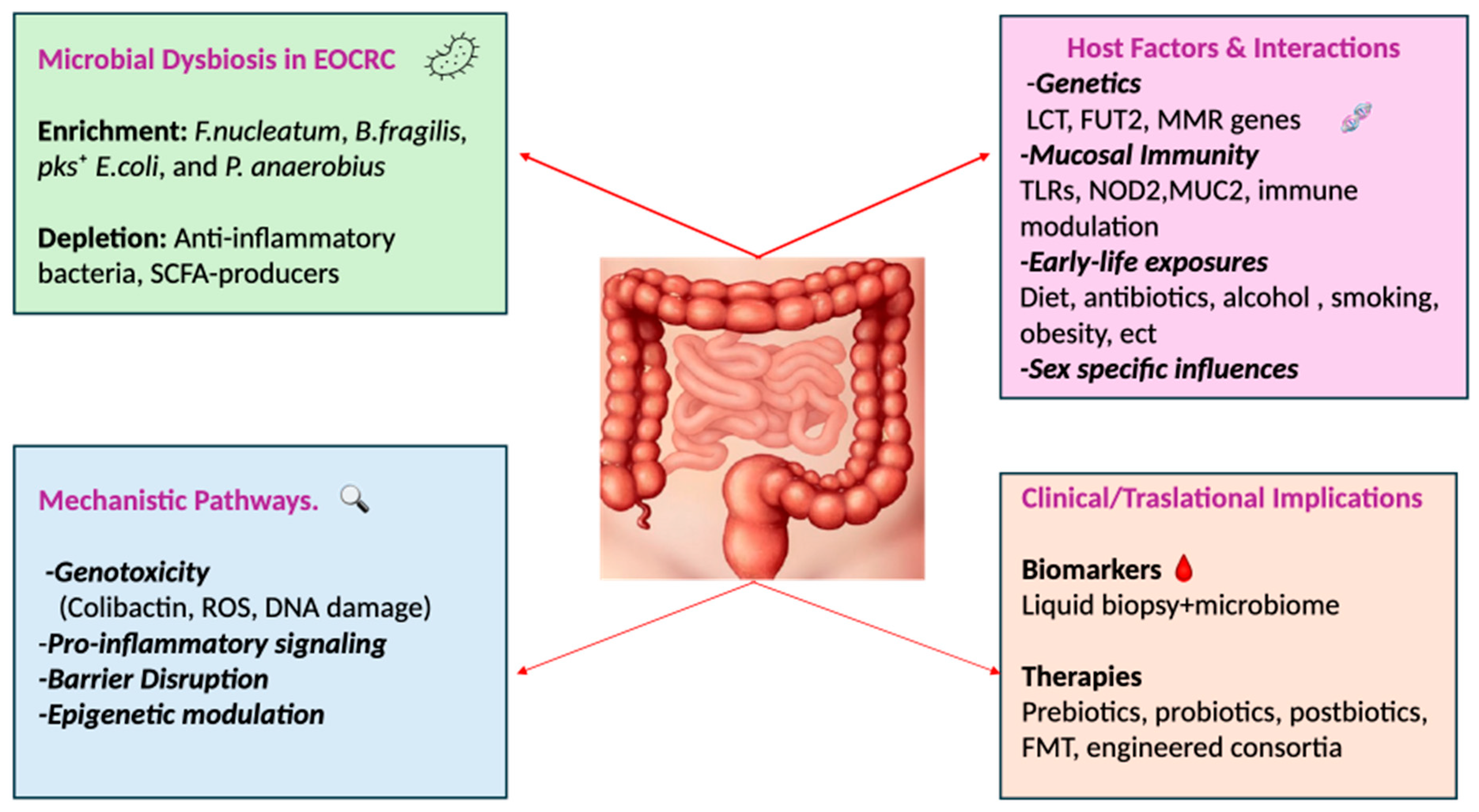
3. Microbial Dysbiosis as a Driver of Colorectal Carcinogenesis
4. Microbial Signatures in EOCRC
5. Host-Microbiome Interactions in Young Patients
5.1. Host Genetics
5.2. Mucosal Immune Control
5.3. Sex
5.4. Early Life and Lifestyle Exposures
6. Gut Microbiome as a Predictor of Therapeutic Response in CRC: Insights from EOCRC
7. Microbiome-Based Therapeutic Strategies in EOCRC
8. Microbiome-Based Implications for Diagnosis, Prognosis, and Future Directions
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CRC | Colorectal Cancer |
| EOCRC | Early-Onset Colorectal Cancer |
| LOCRC | Late-Onset Colorectal Cancer |
| SCFA | Short-Chain Fatty Acids |
| MWAS | Metagenome-Wide Association Study |
| qPCR | quantitative Polymerase Chain Reaction |
| WGS | Whole-Genome Sequencing |
| nCRT | Neoadjuvant Chemoradiotherapy |
| ICI | Immune Checkpoint Inhibitor |
| FMT | Fecal Microbiota Transplantation |
| HAMSB | Butyrylated High-Amylose Maize Starch |
| LCT | Lactase |
References
- Ferlay, J.; Colombet, M.; Soerjomataram, I.; Dyba, T.; Randi, G.; Bettio, M. Cancer incidence and mortality patterns in Europe: Estimates for 40 countries and 25 major cancers in 2018. Eur. J. Cancer 2018, 103, 356–387. [Google Scholar] [CrossRef]
- Dyba, T.; Randi, G.; Bray, F.; Martos, C.; Giusti, F.; Nicholson, N. The European cancer burden in 2020, Incidence and mortality estimates for 40 countries and 25 major cancers. Eur. J. Cancer 2021, 157, 308–347. [Google Scholar] [CrossRef]
- Foppa, C.; Maroli, A.; Lauricella, S.; Luberto, A.; La Raja, C.; Bunino, F. Different oncologic outcomes in early-onset and late-onset sporadic colorectal cancer: A regression analysis on 2073 patients. Cancers 2022, 14, 6239. [Google Scholar] [CrossRef] [PubMed]
- Venugopal, A.; Carethers, J.M. Epidemiology and biology of early onset colorectal cancer. EXCLI J. 2022, 21, 162–182. [Google Scholar] [CrossRef]
- Walker, B.; Jani, C.T.; Liu, W.; Punjwani, S.; Kareff, S.; Ceglowski, P. Does a “Western Lifestyle” confer a higher burden of colorectal cancer? A comparison of EU15+ countries versus global trends between 1990 and 2019. Cancers 2024, 16, 2277. [Google Scholar] [CrossRef]
- Vuik, F.E.; Nieuwenburg, S.A.; Bardou, M.; Lansdorp-Vogelaar, I.; Dinis-Ribeiro, M.; Bento, M.J. Increasing incidence of colorectal cancer in young adults in Europe over the last 25 years. Gut 2019, 68, 1820–1826. [Google Scholar] [CrossRef]
- Santucci, C.; Mignozzi, S.; Malvezzi, M.; Boffetta, P.; Collatuzzo, G.; Levi, F. European cancer mortality predictions for the year 2024 with focus on colorectal cancer. Ann. Oncol. 2024, 35, 308–316. [Google Scholar] [CrossRef]
- Cercek, A.; Chatila, W.K.; Yaeger, R.; Walch, H.; Fernandes, G.D.S.; Krishnan, A.; Palmaira, L.; Maio, A.; Kemel, Y.; Srinivasan, P.; et al. A comprehensive comparison of early-onset and average-onset colorectal cancers. J. Natl. Cancer Inst. 2021, 113, 1683–1692. [Google Scholar] [CrossRef]
- Arnold, M.; Karim-Kos, H.E.; Coebergh, J.W.; Byrnes, G.; Antilla, A.; Ferlay, J.; Renehan, A.G.; Forman, D.; Soerjomataram, I. Recent trends in incidence of five common cancers in 26 European countries since 1988: Analysis of the European Cancer Observatory. Eur. J. Cancer 2015, 51, 1164–1187. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Feng, Q.; Wong, S.H.; Zhang, D.; Liang, Q.Y.; Qin, Y.; Tang, L.; Zhao, H.; Stenvang, J.; Li, Y.; et al. Metagenomic analysis of faecal microbiome as a tool towards targeted non-invasive biomarkers for colorectal cancer. Gut 2017, 66, 70–78. [Google Scholar] [CrossRef] [PubMed]
- Rubinstein, M.R.; Wang, X.; Liu, W.; Hao, Y.; Cai, G.; Han, Y.W. Fusobacterium nucleatum promotes colorectal carcinogenesis by modulating E-cadherin/β-catenin signaling via its FadA adhesin. Cell Host Microbe 2013, 14, 195–206. [Google Scholar] [CrossRef]
- Gur, C.; Ibrahim, Y.; Isaacson, B.; Yamin, R.; Abed, J.; Gamliel, M.; Enk, J.; Bar-On, Y.; Stanietsky-Kaynan, N.; Coppenhagen-Glazer, S.; et al. Binding of the Fap2 protein of Fusobacterium nucleatum to human inhibitory receptor TIGIT protects tumors from immune cell attack. Immunity 2015, 42, 344–355. [Google Scholar] [CrossRef]
- Wu, S.; Rhee, K.J.; Albesiano, E.; Rabizadeh, S.; Wu, X.; Yen, H.R.; Huso, D.L.; Brancati, F.L.; Wick, E.; McAllister, F.; et al. A human colonic commensal promotes colon tumorigenesis via activation of T helper type 17 T cell responses. Nat. Med. 2009, 15, 1016–1022. [Google Scholar] [CrossRef] [PubMed]
- Pleguezuelos-Manzano, C.; Puschhof, J.; Rosendahl Huber, A.; van Hoeck, A.; Wood, H.M.; Nomburg, J.; Gurjao, C.; Manders, F.; Dalmasso, G.; Stege, P.B.; et al. Mutational signature in colorectal cancer caused by genotoxic pks+ E. coli. Nature 2020, 580, 269–273. [Google Scholar] [CrossRef] [PubMed]
- Kong, C.; Liang, L.; Liu, G.; Du, L.; Yang, Y.; Liu, J.; Shi, D.; Li, X.; Ma, Y. Integrated metagenomic and metabolomic analysis reveals distinct gut-microbiome-derived phenotypes in early-onset colorectal cancer. Gut 2023, 72, 1129–1142. [Google Scholar] [CrossRef]
- Qin, Y.; Tong, X.; Mei, W.J.; Cheng, Y.; Zou, Y.; Han, K.; Yu, J.; Jie, Z.; Zhang, T.; Zhu, S.; et al. Consistent signatures in the human gut microbiome of old- and young-onset colorectal cancer. Nat. Commun. 2024, 15, 3396. [Google Scholar] [CrossRef]
- Adnan, D.; Trinh, J.Q.; Sharma, D.; Alsayid, M.; Bishehsari, F. Early-onset colon cancer shows a distinct intestinal microbiome and a host–microbe interaction. Cancer Prev. Res. 2024, 17, 29–38. [Google Scholar] [CrossRef]
- Díaz-Gay, M.; Dos Santos, W.; Moody, S.; Kazachkova, M.; Abbasi, A.; Steele, C.D.; Vangara, R.; Senkin, S.; Wang, J.; Fitzgerald, S.; et al. Geographic and age variations in mutational processes in colorectal cancer. Nature 2025, 643, 230–240. [Google Scholar] [CrossRef]
- Ionescu, V.A.; Diaconu, C.C.; Gheorghe, G.; Mihai, M.M.; Diaconu, C.C.; Bostan, M.; Bleotu, C. Gut Microbiota and Colorectal Cancer: A Balance Between Risk and Protection. Int. J. Mol. Sci. 2025, 26, 3733. [Google Scholar] [CrossRef] [PubMed]
- Sinicrope, F.A. Increasing Incidence of Early-Onset Colorectal Cancer. N. Engl. J. Med. 2022, 386, 1547–1558. [Google Scholar] [CrossRef]
- El Tekle, G.; Andreeva, N.; Garrett, W.S. The Role of the Microbiome in the Etiopathogenesis of Colon Cancer. Annu. Rev. Physiol. 2024, 86, 453–478. [Google Scholar] [CrossRef] [PubMed]
- Wong, C.C.; Yu, J. Gut microbiota in colorectal cancer development and therapy. Nat. Rev. Clin. Oncol. 2023, 20, 429–452. [Google Scholar] [CrossRef]
- Karpiński, T.M.; Ożarowski, M.; Stasiewicz, M. Carcinogenic microbiota and its role in colorectal cancer development. Semin. Cancer Biol. 2022, 86 Pt 3, 420–430. [Google Scholar] [CrossRef]
- Han, Y.W. Fusobacterium nucleatum: A commensal-turned pathogen. Curr. Opin. Microbiol. 2015, 23, 141–147. [Google Scholar] [CrossRef] [PubMed]
- Gao, R.; Wang, Z.; Li, H.; Cao, Z.; Gao, Z.; Chen, H.; Zhang, X.; Pan, D.; Yang, R.; Zhong, H.; et al. Gut microbiota dysbiosis signature is associated with the colorectal carcinogenesis sequence and im-proves the diagnosis of colorectal lesions. J. Gastroenterol. Hepatol. 2020, 35, 2109–2121. [Google Scholar] [CrossRef]
- Wirbel, J.; Pyl, P.T.; Kartal, E.; Zych, K.; Kashani, A.; Milanese, A.; Fleck, J.S.; Voigt, A.Y.; Palleja, A.; Ponnudurai, R.; et al. Meta-analysis of fecal metagenomes reveals global microbial signatures that are specific for colorectal cancer. Nat. Med. 2019, 25, 679–689. [Google Scholar] [CrossRef] [PubMed]
- Zeller, G.; Tap, J.; Voigt, A.Y.; Sunagawa, S.; Kultima, J.R.; Costea, P.I.; Amiot, A.; Böhm, J.; Brunetti, F.; Habermann, N.; et al. Potential of fecal microbiota for early-stage detection of colorectal cancer. Mol. Syst. Biol. 2014, 10, 766. [Google Scholar] [CrossRef]
- Long, X.; Wong, C.C.; Tong, L.; Chu, E.S.H.; Ho Szeto, C.; Go, M.Y.Y.; Coker, O.O.; Chan, A.W.H.; Chan, F.K.L.; Sung, J.J.Y.; et al. Peptostreptococcus anaerobius promotes colorectal carcinogenesis and modulates tumour immunity. Nat. Microbiol. 2019, 4, 2319–2330. [Google Scholar] [CrossRef]
- Song, M.; Chan, A.T.; Sun, J. Influence of the gut microbiome, diet, and environment on risk of colorectal cancer. Gastroenterology 2020, 158, 322–340. [Google Scholar] [CrossRef]
- Jayakrishnan, T.T.; Sangwan, N.; Barot, S.V.; Farha, N.; Mariam, A.; Xiang, S.; Aucejo, F.; Conces, M.; Nair, K.G.; Krishnamurthi, S.S.; et al. Multi-omics machine learning to study host–microbiome interactions in early-onset colorectal cancer. NPJ Precis. Oncol. 2024, 8, 146. [Google Scholar] [CrossRef]
- Flemer, B.; Lynch, D.B.; Brown, J.M.R.; Jeffery, I.B.; Ryan, F.J.; Claesson, M.J.; O’Riordain, M.; Shanahan, F.; O’Toole, P.W. Tumour-associated and non-tumour-associated microbiota in colorectal cancer. Gut 2017, 66, 633–643. [Google Scholar] [CrossRef]
- Kurilshikov, A.; Medina-Gomez, C.; Bacigalupe, R.; van Rheenen, W.; Zhernakova, A.; Demirkan, A.; Le Roy, C.I.; Garay, J.A.R.; Finnicum, C.T.; Liu, X.; et al. Large-scale association analyses identify host factors influencing human gut microbiome composition. Nat. Genet. 2021, 53, 156–165. [Google Scholar] [CrossRef] [PubMed]
- Marchesi, J.R.; Dutilh, B.E.; Hall, N.; Peters, W.H.; Roelofs, R.; Boleij, A.; Tjalsma, H. Towards the human colorectal cancer microbiome. PLoS ONE 2011, 6, e20447. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Wu, X.; Mou, M.; Wang, C.; Wang, L.; Li, F.; Guo, M.; Yin, J.; Xie, W.; Wang, X.; et al. GIMICA: Host genetic and immune factors shaping human microbiota. Nucleic Acids Res. 2021, 49, D715–D722. [Google Scholar] [CrossRef]
- Lynch, S.V.; Pedersen, O. The human intestinal microbiome in health and disease. N. Engl. J. Med. 2016, 375, 2369–2379. [Google Scholar] [CrossRef]
- Dai, R.; Kelly, B.N.; Ike, A.; Berger, D.; Chan, A.; Drew, D.A.; Ljungman, D.; Mutiibwa, D.; Ricciardi, R.; Tumusiime, G.; et al. The Impact of the Gut Microbiome, Environment, and Diet in Early-Onset Colorectal Cancer Development. Cancers 2024, 16, 676. [Google Scholar] [CrossRef] [PubMed]
- Piasecka, B.; Duffy, D.; Urrutia, A.; Quach, H.; Patin, E.; Posseme, C.; Bergstedt, J.; Charbit, B.; Rouilly, V.; MacPherson, C.R.; et al. Distinctive roles of age, sex, and genetics in shaping transcriptional variation of human immune responses to microbial challenges. Proc. Natl. Acad. Sci. USA 2018, 115, E488–E497. [Google Scholar] [CrossRef]
- Bajpai, G.; Nahrendorf, M. Infectious and lifestyle modifiers of immunity and host resilience. Immunity 2021, 54, 1110–1122. [Google Scholar] [CrossRef]
- Müller, L.; Pawelec, G. Aging and immunity—Impact of behavioral intervention. Brain Behav. Immun. 2014, 39, 8–22. [Google Scholar] [CrossRef]
- Mahnič, A.; Rupnik, M. Different host factors are associated with patterns in bacterial and fungal gut microbiota in Slovenian healthy cohort. PLoS ONE 2018, 13, e0209209. [Google Scholar] [CrossRef]
- Markle, J.G.M.; Frank, D.N.; Mortin-Toth, S.; Robertson, C.E.; Feazel, L.M.; Rolle-Kampczyk, U.; von Bergen, M.; McCoy, K.D.; Macpherson, A.J.; Danska, J.S. Sex differences in the gut microbiome drive hormone-dependent regulation of autoimmunity. Science 2013, 339, 1084–1088. [Google Scholar] [CrossRef]
- Shahi, S.K.; Freedman, S.N.; Mangalam, A.K. Gut microbiome in multiple sclerosis: The players involved and the roles they play. Front. Microbiol. 2019, 10, 454. [Google Scholar] [CrossRef]
- Kim, Y.S.; Unno, T.; Kim, B.Y.; Park, M.S. Sex differences in gut microbiota. World J. Mens. Health 2020, 38, 48–60. [Google Scholar] [CrossRef]
- Yang, Y.; Du, L.; Shi, D.; Kong, C.; Liu, J.; Liu, G.; Li, X.; Ma, Y. Dysbiosis of human gut microbiome in young-onset colorectal cancer. Nat. Commun. 2021, 12, 6757. [Google Scholar] [CrossRef]
- Xiong, H.; Wang, J.; Chang, Z.; Hu, H.; Yuan, Z.; Zhu, Y.; Hu, Z.; Wang, C.; Liu, Y.; Wang, Y.; et al. Gut microbiota display alternative profiles in patients with early-onset colorectal cancer. Front. Cell. Infect. Microbiol. 2022, 12, 1036946. [Google Scholar] [CrossRef]
- Grion, B.A.R.; Fonseca, P.L.C.; Kato, R.B.; García, G.J.Y.; Vaz, A.B.M.; Jiménez, B.N.; Dambolenea, A.L.; Garcia-Etxebarria, K.; Brenig, B.; Azevedo, V.; et al. Identification of taxonomic changes in the fecal bacteriome associated with colorectal polyps and cancer: Potential biomarkers for early diagnosis. Front. Microbiol. 2024, 14, 1292490. [Google Scholar] [CrossRef]
- Abdullah, M.; Sukartini, N.; Nursyirwan, S.A.; Pribadi, R.R.; Maulahela, H.; Utari, A.P.; Muzellina, V.N.; Wiraatmadja, A.; Renaldi, K. Gut Microbiota Profiles in Early- and Late-Onset Colorectal Cancer: A Potential Diagnostic Biomarker in the Future. Digestion 2021, 102, 823–832. [Google Scholar] [CrossRef] [PubMed]
- Hong, D.; Tian, R.; Kim, H.; Doering, M.; Cao, Y. The microbiome landscape of early-onset colorectal cancer: A systematic review. J. Clin. Oncol. 2024, 42 (Suppl. 16), e15646. [Google Scholar] [CrossRef]
- Ullah, F.; Pillai, A.B.; Omar, N.; Dima, D.; Harichand, S. Early-Onset Colorectal Cancer: Current Insights. Cancers 2023, 15, 3202. [Google Scholar] [CrossRef] [PubMed]
- Arima, K.; Zhong, R.; Ugai, T.; Zhao, M.; Haruki, K.; Akimoto, N.; Lau, M.C.; Okadome, K.; Mehta, R.S.; Väyrynen, J.P.; et al. Western-style diet, pks island-carrying Escherichia coli, and colorectal cancer: Analyses from two large prospective cohort studies. Gastroenterology 2022, 163, 862–874. [Google Scholar] [CrossRef]
- Puzzono, M.; Mannucci, A.; Grannò, S.; Zuppardo, R.A.; Galli, A.; Danese, S.; Cavestro, G.M. The Role of Diet and Lifestyle in Early-Onset Colorectal Cancer: A Systematic Review. Cancers 2021, 13, 5933. [Google Scholar] [CrossRef]
- Anwar, H.; Iftikhar, A.; Muzaffar, H.; Almatroudi, A.; Allemailem, K.S.; Navaid, S.; Saleem, S.; Khurshid, M. Antibiotic exposure and colorectal cancer risk: Current evidence and future directions. Biomed. Res. Int. 2021, 2021, 5575245. [Google Scholar] [CrossRef]
- McDowell, R.; Perrott, S.; Murchie, P.; Cardwell, C.; Hughes, C.; Samuel, L. Oral antibiotic use and early-onset colorectal cancer: Findings from a case-control study using a national clinical database. Br. J. Cancer 2022, 126, 957–967. [Google Scholar] [CrossRef]
- Puzzono, M.; Mannucci, A.; Di Leo, M.; Zuppardo, R.A.; Russo, M.; Ditonno, I.; Goni, E.; Notaristefano, C.; Azzolini, F.; Fanti, L.; et al. Diet and Lifestyle Habits in Early-Onset Colorectal Cancer: A Pilot Case-Control Study. Dig. Dis. 2022, 40, 710–718. [Google Scholar] [CrossRef]
- Laudes, M.; Geisler, C.; Rohmann, N.; Bouwman, J.; Pischon, T.; Schlicht, K. Microbiota in health and disease—Potential clinical applications. Nutrients 2021, 13, 3866. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Alcoholado, L.; Laborda-Illanes, A.; Otero, A.; Ordóñez, R.; González-González, A.; Plaza-Andrades, I.; Ramos-Molina, B.; Gómez-Millán, J.; Queipo-Ortuño, M.I. Relationships of gut microbiota composition, short-chain fatty acids and polyamines with the pathological response to neoadjuvant radiochemotherapy in colorectal cancer patients. Int. J. Mol. Sci. 2021, 22, 9549. [Google Scholar] [CrossRef]
- Yi, Y.; Shen, L.; Shi, W.; Xia, F.; Zhang, H.; Wang, Y.; Zhang, J.; Wang, Y.; Sun, X.; Zhang, Z.; et al. Gut microbiome components predict response to neoadjuvant chemoradiotherapy in locally advanced rectal cancer. Clin. Cancer Res. 2021, 27, 1329–1340. [Google Scholar] [CrossRef]
- Ajab, S.M.; Zoughbor, S.H.; Labania, L.A.; Östlundh, L.M.; Orsud, H.S.; Olanda, M.A.; Alkaabi, O.; Alkuwaiti, S.H.; Alnuaimi, S.M.; Al Rasbi, Z. Microbiota composition effect on immunotherapy outcomes in colorectal cancer: A systematic review. PLoS ONE 2024, 19, e0307639. [Google Scholar] [CrossRef] [PubMed]
- White, M.G.; Damania, A.; Alshenaifi, J.; Sahasrabhojane, P.; Peacock, O.; Losh, J.; Wong, M.C.B.; Lutter-Berkova, Z.; Chang, G.J.; Futreal, A.; et al. Young-onset rectal cancer: Unique tumoral microbiome and correlation with response to neoadjuvant therapy. Ann. Surg. 2023, 278, 538–548. [Google Scholar] [CrossRef] [PubMed]
- Deng, X.; Li, Z.; Li, G.; Li, B.; Jin, X.; Lyu, G. Comparison of microbiota in patients treated by surgery or chemotherapy by 16S rRNA sequencing reveals potential biomarkers for colorectal cancer therapy. Front. Microbiol. 2018, 9, 1607. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Alcoholado, L.; Ramos-Molina, B.; Otero, A.; Laborda-Illanes, A.; Ordóñez, R.; Medina, J.A.; Gómez-Millán, J.; Queipo-Ortuño, M.I. The role of the gut microbiome in colorectal cancer development and therapy response. Cancers 2020, 12, 1406. [Google Scholar] [CrossRef]
- Mouradov, D.; Greenfield, P.; Li, S.; In, E.J.; Storey, C.; Sakthianandeswaren, A.; Georgeson, P.; Buchanan, D.D.; Ward, R.L.; Hawkins, N.J.; et al. Oncomicrobial community profiling identifies clinicomolecular and prognostic subtypes of colorectal cancer. Gastroenterology 2023, 165, 104–120. [Google Scholar] [CrossRef]
- Pandey, H.; Tang, D.W.T.; Wong, S.H.; Lal, D. Gut Microbiota in Colorectal Cancer: Biological Role and Therapeutic Opportunities. Cancers 2023, 15, 866. [Google Scholar] [CrossRef]
- Sivan, A.; Corrales, L.; Hubert, N.; Williams, J.B.; Aquino-Michaels, K.; Earley, Z.M.; Benyamin, F.W.; Lei, Y.M.; Jabri, B.; Alegre, M.-L.; et al. Commensal Bifidobacterium promotes antitumor immunity and facilitates anti-PD-L1 efficacy. Science 2015, 350, 1084–1089. [Google Scholar] [CrossRef]
- Kvakova, M.; Kamlarova, A.; Stofilova, J.; Benetinova, V.; Bertkova, I. Probiotics and postbiotics in colorectal cancer: Prevention and complementary therapy. World J. Gastroenterol. 2022, 28, 3370–3382. [Google Scholar] [CrossRef]
- Chaturvedi, P.; Pathak, R.; Dayal, R.; Parihar, H.; Kathireshan, A.K.; Tirumalai, P.S. Colorectal Cancer Mitigation Through Probiotics: Current Evidence and Future Directions. Curr. Microbiol. 2025, 82, 339. [Google Scholar] [CrossRef] [PubMed]
- Khavandegar, A.; Heidarzadeh, A.; Angoorani, P.; Hasani-Ranjbar, S.; Ejtahed, H.S.; Larijani, B.; Qorbani, M. Adherence to the Mediterranean diet can beneficially affect the gut microbiota composition: A systematic review. BMC Med. Genom. 2024, 17, 91. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.; Zhou, J. Exploring the potential of high-amylose maize starch butyrate (HAMSB) in colorectal health. Front. Nutr. 2024, 11, 1285169. [Google Scholar] [CrossRef]
- Xie, W.; Zhong, Y.S.; Li, X.J.; Kang, Y.K.; Peng, Q.Y.; Ying, H.Z. Postbiotics in colorectal cancer: Intervention mechanisms and perspectives. Front. Microbiol. 2024, 15, 1360225. [Google Scholar] [CrossRef]
- Zdybel, K.; Śliwka, A.; Polak-Berecka, M.; Polak, P.; Waśko, A. Postbiotics formulation and therapeutic effect in inflammation: A systematic review. Nutrients 2025, 17, 2187. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Tu, H.; Chen, T. Postbiotics in human health: A narrative review. Nutrients 2023, 15, 291. [Google Scholar] [CrossRef]
- Ooijevaar, R.E.; Terveer, E.M.; Verspaget, H.W.; Kuijper, E.J.; Keller, J.J. Clinical application and potential of fecal microbiota transplantation. Annu. Rev. Med. 2019, 70, 335–351. [Google Scholar] [CrossRef] [PubMed]
- Fong, W.; Li, Q.; Yu, J. Gut microbiota modulation: A novel strategy for prevention and treatment of colorectal cancer. Oncogene 2020, 39, 4925–4943. [Google Scholar] [CrossRef]
- Yu, H.; Li, X.X.; Han, X.; Chen, B.X.; Zhang, X.H.; Gao, S.; Xu, D.Q.; Wang, Y.; Gao, Z.K.; Yu, L.; et al. Fecal microbiota transplantation inhibits colorectal cancer progression: Reversing intestinal microbial dysbiosis to enhance anti-cancer immune responses. Front. Microbiol. 2023, 14, 1126808. [Google Scholar] [CrossRef] [PubMed]
- Song, Q.; Gao, Y.; Liu, K.; Tang, Y.; Man, Y.; Wu, H. Gut microbial and metabolomics profiles reveal the potential mechanism of fecal microbiota transplantation in modulating the progression of colitis-associated colorectal cancer in mice. J. Transl. Med. 2024, 22, 1028. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.W.; Lee, H.C.; Li, L.H.; Chiang Chiau, J.S.; Wang, T.E.; Chuang, W.H.; Chen, M.J.; Wang, H.Y.; Shih, S.C.; Liu, C.Y.; et al. Fecal microbiota transplantation prevents intestinal injury, upregulation of toll-like receptors, and 5-fluorouracil/oxaliplatin-induced toxicity in colorectal cancer. Int. J. Mol. Sci. 2020, 21, 386. [Google Scholar] [CrossRef]
- Veettil, S.K.; Wong, T.Y.; Loo, Y.S.; Playdon, M.C.; Lai, N.M.; Giovannucci, E.L.; Chaiyakunapruk, N.; Wu, J.H.Y.; Zheng, Y.; Thongprayoon, C.; et al. Role of Diet in Colorectal Cancer Incidence: Umbrella Review of Meta-analyses of Prospective Observational Studies. JAMA Netw. Open 2021, 4, e2037341. [Google Scholar] [CrossRef]
- Vivarelli, S.; Salemi, R.; Candido, S.; Falzone, L.; Santagati, M.; Stefani, S.; Torino, F.; Banna, G.L.; Tonini, G.; Libra, M. Gut Microbiota and Cancer: From Pathogenesis to Therapy. Cancers 2019, 11, 38. [Google Scholar] [CrossRef]
- Davar, D.; Dzutsev, A.K.; McCulloch, J.A.; Rodrigues, R.R.; Chauvin, J.M.; Morrison, R.M.; Deblasio, R.N.; Menna, C.; Ding, Q.; Pagliano, O.; et al. Fecal microbiota transplant overcomes resistance to anti–PD-1 therapy in melanoma patients. Science 2021, 371, 595–602. [Google Scholar] [CrossRef]
- Wang, Y.; Li, H. Gut microbiota modulation: A tool for the management of colorectal cancer. J. Transl. Med. 2022, 20, 178. [Google Scholar] [CrossRef]
- Lawson, C.E.; Harcombe, W.R.; Hatzenpichler, R.; Lindemann, S.R.; Löffler, F.E.; O’Malley, M.A.; García Martín, H.; Pfleger, B.F.; Raskin, L.; Venturelli, O.S.; et al. Common principles and best practices for engineering microbial communities. Nat. Rev. Microbiol. 2019, 17, 481–496. [Google Scholar] [CrossRef]
- Almeida, A.; Mitchell, A.L.; Boland, M.; Forster, S.C.; Gloor, G.B.; Tarkowska, A.; Lawley, T.D.; Finn, R.D. A new genomic blue-print of the human gut microbiota. Nature 2019, 568, 499–504. [Google Scholar] [CrossRef]
- Mimee, M.; Citorik, R.J.; Lu, T.K. Microbiome therapeutics—Advances and challenges. Adv. Drug Deliv. Rev. 2016, 105 Pt A, 44–54. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Zheng, Z.; Yang, L.; Li, R.; Ma, X.; Zhang, J.; Yin, F.; Liu, L.; Xu, Q.; Shen, Q.; et al. A novel promising diagnosis model for colorectal advanced adenoma and carcinoma based on the progressive gut microbiota gene biomarkers. Cell Biosci. 2022, 12, 208. [Google Scholar] [CrossRef]
- Ai, L.; Tian, H.; Chen, Z.; Chen, H.; Xu, J.; Fang, J.Y. Systematic evaluation of supervised classifiers for fecal microbiota-based prediction of colorectal cancer. Oncotarget 2017, 8, 9546–9556. [Google Scholar] [CrossRef]
- Wu, Y.; Jiao, N.; Zhu, R.; Zhang, Y.; Wu, D.; Wang, A.J.; Fang, S.; Tao, L.; Li, Y.; Cheng, S.; et al. Identification of microbial markers across populations in early detection of colorectal cancer. Nat. Commun. 2021, 12, 3063. [Google Scholar] [CrossRef] [PubMed]
- Mima, K.; Nishihara, R.; Qian, Z.R.; Cao, Y.; Sukawa, Y.; Nowak, J.A.; Yang, J.; Dou, R.; Masugi, Y.; Song, M.; et al. Fusobacterium nucleatum in colorectal carcinoma tissue and patient prognosis. Gut 2016, 65, 1973–1980. [Google Scholar] [CrossRef] [PubMed]
- Flanagan, L.; Schmid, J.; Ebert, M.; Soucek, P.; Kunicka, T.; Liška, V.; Bruha, J.; Neary, P.; DeZeeuw, N.; Tommasino, M.; et al. Fusobacterium nucleatum associates with stages of colorectal neoplasia development, colorectal cancer and disease outcome. Eur. J. Clin. Microbiol. Infect. Dis. 2014, 33, 1381–1390. [Google Scholar] [CrossRef]
- Gao, R.; Wu, C.; Zhu, Y.; Kong, C.; Zhu, Y.; Gao, Y.; Zhang, X.; Yang, R.; Zhong, H.; Xiong, X.; et al. Integrated Analysis of Colorectal Cancer Reveals Cross-Cohort Gut Microbial Signatures and Associated Serum Metabolites. Gastroenterology 2022, 163, 1024–1037.e9. [Google Scholar] [CrossRef]
- Zwezerijnen-Jiwa, F.H.; Sivov, H.; Paizs, P.; Zafeiropoulou, K.; Kinross, J. A systematic review of microbiome-derived biomarkers for early colorectal cancer detection. Neoplasia 2023, 36, 100868. [Google Scholar] [CrossRef]
- Herlo, L.F.; Salcudean, A.; Sirli, R.; Iurciuc, S.; Herlo, A.; Nelson-Twakor, A.; Alexandrescu, L.; Dumache, R. Gut Microbiota Signatures in Colorectal Cancer as a Potential Diagnostic Biomarker in the Future: A Systematic Review. Int. J. Mol. Sci. 2024, 25, 7937. [Google Scholar] [CrossRef] [PubMed]
- Raza, A.; Khan, A.Q.; Inchakalody, V.P.; Mestiri, S.; Yoosuf, Z.S.K.M.; Bedhiafi, T.; El-Ella, D.M.A.; Taib, N.; Hydrose, S.; Akbar, S.; et al. Dynamic liquid biopsy components as predictive and prognostic biomarkers in colorectal cancer. J. Exp. Clin. Cancer Res. 2022, 41, 99. [Google Scholar] [CrossRef] [PubMed]
- Koulouris, A.; Tsagkaris, C.; Messaritakis, I.; Gouvas, N.; Sfakianaki, M.; Trypaki, M.; Spyrou, V.; Christodoulakis, M.; Athanasakis, E.; Xynos, E.; et al. Resectable Colorectal Cancer: Current Perceptions on the Correlation of Recurrence Risk, Microbiota and Detection of Genetic Mutations in Liquid Biopsies. Cancers 2021, 13, 3522. [Google Scholar] [CrossRef]
- Ziranu, P.; Pretta, A.; Saba, G.; Spanu, D.; Donisi, C.; Ferrari, P.A.; Cau, F.; D’agata, A.P.; Piras, M.; Mariani, S.; et al. Navigating the Landscape of Liquid Biopsy in Colorectal Cancer: Current Insights and Future Directions. Int. J. Mol. Sci. 2025, 26, 7619. [Google Scholar] [CrossRef]
- Human Microbiome Project Consortium. Structure, function and diversity of the healthy human microbiome. Nature 2012, 486, 207–214. [Google Scholar] [CrossRef] [PubMed]
- Qin, J.; Li, R.; Raes, J.; Arumugam, M.; Burgdorf, K.S.; Manichanh, C.; Nielsen, T.; Pons, N.; Levenez, F.; Yamada, T.; et al. A human gut microbial gene catalogue established by metagenomic sequencing. Nature 2010, 464, 59–65. [Google Scholar] [CrossRef]
- Lee, J.W.J.; Plichta, D.R.; Asher, S.; Delsignore, M.; Jeong, T.; McGoldrick, J.; Staller, K.; Khalili, H.; Xavier, R.J.; Chung, D.C. Association of distinct microbial signatures with premalignant colorectal adenomas. Cell Host Microbe 2023, 31, 827–838.e3. [Google Scholar] [CrossRef]
| Authors | Population | Methods | Key Findings | Limitations |
|---|---|---|---|---|
| Yu et al., 2017 [10] | 74 CRC + 54 controls, validation in Denmark, France, Austria | Fecal metagenomics, MWAS, qPCR validation | Identified CRC-associated taxa (Fusobacterium nucleatum, Parvimonas micra, Solobacterium moorei); developed and validated non-invasive biomarkers with high diagnostic accuracy (AUC up to 0.84) | Limited sample sizes in validation cohorts; potential geographic bias |
| Rubinstein et al., 2013 [11] | Human CRC tissues, cell lines | Functional/mechanistic assays on FadA adhesin | Fusobacterium nucleatum FadA binds E-cadherin, activates β-catenin signaling, promotes CRC cell growth; FadA upregulated in adenomas and carcinomas | Mainly mechanistic, not large-scale clinical validation |
| Gur et al., 2015 [12] | Human CRC tissues, cell culture, immune assays | TIGIT–Fap2 interaction analysis | Fusobacterium nucleatum Fap2 protein inhibits NK and T-cell activity via TIGIT, enabling immune evasion | Focused on immune modulation; lacks epidemiological cohort |
| Wu et al., 2009 [13] | Mouse model (Min mice), ETBF vs. NTBF colonization | Colonization + immunological assays | ETBF induces colitis, Stat3/Th17 activation, and tumorigenesis; IL-17/IL-23 blockade prevents tumor formation | Preclinical study; limited human data |
| Pleguezuelos-Manzano et al., 2020 [14] | Human intestinal organoids; 5876 human cancer genomes | Organoid exposure to pks+ Escherichia coli, WGS, and mutational signature analysis. | Identified distinct mutational signature of colibactin in CRC; colibactin linked to APC driver mutations | Organoid model may not capture full in vivo complexity; prevalence in general population uncertain |
| Kong et al., 2023 [15] | 114 EOCRC, 130 LOCRC, 197 controls; independent validation cohort | Multi-omics: metagenomics + metabolomics | EOCRC associated with Flavonifractor plautii, altered tryptophan/bile acid/choline metabolism; predictive multi-omics classifier performed well | Single-country cohorts; dietary/lifestyle confounders |
| Qin et al., 2024 [16] | Large yCRC and oCRC metagenomes from 2 independent cohorts (China) | Shotgun metagenomic sequencing | Consistent CRC microbial signatures (e.g., Fusobacterium nucleatum, Bacteroides fragilis) across young- and old-onset patients; microbiome-based models equally accurate across age groups | Mostly Chinese cohorts: functional validation limited |
| Adnan et al., 2024 [17] | 701 CRC vs. 693 controls (fecal metagenomes, CMGData) + 85 tumor microbiomes (TCGA) | Bioinformatics, fecal and tumor microbiome, host transcriptomics | Age-specific microbial differences; stronger host–microbe interactions in EOCRC tumors | Secondary data analysis; heterogeneous datasets |
| Díaz-Gay et al., 2025 [18] | 981 CRC genomes from 11 countries | WGS, mutational signature analysis | Geographic and age-related variation in mutational processes; SBS88/ID18 (colibactin) enriched in EOCRC; ~25% APC indels linked to colibactin | Correlation with microbiome exposure inferred, not directly measured |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lauricella, S.; Brucchi, F.; Cirocchi, R.; Cassini, D.; Vitellaro, M. The Gut Microbiome in Early-Onset Colorectal Cancer: Distinct Signatures, Targeted Prevention and Therapeutic Strategies. J. Pers. Med. 2025, 15, 552. https://doi.org/10.3390/jpm15110552
Lauricella S, Brucchi F, Cirocchi R, Cassini D, Vitellaro M. The Gut Microbiome in Early-Onset Colorectal Cancer: Distinct Signatures, Targeted Prevention and Therapeutic Strategies. Journal of Personalized Medicine. 2025; 15(11):552. https://doi.org/10.3390/jpm15110552
Chicago/Turabian StyleLauricella, Sara, Francesco Brucchi, Roberto Cirocchi, Diletta Cassini, and Marco Vitellaro. 2025. "The Gut Microbiome in Early-Onset Colorectal Cancer: Distinct Signatures, Targeted Prevention and Therapeutic Strategies" Journal of Personalized Medicine 15, no. 11: 552. https://doi.org/10.3390/jpm15110552
APA StyleLauricella, S., Brucchi, F., Cirocchi, R., Cassini, D., & Vitellaro, M. (2025). The Gut Microbiome in Early-Onset Colorectal Cancer: Distinct Signatures, Targeted Prevention and Therapeutic Strategies. Journal of Personalized Medicine, 15(11), 552. https://doi.org/10.3390/jpm15110552







