From Architecture to Outcomes: Mapping the Landscape of Digital Twins for Personalized Diabetes Care—A Scoping Review
Abstract
1. Introduction
2. Methods
2.1. Methodological Framework and Protocol
2.2. Key Definitions
2.3. Research Question and PCC Framework
2.4. Search Strategy
2.5. Study Selection Process
2.6. Data Extraction
2.7. Statistical Analysis and Data Synthesis
2.8. Methodological Considerations
3. Results
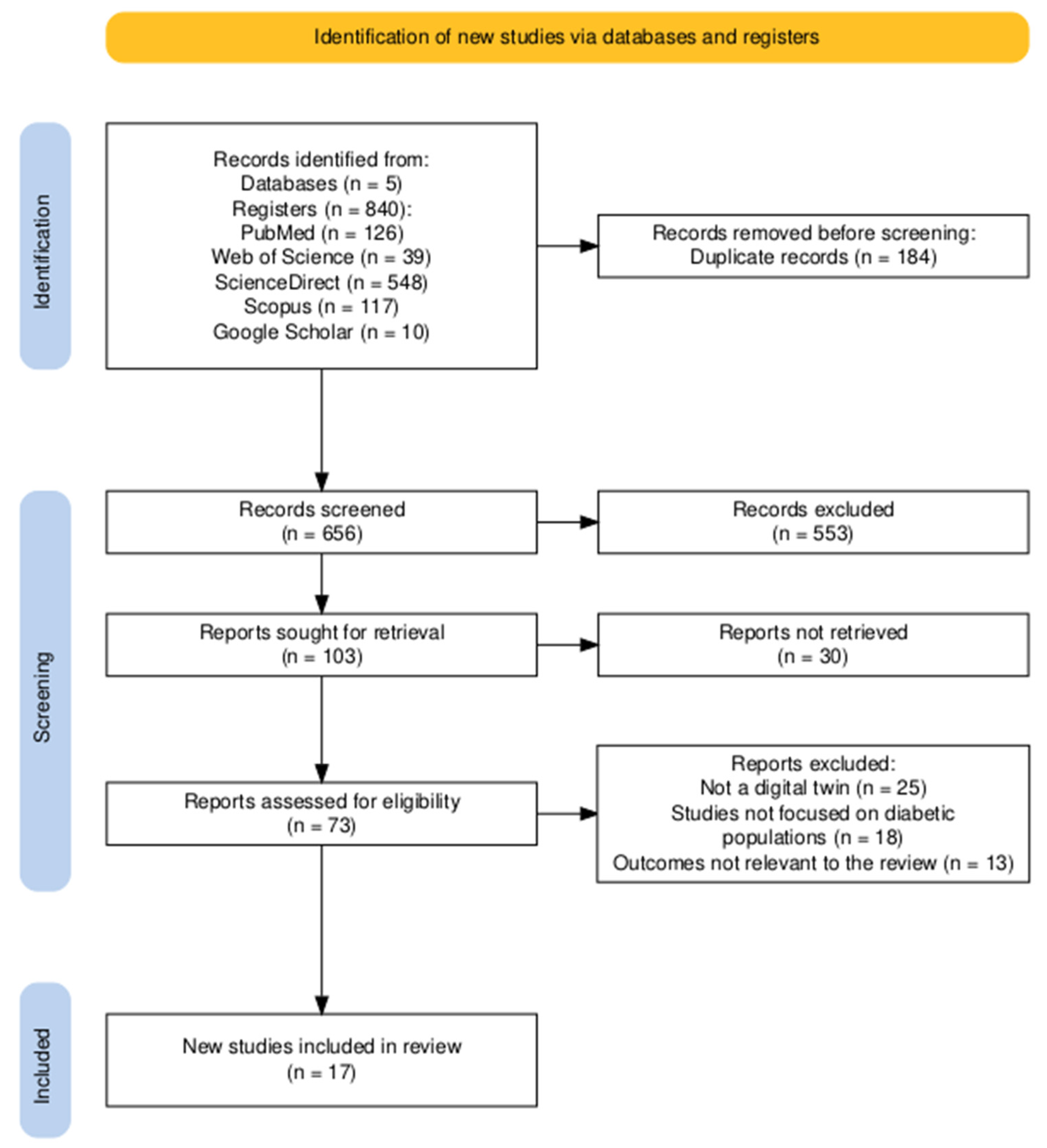
3.1. Study Selection
3.2. Bibliographic and Methodological Characteristics
3.3. Population Characteristics
3.4. Digital Twin Implementation and Technology
3.5. Performance and Clinical Outcomes
3.6. System Integration and Personalization
3.7. Clinical Outcomes and Safety of Interventional Studies
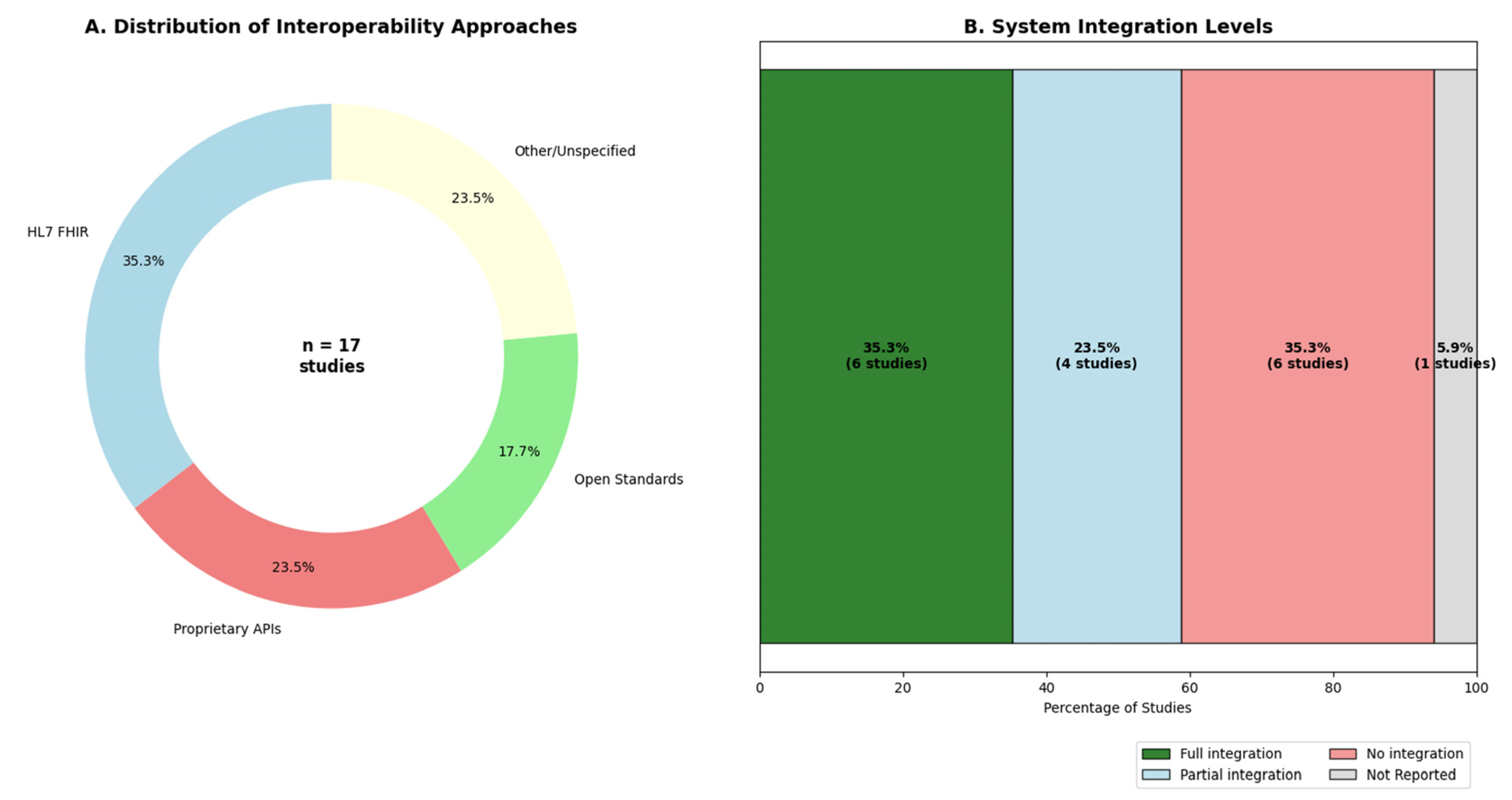
3.8. Interoperability and EHR Integration
4. Discussion
4.1. Principal Findings
4.2. Technological Architecture: Convergence Toward Intelligent Personalization
4.3. Clinical Impact: Transformative Outcomes Amid Implementation Complexities
4.4. Comparative Analysis with Contemporary Literature: Positioning Our Findings
4.5. Critical Limitations and Methodological Considerations
4.6. Implications for Clinical Practice: Bridging Innovation and Implementation
4.7. Future Research Directions: Toward Mature Digital Twin Ecosystems
4.8. Regulatory and Ethical Imperatives: Ensuring Responsible Innovation
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AI | Artificial Intelligence |
| AUC | Area Under Curve |
| AUC-ROC | Area Under Receiver Operating Characteristic Curve |
| BP | Blood Pressure |
| CDS | Clinical Decision Support |
| CGM | Continuous Glucose Monitoring |
| CKD | Chronic Kidney Disease |
| CVD | Cardiovascular Disease |
| DL | Deep Learning |
| DSS | Decision Support System |
| DT | Digital Twin |
| EHR | Electronic Health Record |
| FGM | Flash Glucose Monitoring |
| GDM | Gestational Diabetes Mellitus |
| GMF | Generalized Metabolic Fluxes |
| GV | Glycemic Variability |
| HbA1c | Hemoglobin A1c |
| HPC | High-Performance Computing |
| HTN | Hypertension |
| IoT | Internet of Things |
| JBI | Joanna Briggs Institute |
| LTI | Linear Time-Invariant |
| LSTM | Long Short-Term Memory |
| MAFLD | Metabolic Dysfunction-Associated Fatty Liver Disease |
| MARD | Mean Absolute Relative Difference |
| mHealth | Mobile Health |
| ML | Machine Learning |
| NAFLD | Non-Alcoholic Fatty Liver Disease |
| NN | Neural Networks |
| NPV | Negative Predictive Value |
| PPV | Positive Predictive Value |
| PRISMA-ScR | Preferred Reporting Items for Systematic Reviews and Meta-Analyses Extension for Scoping Reviews |
| PRO | Patient-Reported Outcomes |
| QoL | Quality of Life |
| RCT | Randomized Controlled Trial |
| RNN | Recurrent Neural Network |
| RWE | Real-World Evidence |
| SC | Standard Care |
| SMBG | Self-Monitoring of Blood Glucose |
| SOGMM | Subcutaneous Oral Glucose Minimal Model |
| SVM | Support Vector Machines |
| T1DM | Type 1 Diabetes Mellitus |
| T2DM | Type 2 Diabetes Mellitus |
| TAR | Time Above Range |
| TBR | Time Below Range |
| TIR | Time in Range |
| XAI | Explainable AI |
References
- Sarani Rad, F.; Hendawi, R.; Yang, X.; Li, J. Personalized Diabetes Management with Digital Twins: A Patient-Centric Knowledge Graph Approach. J. Pers. Med. 2024, 14, 359. [Google Scholar] [CrossRef]
- Zhang, Y.; Qin, G.; Aguilar, B.; Rappaport, N.; Yurkovich, J.T.; Pflieger, L.; Huang, S.; Hood, L.; Shmulevich, I. A Framework towards Digital Twins for Type 2 Diabetes. Front. Digit. Health 2024, 6, 1336050. [Google Scholar] [CrossRef]
- Pacichana, J.A.; Osorio, L.M.; Restrepo, K.; García, A.F.; Rivas, G.; Liscano, Y. Diabetic Ketoacidosis as a Debut and Immune-Mediated Complication Caused by Pembrolizumab: Case Report. Diabetology 2024, 5, 600–607. [Google Scholar] [CrossRef]
- Cappon, G.; Vettoretti, M.; Sparacino, G.; Favero, S.D.; Facchinetti, A. ReplayBG: A Digital Twin-Based Methodology to Identify a Personalized Model from Type 1 Diabetes Data and Simulate Glucose Concentrations to Assess Alternative Therapies. IEEE Trans. Biomed. Eng. 2023, 70, 3227–3238. [Google Scholar] [CrossRef] [PubMed]
- Jones, D.E.; Snider, C.; Nassehi, A.; Yon, J.M.; Hicks, B.J. Characterising the Digital Twin: A Systematic Literature Review. CIRP J. Manuf. Sci. Technol. 2020, 29, 36–52. [Google Scholar] [CrossRef]
- Balasubramanyam, A.; Ramesh, R.; Sudheer, R.; Honnavalli, P.B. Revolutionizing Healthcare: A Review Unveiling the Transformative Power of Digital Twins. IEEE Access 2024, 12, 69652–69676. [Google Scholar] [CrossRef]
- Ringeval, M.; Sosso, F.A.E.; Cousineau, M.; Paré, G. Advancing Health Care with Digital Twins: Meta-Review of Applications and Implementation Challenges. J. Med. Internet Res. 2024, 27, e69544. [Google Scholar] [CrossRef]
- Angulo Medina, A.S.; Aguilar Bonilla, M.I.; Rodríguez Giraldo, I.D.; Montenegro Palacios, J.F.; Cáceres Gutiérrez, D.A.; Liscano, Y. Electroencephalography-Based Brain-Computer Interfaces in Rehabilitation: A Bibliometric Analysis (2013–2023). Sensors 2024, 24, 7125. [Google Scholar] [CrossRef]
- Meijer, C.; Uh, H.-W.; El Bouhaddani, S. Digital Twins in Healthcare: Methodological Challenges and Opportunities. J. Pers. Med. 2023, 13, 1522. [Google Scholar] [CrossRef]
- Santos, W.M.D.; Secoli, S.R.; Püschel, V.A.D.A. The Joanna Briggs Institute Approach for Systematic Reviews. Rev. Latino Am. Enfermagem 2018, 26, e3074. [Google Scholar] [CrossRef]
- Tricco, A.C.; Lillie, E.; Zarin, W.; O’Brien, K.K.; Colquhoun, H.; Levac, D.; Moher, D.; Peters, M.D.J.; Horsley, T.; Weeks, L.; et al. PRISMA Extension for Scoping Reviews (PRISMA-ScR): Checklist and Explanation. Ann. Intern. Med. 2018, 169, 467–473. [Google Scholar] [CrossRef]
- Haddaway, N.R.; Page, M.J.; Pritchard, C.C.; McGuinness, L.A. PRISMA2020: An R Package and Shiny App for Producing PRISMA 2020-Compliant Flow Diagrams, with Interactivity for Optimised Digital Transparency and Open Synthesis. Campbell Syst. Rev. 2022, 18, e1230. [Google Scholar] [CrossRef] [PubMed]
- Shamanna, P.; Saboo, B.; Damodharan, S.; Mohammed, J.; Mohamed, M.; Poon, T.; Kleinman, N.; Thajudeen, M. Reducing HbA1c in Type 2 Diabetes Using Digital Twin Technology-Enabled Precision Nutrition: A Retrospective Analysis. Diabetes Ther. 2020, 11, 2703–2714. [Google Scholar] [CrossRef]
- Shamanna, P.; Dharmalingam, M.; Sahay, R.; Mohammed, J.; Mohamed, M.; Poon, T.; Kleinman, N.; Thajudeen, M. Retrospective Study of Glycemic Variability, BMI, and Blood Pressure in Diabetes Patients in the Digital Twin Precision Treatment Program. Sci. Rep. 2021, 11, 14892. [Google Scholar] [CrossRef] [PubMed]
- Shamanna, P.; Joshi, S.; Shah, L.; Dharmalingam, M.; Saboo, B.; Mohammed, J.; Mohamed, M.; Poon, T.; Kleinman, N.; Thajudeen, M.; et al. Type 2 Diabetes Reversal with Digital Twin Technology-Enabled Precision Nutrition and Staging of Reversal: A Retrospective Cohort Study. Clin. Diabetes Endocrinol. 2021, 7, 21. [Google Scholar] [CrossRef] [PubMed]
- Joshi, S.; Shamanna, P.; Dharmalingam, M.; Vadavi, A.; Keshavamurthy, A.; Shah, L.; Mechanick, J.I. Digital Twin-Enabled Personalized Nutrition Improves Metabolic Dysfunction-Associated Fatty Liver Disease in Type 2 Diabetes: Results of a 1-Year Randomized Controlled Study. Endocr. Pract. 2023, 29, 960–970. [Google Scholar] [CrossRef]
- Shamanna, P.; Joshi, S.; Thajudeen, M.; Shah, L.; Poon, T.; Mohamed, M.; Mohammed, J. Personalized Nutrition in Type 2 Diabetes Remission: Application of Digital Twin Technology for Predictive Glycemic Control. Front. Endocrinol. 2024, 15, 1485464. [Google Scholar] [CrossRef]
- Shamanna, P.; Erukulapati, R.S.; Shukla, A.; Shah, L.; Willis, B.; Thajudeen, M.; Kovil, R.; Baxi, R.; Wali, M.; Damodharan, S.; et al. One-Year Outcomes of a Digital Twin Intervention for Type 2 Diabetes: A Retrospective Real-World Study. Sci. Rep. 2024, 14, 25478. [Google Scholar] [CrossRef]
- Shamanna, P.; Joshi, S.; Dharmalingam, M.; Vadavi, A.; Keshavamurthy, A.; Shah, L.; Samajdar, S.S.; Mechanick, J.I. Digital Twin in Managing Hypertension Among People with Type 2 Diabetes. JACC Adv. 2024, 3, 101172. [Google Scholar] [CrossRef]
- Colmegna, P.; Wang, K.; Garcia-Tirado, J.; Breton, M.D. Mapping Data to Virtual Patients in Type 1 Diabetes. Control Eng. Pract. 2020, 103, 104605. [Google Scholar] [CrossRef]
- Hughes, J.; Gautier, T.; Colmegna, P.; Fabris, C.; Breton, M.D. Replay Simulations with Personalized Metabolic Model for Treatment Design and Evaluation in Type 1 Diabetes. J. Diabetes Sci. Technol. 2021, 15, 1326–1336. [Google Scholar] [CrossRef] [PubMed]
- Surian, N.U.; Batagov, A.; Wu, A.; Lai, W.B.; Sun, Y.; Bee, Y.M.; Dalan, R. A Digital Twin Model Incorporating Generalized Metabolic Fluxes to Identify and Predict Chronic Kidney Disease in Type 2 Diabetes Mellitus. npj Digit. Med. 2024, 7, 140. [Google Scholar] [CrossRef]
- Visentin, R.; Man, C.D.; Cobelli, C. One-Day Bayesian Cloning of Type 1 Diabetes Subjects: Toward a Single-Day UVA/Padova Type 1 Diabetes Simulator. IEEE Trans. Biomed. Eng. 2016, 63, 2416–2424. [Google Scholar] [CrossRef]
- Thamotharan, P.; Srinivasan, S.; Kesavadev, J.; Krishnan, G.; Mohan, V.; Seshadhri, S.; Bekiroglu, K.; Toffanin, C. Human Digital Twin for Personalized Elderly Type 2 Diabetes Management. J. Clin. Med. 2023, 12, 2094. [Google Scholar] [CrossRef]
- Young, G.; Dodier, R.; Youssef, J.E.; Castle, J.R.; Wilson, L.; Riddell, M.C.; Jacobs, P.G. Design and In Silico Evaluation of an Exercise Decision Support System Using Digital Twin Models. J. Diabetes Sci. Technol. 2024, 18, 324–334. [Google Scholar] [CrossRef]
- Deichmann, J.; Bachmann, S.; Burckhardt, M.-A.; Pfister, M.; Szinnai, G.; Kaltenbach, H.-M. New Model of Glucose-Insulin Regulation Characterizes Effects of Physical Activity and Facilitates Personalized Treatment Evaluation in Children and Adults with Type 1 Diabetes. PLoS Comput. Biol. 2023, 19, e1010289. [Google Scholar] [CrossRef]
- Katsoulakis, E.; Wang, Q.; Wu, H.; Shahriyari, L.; Fletcher, R.; Liu, J.; Achenie, L.; Liu, H.; Jackson, P.; Xiao, Y.; et al. Digital Twins for Health: A Scoping Review. npj Digit. Med. 2024, 7, 77. [Google Scholar] [CrossRef]
- Sun, S.; Simonsson, O.; McGarvey, S.; Torous, J.; Goldberg, S.B. Mobile Phone Interventions to Improve Health Outcomes among Patients with Chronic Diseases: An Umbrella Review and Evidence Synthesis from 34 Meta-Analyses. Lancet Digit. Health 2024, 6, e857–e870. [Google Scholar] [CrossRef]
- Shen, S.; Qi, W.; Liu, X.; Zeng, J.; Li, S.; Zhu, X.; Dong, C.; Wang, B.; Shi, Y.; Yao, J.; et al. From Virtual to Reality: Innovative Practices of Digital Twins in Tumor Therapy. J. Transl. Med. 2025, 23, 348. [Google Scholar] [CrossRef]
- Zhou, X.; Siegel, K.R.; Ng, B.P.; Jawanda, S.; Proia, K.K.; Zhang, X.; Albright, A.L.; Zhang, P. Cost-Effectiveness of Diabetes Prevention Interventions Targeting High-Risk Individuals and Whole Populations: A Systematic Review. Diabetes Care 2020, 43, 1593–1616. [Google Scholar] [CrossRef]
- Bosetti, R.; Tabatabai, L.; Naufal, G.; Menser, T.; Kash, B. Comprehensive Cost-Effectiveness of Diabetes Management for the Underserved in the United States: A Systematic Review. PLoS ONE 2021, 16, e0260139. [Google Scholar] [CrossRef]
- Vallée, A. Digital Twin for Healthcare Systems. Front. Digit. Health 2023, 5, 1253050. [Google Scholar] [CrossRef]
- Grieb, N.; Schmierer, L.; Kim, H.U.; Strobel, S.; Schulz, C.; Meschke, T.; Kubasch, A.S.; Brioli, A.; Platzbecker, U.; Neumuth, T.; et al. A Digital Twin Model for Evidence-Based Clinical Decision Support in Multiple Myeloma Treatment. Front. Digit. Health 2023, 5, 1324453. [Google Scholar] [CrossRef]
- Riahi, V.; Diouf, I.; Khanna, S.; Boyle, J.; Hassanzadeh, H. Digital Twins for Clinical and Operational Decision-Making: Scoping Review. J. Med. Internet Res. 2025, 27, e55015. [Google Scholar] [CrossRef]
- Bertelsen, N.; Dewulf, L.; Ferrè, S.; Vermeulen, R.; Schroeder, K.; Gatellier, L.; Sargeant, I.; Luzuriaga, D.; Chapman, H.; Brooke, N. Patient Engagement and Patient Experience Data in Regulatory Review and Health Technology Assessment: A Global Landscape Review. Ther. Innov. Regul. Sci. 2024, 58, 63–78. [Google Scholar] [CrossRef] [PubMed]
- Levin, L.; Sheldon, M.; McDonough, R.S.; Aronson, N.; Rovers, M.; Gibson, C.M.; Tunis, S.R.; Kuntz, R.E. Early Technology Review: Towards an Expedited Pathway. Int. J. Technol. Assess. Health Care 2024, 40, e13. [Google Scholar] [CrossRef]
- Tan, B.; Matta, A. The Digital Twin Synchronization Problem: Framework, Formulations, and Analysis. IISE Trans. 2024, 56, 652–665. [Google Scholar] [CrossRef]
- De Simone, B.; Deeken, G.; Catena, F. Balancing Ethics and Innovation: Can Artificial Intelligence Safely Transform Emergency Surgery? A Narrative Perspective. J. Clin. Med. 2025, 14, 3111. [Google Scholar] [CrossRef]
- Naik, N.; Hameed, B.M.Z.; Shetty, D.K.; Swain, D.; Shah, M.; Paul, R.; Aggarwal, K.; Ibrahim, S.; Patil, V.; Smriti, K.; et al. Legal and Ethical Consideration in Artificial Intelligence in Healthcare: Who Takes Responsibility? Front. Surg. 2022, 9, 862322. [Google Scholar] [CrossRef]
- Micheli, M.; Ponti, M.; Craglia, M.; Berti Suman, A. Emerging Models of Data Governance in the Age of Datafication. Big Data Soc. 2020, 7, 2053951720948087. [Google Scholar] [CrossRef]


| Inclusion Criteria | Exclusion Criteria |
|---|---|
| Study Types: | Study Types: |
| • Empirical studies (observational, experimental, modeling) | • Studies without empirical data, systematic or scoping reviews or metanalysis |
| • Conceptual and theoretical models that lack a detailed architectural framework or a clear pathway for future clinical validation | |
| • Original research published in peer-reviewed journals | • Opinion articles, editorials, commentaries |
| • Full-text articles available | • Conference abstracts, preprints, theses |
| • English | • Brief communications without original data |
| Population: | Population: |
| • Human patients with diabetes mellitus (any type) | • Non-human studies (animal models, in vitro) |
| • Type 1, Type 2, gestational, or other diabetes variants | • Studies not focused on diabetic populations |
| • Any age group (pediatric, adult, elderly) | • Healthy populations without diabetes |
| • Any geographical location | • Mixed populations where diabetes data cannot be extracted |
| Concept/Technology: | Concept/Technology: |
| • Digital twins implementation in diabetes management | • Traditional monitoring systems only |
| • Virtual patient models with real-time data integration | • Studies without digital twin components |
| • Predictive modeling for diabetes complications | • Simple mobile apps without modeling capabilities |
| • Personalized treatment optimization systems | • Basic data collection tools without analytics |
| • Integration of wearable devices, CGM, IoT sensors | • Single-parameter monitoring systems |
| • Machine learning/AI applications in diabetes care | • Static risk calculators |
| Outcomes/Context: | Outcomes/Context: |
| • Clinical effectiveness metrics reported | • Unclear or insufficient outcome data |
| • Performance measures (sensitivity, specificity, accuracy) | • Studies with incomplete results |
| • Integration with traditional healthcare systems | • Purely technical/engineering focus without clinical application |
| • Real-world implementation evidence | • Simulation studies without real patient data |
| • Patient-reported outcomes and acceptability | • Studies focusing solely on system architecture |
| • Cost-effectiveness or feasibility data | • No validation against clinical standards |
| Variable Category | Specific Variables Extracted |
|---|---|
| Bibliographic and Methodological Information | |
| • Author names and publication year | |
| • Study design and duration | |
| • Geographical location and setting | |
| • Sample size and power calculations (when available) | |
| • Demographic characteristics of study populations | |
| Population Characteristics | |
| • Diabetes type (type 1, type 2, gestational, monogenic, other variants) | |
| • Age distribution and sex composition | |
| • Diabetes duration and disease severity | |
| • Comorbidity profiles and complications history | |
| • Previous pharmacological treatments and management approaches | |
| Digital Twin Implementation Variables | |
| • Data sources: continuous glucose monitors, flash glucose monitoring systems | |
| • Wearable activity trackers, smart insulin pens, mobile health applications | |
| • Electronic health record integration | |
| • System architecture and technological platforms | |
| • Cloud computing infrastructure, edge computing capabilities | |
| • Data processing frameworks | |
| • Analytical algorithms: machine learning (random forests, SVM, neural networks) | |
| • Statistical models (time series analysis, regression methods) | |
| • Deep learning architectures and ensemble modeling techniques | |
| Performance and Validation Metrics | |
| • Predictive accuracy: sensitivity, specificity, PPV, NPV | |
| • Area under receiver operating characteristic curve (AUC-ROC) | |
| • Calibration metrics | |
| • Correlations with traditional methods (HbA1c, SMBG, clinical assessments) | |
| • Temporal advantages: lead time measurements, early warning capabilities | |
| • Glycemic control: time in range, time above/below range | |
| • Glycemic variability indices, mean absolute relative difference | |
| Integration and Implementation Variables | |
| • Validation methods: cross-validation, external validation, real-world evidence | |
| • EHR compatibility, clinical decision support integration | |
| • Healthcare provider workflow incorporation | |
| • Impact on clinical decisions and treatment adherence | |
| • Patient-reported outcomes and quality of life measures | |
| • Healthcare utilization patterns | |
| Contextual and Feasibility Variables | |
| • Spatial and temporal data granularity specifications | |
| • Ethical considerations and privacy protection measures | |
| • Regulatory compliance and approval status | |
| • Technological barriers and implementation challenges | |
| • User acceptability (patients and healthcare providers) | |
| • Training requirements for clinical staff | |
| • Cost-effectiveness analyses and budget impact assessments |
| Authors | Year | Title | Journal | Country | Study Type |
|---|---|---|---|---|---|
| Shamanna et al. [13] | 2020 | Reducing HbA1c in Type 2 Diabetes Using Digital Twin Technology-Enabled Precision Nutrition: A Retrospective Analysis | Diabetes Therapy | India | Retrospective |
| Shamanna et al. [14] | 2021 | Retrospective study of glycemic variability, BMI, and blood pressure in diabetes patients in the Digital Twin Precision Treatment Program | Scientific Reports | India | Retrospective cohort |
| Shamanna et al. [15] | 2021 | Type 2 diabetes reversal with digital twin technology-enabled precision nutrition and staging of reversal: a retrospective cohort study | Clinical Diabetes and Endocrinology | India | Retrospective cohort |
| Joshi, Shamanna et al. [16] | 2023 | Digital Twin-Enabled Personalized Nutrition Improves Metabolic Dysfunction-Associated Fatty Liver Disease in Type 2 Diabetes: Results of a 1-Year Randomized Controlled Study | Endocrine Practice | India | RCT |
| Shamanna et al. [17] | 2024 | Personalized nutrition in type 2 diabetes remission: application of digital twin technology for predictive glycemic control | Frontiers in Endocrinology | India | RCT |
| Shamanna et al. [18] | 2024 | One-year outcomes of a digital twin intervention for type 2 diabetes: a retrospective real-world study | Scientific Reports | India | Retrospective |
| Shamanna et al. [19] | 2024 | Digital twin in managing hypertension among people with type 2 diabetes 1-year randomized controlled trial | JACC: Advances | India | RCT |
| Colmegna et al. [20] | 2020 | Mapping data to virtual patients in type 1 diabetes | Control Engineering Practice | Spain/USA | In silico simulation |
| Hughes et al. [21] | 2021 | Replay Simulations with Personalized Metabolic Model for Treatment Design and Evaluation in Type 1 Diabetes | Journal of Diabetes Science and Technology | USA | In silico validation |
| Cappon et al. [4] | 2023 | ReplayBG: A Digital Twin-Based Methodology to Identify a Personalized Model From Type 1 Diabetes Data and Simulate Glucose Concentrations to Assess Alternative Therapies | IEEE Transactions on Biomedical Engineering | Italy | Technical validation |
| Sarani Rad et al. [1] | 2024 | Personalized Diabetes Management with Digital Twins: A Patient-Centric Knowledge Graph Approach | Journal of Personalized Medicine | USA | Conceptual framework |
| Surian et al. [22] | 2024 | A digital twin model incorporating generalized metabolic fluxes to identify and predict chronic kidney disease in type 2 diabetes mellitus | NPJ Digital Medicine | Australia/Singapore | Model development |
| Zhang et al. [2] | 2024 | A framework towards digital twins for type 2 diabetes | Frontiers in Digital Health | China/USA | Conceptual framework |
| Visentin et al. [23] | 2016 | One-Day Bayesian Cloning of Type 1 Diabetes Subjects: Towards a Single-Day UVA/Padova Type 1 Diabetes Simulator | IEEE Transactions on Biomedical Engineering | Italy | Model development |
| Thamotharan et al. [24] | 2023 | Human digital twin for personalized elderly type 2 diabetes management | Journal of Clinical Medicine | India | Conceptual |
| Young et al. [25] | 2024 | Design and In Silico Evaluation of an Exercise Decision Support System Using Digital Twin Models | Journal of Diabetes Science and Technology | USA | In silico evaluation |
| Deichmann et al. [26] | 2023 | New model of glucose-insulin regulation characterizes effects of physical activity and facilitates personalized treatment evaluation in children and adults with type 1 diabetes | PLOS Computational Biology | Switzerland | Model development |
| Authors | Diabetes Type | Sample Size | Age (Years) | Female (%) | Diabetes Duration | Comorbidities | Baseline HbA1c (%) | Key Inclusion/Exclusion Criteria |
|---|---|---|---|---|---|---|---|---|
| Shamanna et al. [13] | T2DM | 64 | 52.4 ± 10.0 | 29.7% | 8.4 ± 6.5 years | Adequate hepatic/renal function required | 8.8 ± 2.2 | Incl: T2D, adequate hepatic/renal function. |
| Shamanna et al. [14] | T2DM | 64 | 52.44 ± 9.9 | 29.69% | 8.43 ± 6.5 years | No recent cardiovascular events | 8.8 ± 2.2 | Incl: T2D, adequate hepatic/renal function. |
| Shamanna et al. [15] | T2DM | 463–475 | 48.5 ± 10.6 | 27.4% | 9.1 ± 7.5 years | Adequate hepatic/renal function | 9.0 ± 1.9 | Incl: T2D, adequate hepatic/renal function. |
| Joshi, Shamanna et al. [16] | T2DM | 319 | 18–70 | Not reported | ≥8 years | Normal hepatic/renal function | DT: 9.0 ± 1.9 SC: 8.5 ± 1.9 | Incl: Age 18–70, T2D < 8 years, normal hepatic/renal function. |
| Shamanna et al. [17] | T2DM | 319 | 18–70 | Not reported | ≤8 years | HTN, MAFLD, CVD, neuropathy | DT: 9.0 ± 1.9 SC: 8.5 ± 1.9 | Incl: Age 18–70, T2D < 8 years. |
| Shamanna et al. [18] | T2DM | 1853 | 50.9 ± 9.9 | 20.5% | 6.7 ± 6.2 years | Obesity (36.9%), HTN (38.2%), NAFLD (83.1%) | 8.1 ± 1.7 | Incl: Age 18–80, T2D diagnosis. |
| Shamanna et al. [19] | T2DM | 319 | DT: 43.6 ± 8.6, SC: 51.8 ± 10.5 | DT: 16.3%, SC: 53.5% | 3.8 ± 2.6 years | Hypertension | DT: 9.0 ± 1.9 SC: 8.4 ± 1.9 | Incl: Age 18–70, T2D ≤ 8 years, normal hepatic/renal function. |
| Colmegna et al. [20] | T1DM | 15 | 41 ± 11 | 73% | 25 ± 10 years | None reported | 7.41 ± 0.97 | Participants from a previous clinical trial (NCT02558491). |
| Hughes et al. [21] | T1DM | 100 (virtual) | Adult virtual subjects | N/A | N/A | N/A (simulation) | N/A | N/A (in silico study) |
| Cappon et al. [4] | T1DM | 100 (virtual) + 1 real | Adult virtual subjects | N/A | N/A | N/A (simulation) | N/A | N/A (in silico study) |
| Sarani Rad et al. [1] | Not specified | N/A (model) | N/A | N/A | N/A | N/A (conceptual framework) | N/A | N/A (conceptual framework) |
| Surian et al. [22] | T2DM | 7072 | EVAS: 54 ± 11.1, NHANES: 59 ± 11.9, SDR: 61.2 ± 11.0, CDMD: 57 ± 12.4 | ~48–50% | Not reported | Hypertension, CKD | EVAS: 8.6 ± 1.8 NHANES: 7.6 ± 1.9 SDR: 7.4 ± 1.6 CDMD: 8.0 ± 1.8 | T2DM patients from four existing multi-ethnic cohorts, aged 20–80. |
| Zhang et al. [2] | T2DM | 1131 | 49.53 ± 11.29 | Not reported | Not applicable | CKD progression focus | 5.51 ± 0.43 | Participants from Arivale dataset with multi-omic data. |
| Visentin et al. [23] | T1DM | 47 | 42.0 ± 10.1 | Not reported | Not reported | Not reported | Not reported | T1DM subjects from the AP@home FP7-EU project clinical trial. |
| Thamotharan et al. [24] | T2DM | 15 | 36–80 | 53.3% | Not reported | HTN, dyslipidemia, CKD, heart disease | Not reported | Elderly T2D patients with comorbidities recruited at JDRC, India. |
| Young et al. [25] | T1DM | 247 | Not reported | Not reported | Not reported | Not reported | N/A | Digital twins matched to T1Dexi study participants based on insulin sensitivity and weight. |
| Deichmann et al. [26] | T1DM | 5 | ago-14 | Not reported | Not reported | Not reported | Not reported | Children with T1D from the ‘DiaActive’ study. |
| Authors | Technological Components | Algorithms/Models | Data Sources | Captured Parameters | Update Frequency |
|---|---|---|---|---|---|
| Shamanna et al. [13] | IoT, Cloud, Mobile app, Wearables (Fitbit), Bluetooth devices | Machine learning (gradient boosted trees, deep learning, LSTM) | CGM (Abbott Libre Pro), Fitbit, BP monitor, smart scale, food app | Glucose (96/day), activity, sleep, weight, BP, ketones, nutrition | Daily |
| Shamanna et al. [14] | Digital platform, TPT system, IoT sensors, Mobile app | Machine learning algorithms, precision nutrition algorithm | CGM, wearables, clinical data, food logging | Glucose, activity, nutrition, clinical biomarkers | Daily |
| Shamanna et al. [15] | Digital twin platform, Mobile app, Cloud-based | ML algorithms, clustering models | CGM, clinical assessments, nutrition data | Glucose, HbA1c, insulin resistance markers | 90-day intervals |
| Joshi, Shamanna et al. [16] | Digital twin platform, Mobile devices, Wearables | ML, precision nutrition algorithms | CGM, wearables, mHealth devices | >100 physiological signals, glucose, liver markers | Real-time to monthly |
| Shamanna et al. [17] | DT platform, Mobile app, Wearables | ML algorithms, predictive models | CGM, wearables, clinical data | Glucose, physiological markers, lifestyle | Real-time |
| Shamanna et al. [18] | Digital platform, Mobile app, IoT devices | ML algorithms, data analytics | CGM, wearables, clinical assessments | Glucose, weight, BP, activity, nutrition | Daily to monthly |
| Shamanna et al. [19] | Twin Health platform, Mobile app, Sensors | ML, AI algorithms | CGM, wearables, clinical data, BP monitoring | Glucose, BP, weight, activity | Real-time |
| Colmegna et al. [20] | UVA/Padova simulator, HPC systems, Matlab toolbox | Mathematical model, Fourier series, optimization | CGM, insulin pumps, meal data | Glucose, insulin, carbohydrates, 8 physiological parameters | 5 min sampling, daily parameter updates |
| Hughes et al. [21] | UVa/Padova simulator, Computational platform | SOGMM, LTI model, regularized deconvolution | In silico data, CGM, insulin/meal records | Glucose, insulin sensitivity, meal absorption parameters | Per day (SI, f), per meal (absorption) |
| Cappon et al. [4] | UVa/Padova simulator, Digital twin framework | Personalized metabolic models, deconvolution algorithms | CGM, insulin pumps, carbohydrate intake | Glucose, insulin, carbohydrates, physiological parameters | Daily parameter identification |
| Sarani Rad et al. [1] | Knowledge graph platform, Digital twin framework | Machine learning, knowledge graphs | PHKGs, clinical data, IoT sensors | Patient health knowledge, glucose patterns | Variable/real-time |
| Surian et al. [22] | Cloud-based ML pipeline, HealthVector Diabetes® | Logistic regression, GMF-based models | EHR, clinical labs, multi-ethnic cohorts | 33 clinical variables, metabolic fluxes | Episodic (yearly aggregation) |
| Zhang et al. [2] | Multilevel system, Cloud platform | Neural networks, deep learning, multi-omics integration | Multi-omics data, clinical records, longitudinal data | >200 integrated variables, biomarkers | Variable |
| Visentin et al. [23] | UVA/Padova simulator, Bayesian framework | Bayesian identification, compartmental model | Clinical trial data, glucose/insulin measurements | Glucose, insulin, model parameters | Single-day identification |
| Thamotharan et al. [24] | Mobile platform, IoT sensors, Cloud | Digital twin algorithms, personalized models | Clinical data, sensor data, patient records | Glucose, clinical parameters, lifestyle factors | Variable |
| Young et al. [25] | Digital twin simulator, Exercise DSS, OHSU metabolic simulator | Digital twin models, exercise algorithms | Heart rate, insulin, meal data, T1Dexi dataset | Glucose, exercise parameters, insulin | 30 min exercise sessions |
| Deichmann et al. [26] | Computational platform | Mathematical glucose-insulin regulation model | CGM, accelerometer, logbook data | Glucose, insulin, physical activity, carbohydrates | Multi-day continuous |
| Authors | Clinical Metrics | Validation Type | System Integration | User Interface | Interoperability | Personalization |
|---|---|---|---|---|---|---|
| Shamanna et al. [13] | HbA1c (−1.9%), Weight (−4.8 kg), TIR maintained 87.1%, HOMA-IR (−56.9%) | Retrospective | TPN system integrates CGM (Abbott Libre Pro), Fitbit Charge 2, Bluetooth BP monitor (TaiDoc TD-3140), smart scale (PowerMax BCA-130), secure cellular network transmission | TPN mobile app for food logging (>2000 USDA foods), biometric feedback, interaction with nutritional coaches | Compatible with commercial health devices, integration with USDA FoodData Central databases | ML algorithms analyze CGM and food intake for personalized PPGR predictions, individualized dietary recommendations without caloric limits |
| Shamanna et al. [14] | HbA1c reduction, BMI decrease, BP reduction, TIR > 70%, CV 17.34 ± 4.35% | Retrospective | TPT platform integrates body sensors (Fitbit Charge 2), Bluetooth BP monitor (TAIDOC TD-3140), smart scale (Powermax BCA-130), CGM (Abbott Libre Pro), secure cellular transmission | Twin mobile app for food logging (>2000 foods), biometric data visualization, health coach assistance | Interoperability with multiple devices from different brands via Bluetooth and cellular networks, integration with external nutritional databases | Dynamic personalized digital twin with ML, individual PPGR predictions, daily nutrition, exercise and sleep recommendations adapted to specific metabolism |
| Shamanna et al. [15] | Diabetes reversal staging progression | Retrospective | TPN system integrates CGM, sensor watches, BP monitors, smart scales, detailed food intake logging via mobile app, transmission to web platform | Mobile app with database of >50,000 foods with complete nutritional values, nutritional guidance by coaches via app and telephone | Integration with multiple data sources (CGM, sensors, blood analysis) through IoT technologies | ML analyzes macronutrients, micronutrients, biota nutrients for personalized PPGR predictions, dynamic representation of patient-specific metabolism |
| Joshi, Shamanna et al. [16] | HbA1c improvement, MAFLD markers improvement, MRI-PDFF reduction | RCT | DT system integrates CGM (Abbott FreeStyle Libre Pro), smartwatch (Fitbit Charge 2), smart scale (Powermax BCA-130), BP monitor (TAIDOC TD-3140), secure cellular transmission | Mobile app with color-coded foods (red/yellow/green), real-time recommendations, communication with health coaches | Integration with IoT devices from different manufacturers, processing via ML (gradient-boosted decision trees, deep learning, LSTM) | Individualized PPGR predictions, personalized food recommendations based on specific glycemic response, adaptive treatment phases |
| Shamanna et al. [17] | T2D remission (76.5%), improved glycemic control | RCT | DT platform integrates CGM, IoT devices, activity/sleep sensors, BP monitors, scale, secure cellular transmission | Twin app (WBDT) with technological nudge system, remote health coach assistance | IoT integration with >200 feature analysis via ML, compatibility with multiple commercial devices | AI-driven personalization with PPGR predictions, adaptive color coding, recommendations specific to individual moment and condition |
| Shamanna et al. [18] | HbA1c (−1.8%), Weight (−4.8 kg), TIR (69.7% to 86.9%), 74% medication reduction | Retrospective | DT system integrates CGM (Abbott FreeStyle Libre Pro), smartwatch (Fitbit Charge 2), smart scale (Powermax BCA-130), continuous data transmission via cellular network | Twin app with Daily Action Score aggregating health modules (sleep, breathing, activity, nutrition), simplified mobile interface | Automatic synchronization with multiple health devices, cross-validation between biometric sources | Personalized ML for >200 variables, dynamic recommendations adapted to individual metabolic profile, non-intrusive nudge system |
| Shamanna et al. [19] | BP reduction, hypertension management improvement | RCT | DT platform integrates multiple health devices (CGM, smartwatch, scale, BP monitor) via IoT sensors, secure cellular transmission | App with real-time technological nudges, color-coded foods, remote coach assistance | IoT connectivity with data exchange between devices and systems, integrated ML analysis | Personalized PPGR predictions, individual-specific color-coded food recommendations, adaptive phase-based approach |
| Colmegna et al. [20] | MARD < 10%, improved glucose prediction accuracy | In silico simulation | Integration of field-collected data (CGM, insulin pump, meal records) with time-invariant UVA/Padova model plus variability component | Conceptual/simulation system without described end-user interface | Interoperability with UVA/Padova simulator, compatibility with standard commercial device data | Personalization through identification of most sensitive UVA/Padova model parameters and subject-specific variability component |
| Hughes et al. [21] | Model validation metrics, glucose prediction accuracy | In silico validation | Personalized simulator integrated with UVa/Padova T1DM data, individualized physiological parameter identification | No specific user interface described, research simulation system | Integration with UVa/Padova simulator, acceptance of real clinical data for personalized analysis | Strong personalization through physiological parameter adjustment (insulin sensitivity, carbohydrate absorption) specific per subject and day |
| Cappon et al. [4] | ReplayBG validation, accurate therapy simulation | Technical validation | ReplayBG (University of Padova, Padova, Italy) open-source software in MATLAB (Mathworks Inc., Natick, MA, USA), two-step methodology for personalized model identification and simulation | No graphical interface described, available as MATLAB code on GitHub | Open-source software, compatible with standard CGM/insulin/carbohydrate data, interoperability via common data formats | Advanced personalization through Bayesian identification of individual non-linear glucose-insulin dynamics models specific per patient |
| Sarani Rad et al. [1] | Accurate glucose prediction and control | Conceptual validation | Framework integrates EHRs, wearable devices, mobile health apps, patient-generated data using GLAV framework | Healthcare Data Digital Twin Explorer with keyword search and dropdown tree navigation | Ontology aligned with HL7 FHIR standards for interoperability, integration with multiple heterogeneous data sources | Adaptive PHKGs (Personal Health Knowledge Graphs) that expand with new patient data, personalized meal recommendations |
| Surian et al. [22] | CKD prediction, NPV: 84–85%, PPV: 65–66% | Internal/External validation | GMF model integrates complete and incomplete clinical data from multiple datasets (EVAS, NHANES, SDR, CDMD) | Standard implementation in commercial tool “HealthVector Diabetes®”, graphical visualization of GMF profiles | Interoperability with multiple data sources, automatic handling of missing parameters without imputation | Personalized digital twin based on individual biological data, prediction of patient-specific disease trajectories |
| Zhang et al. [2] | Framework validation for T2D progression | Conceptual framework | Framework integrates ML, knowledge graphs (SPOKE with 41 databases), mechanistic models for multi-omic analysis | Future dashboard and UI suggested with natural language interfaces and LLMs | Interoperability through SPOKE graph consolidating 41 biomedical databases | Personalized design with different abstraction levels adapted to specific applications, individualized monitoring and intervention |
| Visentin et al. [23] | Bayesian parameter identification accuracy | Model validation | UVA/Padova simulator with mathematical model, in silico population, interface for scenario configuration | Interface mentioned for simulation scenario setup, running tools, displaying/saving results | No specific interoperability with external systems mentioned | Personalization through individual Bayesian cloning, incorporation of diurnal and inter-individual variability in glucose absorption and insulin sensitivity |
| Thamotharan et al. [24] | Personalized glucose management | Case studies | HDT framework integrates IoT/IoMT devices (wearable sensors, CGM), edge computing (Raspberry Pi), cloud services | Flask/HTML web interface with data visualization, mobile app for real-time vital signs monitoring | Integration of multiple sensor types (BLE, NFC, WiFi, RF), automatic device discovery by UUID | Adaptive patient model with ML (LSTM, predictive control), personalization of insulin infusion, activity/food recommendations |
| Young et al. [25] | Exercise DSS safety and efficacy validation | In silico evaluation | exDSS system integrates CGM, insulin pumps, meal logs, heart rate monitors, digital twins from OHSU metabolic simulator | No detailed user interface described, pre-exercise recommendation system | Functional interoperability between diverse medical devices (CGM, pumps, heart monitors) | Personalized digital twins calibrated by insulin sensitivity and weight, context-specific recommendations per individual physiological condition |
| Deichmann et al. [26] | Model fit to clinical data, glucose-insulin dynamics | Model validation | Modular model integrates CGM data, accelerometers, meal/insulin logs, compatible with existing pharmacokinetic modules | Open-source Python code available, no end-user interface described | Compatible with everyday device data (CGM, activity trackers), modular structure for integration | Robust personalization through least squares regression for individual parameters (insulin sensitivity, meal absorption, basal glucose) |
| Authors | Study Design & Follow-Up | Baseline & Key Inclusion Criteria | Key Clinical Outcomes (Effect Size) | Adverse Events Reported |
|---|---|---|---|---|
| Joshi, Shamanna et al. [16,19] | RCT 1-year follow-up | N = 319. T2D < 8 years, age 18–70, normal hepatic/renal function. Baseline HbA1c: 9.0% (DT) vs. 8.5% (SC). | • HbA1c change: −2.9% (DT) vs. −0.3% (SC), p < 0.001 • T2D Remission: 72.7% in DT group • HTN Remission: 50% in DT group vs. 0% in SC, p < 0.0001 | Not explicitly reported |
| Shamanna et al. [18] | Retrospective 1-year follow-up | N = 1853. T2D, age 18–80. Baseline HbA1c: 8.1%. | • HbA1c change: −1.8% (SD 1.7), p < 0.001 • Weight change: −4.8 kg (SD 6.0), p < 0.001 • Medication reduction: 74% | Not reported |
| Shamanna et al. [13] | Retrospective 90-day follow-up | N = 64. T2D, adequate hepatic/renal function. Baseline HbA1c: 8.8%. | • HbA1c change: −1.9 percentage points (p < 0.0001) • Weight change: −4.8 kg (p < 0.0001) • Insulin stopped: 100% of users | Non-serious AEs (headache, tiredness) were transient. No serious events reported |
| Thamotharan et al. [24] | Case Studies 15-day illustration | N = 15. Elderly T2D patients with comorbidities. | • TIR: Improved to 86–97% from baseline of 3–75% • Insulin reduction: 14–29% | Not reported |
| Authors | Interoperability Standard/Method | Level of EHR Integration | Reported Barriers/Challenges |
|---|---|---|---|
| Shamanna et al. [13,14,18] | IoT integration with commercial devices (Fitbit, scales) via Bluetooth/cellular network; proprietary platform. | Not reported. | Patient adherence to device usage and data logging. |
| Joshi, Shamanna et al. [16,19] | IoT integration; proprietary ML processing on a central platform. | Not reported. | (Implied) Complexity of managing multiple data streams in an RCT context. |
| Sarani Rad et al. [1] | Ontology aligned with HL7 FHIR standards. | Direct integration planned (EHRs are a key data source in the framework). | Data quality, privacy, security, and potential for biases. |
| Surian et al. [22] | Integration from multiple data sources, including EHR and clinical labs. Handles missing data without imputation. | High (integrates data directly from multiple EHR/lab systems). | Harmonizing data across different multi-ethnic cohorts. |
| Zhang et al. [2] | Knowledge graph (SPOKE) integrates 41 biomedical databases. | Future integration suggested via dashboards and LLMs. | Computational complexity of multi-omic data; scalability. |
| Thamotharan et al. [24] | Integration of multiple sensor types (BLE, NFC, WiFi) via IoT/IoMT devices. | Data from “patient records” used, implying some level of integration. | Real-time data processing at the edge; device discovery. |
| Cappon et al. [4] | Open-source software (MATLAB); compatible with standard CGM/pump data formats. | Not applicable. | Computational burden of the identification procedure. |
| Hughes et al. [21] | Interoperability with UVA/Padova simulator; accepts standard clinical data formats. | Not applicable. | Dependence of the “net-effect” signal on model inputs. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cáceres-Gutiérrez, D.A.; Bonilla-Bonilla, D.M.; Liscano, Y.; Díaz Vallejo, J.A. From Architecture to Outcomes: Mapping the Landscape of Digital Twins for Personalized Diabetes Care—A Scoping Review. J. Pers. Med. 2025, 15, 504. https://doi.org/10.3390/jpm15110504
Cáceres-Gutiérrez DA, Bonilla-Bonilla DM, Liscano Y, Díaz Vallejo JA. From Architecture to Outcomes: Mapping the Landscape of Digital Twins for Personalized Diabetes Care—A Scoping Review. Journal of Personalized Medicine. 2025; 15(11):504. https://doi.org/10.3390/jpm15110504
Chicago/Turabian StyleCáceres-Gutiérrez, Danilo Andrés, Diana Marcela Bonilla-Bonilla, Yamil Liscano, and Jhony Alejandro Díaz Vallejo. 2025. "From Architecture to Outcomes: Mapping the Landscape of Digital Twins for Personalized Diabetes Care—A Scoping Review" Journal of Personalized Medicine 15, no. 11: 504. https://doi.org/10.3390/jpm15110504
APA StyleCáceres-Gutiérrez, D. A., Bonilla-Bonilla, D. M., Liscano, Y., & Díaz Vallejo, J. A. (2025). From Architecture to Outcomes: Mapping the Landscape of Digital Twins for Personalized Diabetes Care—A Scoping Review. Journal of Personalized Medicine, 15(11), 504. https://doi.org/10.3390/jpm15110504







