Impact of Complex Genetic and Drug–Drug Interactions on Tamoxifen Metabolism and Efficacy
Abstract
1. Introduction
2. Materials and Methods
3. Genetic Variation Impact on Tamoxifen Therapy
3.1. Tamoxifen Metabolism
3.2. Drug-Phase I Metabolizing Enzyme Interactions
3.3. Drug-Phase II Metabolizing Enzyme Interactions
3.4. Drug Transporter Interactions
4. Multigenic Variations Influence on Tamoxifen Therapy
5. Pharmacogenetic and Drug–Drug Interactions Effects on Tamoxifen Therapy
6. Clinical Management of Tamoxifen Response Variability
6.1. Pharmacogenetic Testing
6.2. Therapeutic Drug Monitoring of Endoxifen
6.3. Phenotyping CYPs Activities Using Validated Probe Drugs
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| 4-Oh-tam | 4-Hydroxy-Tamoxifen |
| ABC | ATP-Binding Cassette transporters |
| ADR | Adverse Drug Reaction |
| AIs | Aromatase Inhibitors |
| ART | Antiretroviral Therapy |
| CYP | Cytochrome P450 enzymes |
| DDIs | Drug–Drug Interactions |
| DDGIs | Drug–Drug–Gene Interactions |
| DGGIs | Drug–Gene–Gene Interactions |
| DGIs | Drug–Gene Interactions |
| DM-TAM | Desmethyl-Tamoxifen |
| DMEs | Drug Metabolism Enzymes |
| EHR | Electronic Health Records |
| ER+ | Estrogen Receptor–Positive |
| GWAS | Genome-Wide Association Studies |
| IM | Intermediate Metabolizer |
| NM | Normal Metabolizer |
| NorEND | N-desmethyl-4-hydroxy-tamoxifen |
| OATPs | Organic Anion Transporting Polypeptides |
| P-gp | P-glycoprotein |
| PM | Poor Metabolizer |
| SLC | Solute Carrier transporters |
| SNRIs | Serotonin–Norepinephrine Reuptake Inhibitors |
| SSRIs | Selective Serotonin Reuptake Inhibitors |
| SULT | Sulfotransferases |
| TDM | Therapeutic Drug Monitoring |
| UGTs | UDP-glucuronosyltransferases |
| UM | Ultrarapid Metabolizer |
References
- Giaquinto, A.N.; Sung, H.; Miller, K.D.; Kramer, J.L.; Newman, L.A.; Minihan, A.; Jemal, A.; Siegel, R.L. Breast Cancer Statistics, 2022. CA Cancer J. Clin. 2022, 72, 524–541. [Google Scholar] [CrossRef]
- Jayasekera, J.; Zhao, A.; Schechter, C.; Lowry, K.; Yeh, J.M.; Schwartz, M.D.; O’Neill, S.; Wernli, K.J.; Stout, N.; Mandelblatt, J.; et al. Reassessing the Benefits and Harms of Risk-Reducing Medication Considering the Persistent Risk of Breast Cancer Mortality in Estrogen Receptor–Positive Breast Cancer. J. Clin. Oncol. 2023, 41, 859–870. [Google Scholar] [CrossRef]
- Eggemann, H.; Brucker, C.; Schrauder, M.; Thill, M.; Flock, F.; Reinisch, M.; Costa, S.-D.; Ignatov, A. Survival Benefit of Tamoxifen in Male Breast Cancer: Prospective Cohort Analysis. Br. J. Cancer 2020, 123, 33–37. [Google Scholar] [CrossRef] [PubMed]
- Early Breast Cancer Trialists’ Collaborative Group. Effects of Chemotherapy and Hormonal Therapy for Early Breast Cancer on Recurrence and 15-Year Survival: An Overview of the Randomised Trials. Lancet 2005, 365, 1687–1717. [Google Scholar] [CrossRef] [PubMed]
- Hahn, M.; Roll, S.C. The Influence of Pharmacogenetics on the Clinical Relevance of Pharmacokinetic Drug–Drug Interactions: Drug–Gene, Drug–Gene–Gene and Drug–Drug–Gene Interactions. Pharmaceuticals 2021, 14, 487. [Google Scholar] [CrossRef]
- Jeiziner, C.; Stäuble, C.K.; Lampert, M.L.; Hersberger, K.E.; Meyer, Z.; Schwabedissen, H.E. Enriching Medication Review with a Pharmacogenetic Profile—A Case of Tamoxifen Adverse Drug Reactions. Pharmgenom. Pers. Med. 2021, 14, 279–286. [Google Scholar] [CrossRef]
- Mürdter, T.E.; Schroth, W.; Bacchus-Gerybadze, L.; Winter, S.; Heinkele, G.; Simon, W.; Fasching, P.A.; Fehm, T.; German Tamoxifen and AI Clinicians Group; Eichelbaum, M.; et al. Activity Levels of Tamoxifen Metabolites at the Estrogen Receptor and the Impact of Genetic Polymorphisms of Phase I and II Enzymes on Their Concentration Levels in Plasma. Clin. Pharmacol. Ther. 2011, 89, 708–717. [Google Scholar] [CrossRef]
- Hodel, F.; De Min, M.B.; Thorball, C.W.; Redin, C.; Vollenweider, P.; Girardin, F.; Fellay, J. Prevalence of Actionable Pharmacogenetic Variants and High-risk Drug Prescriptions: A Swiss Hospital-based Cohort Study. Clin. Transl. Sci. 2024, 17, e70009. [Google Scholar] [CrossRef]
- Nunez-Torres, R.; Pita, G.; Peña-Chilet, M.; López-López, D.; Zamora, J.; Roldán, G.; Herráez, B.; Álvarez, N.; Alonso, M.R.; Dopazo, J.; et al. A Comprehensive Analysis of 21 Actionable Pharmacogenes in the Spanish Population: From Genetic Characterisation to Clinical Impact. Pharmaceutics 2023, 15, 1286. [Google Scholar] [CrossRef] [PubMed]
- Jithesh, P.V.; Abuhaliqa, M.; Syed, N.; Ahmed, I.; El Anbari, M.; Bastaki, K.; Sherif, S.; Umlai, U.-K.; Jan, Z.; Gandhi, G.; et al. A Population Study of Clinically Actionable Genetic Variation Affecting Drug Response from the Middle East. Npj Genom. Med. 2022, 7, 10. [Google Scholar] [CrossRef]
- Goetz, M.P.; Sangkuhl, K.; Guchelaar, H.J.; Schwab, M.; Province, M.; Whirl-Carrillo, M.; Symmans, W.F.; McLeod, H.L.; Ratain, M.J.; Zembutsu, H.; et al. Clinical Pharmacogenetics Implementation Consortium (CPIC) Guideline for CYP2D6 and Tamoxifen Therapy. Clin. Pharmacol. Ther. 2018, 103, 770–777. [Google Scholar] [CrossRef] [PubMed]
- Helland, T.; Henne, N.; Bifulco, E.; Naume, B.; Borgen, E.; Kristensen, V.N.; Kvaløy, J.T.; Lash, T.L.; Alnæs, G.I.G.; van Schaik, R.H.; et al. Serum concentrations of active tamoxifen metabolites predict long-term survival in adjuvantly treated breast cancer patients. Breast Cancer Res. 2017, 19, 125. [Google Scholar] [CrossRef]
- Hammarström, M.; Gabrielson, M.; Bergqvist, J.; Lundholm, C.; Crippa, A.; Bäcklund, M.; Wengström, Y.; Borgquist, S.; Eliasson, E.; Eriksson, M.; et al. Influence of endoxifen on mammographic density: Results from the KARISMA-Tam trial. J. Natl. Cancer Inst. 2025, 117, 629–636. [Google Scholar] [CrossRef]
- Tamoxifen Pathway, Pharmacokinetics. Available online: https://www.pharmgkb.org/pathway/PA145011119 (accessed on 17 October 2025).
- Chen, Y.; Marcath, L.A.; Eliassen, F.M.; Lende, T.H.; Soiland, H.; Mellgren, G.; Helland, T.; Hertz, D.L. Effect of Genetic Variability in 20 Pharmacogenes on Concentrations of Tamoxifen and Its Metabolites. J. Pers. Med. 2021, 11, 507. [Google Scholar] [CrossRef]
- Powers, J.L.; Buys, S.S.; Fletcher, D.; Melis, R.; Johnson-Davis, K.L.; Lyon, E.; Malmberg, E.M.; McMillin, G.A. Multigene and Drug Interaction Approach for Tamoxifen Metabolite Patterns Reveals Possible Involvement of CYP2C9, CYP2C19, and ABCB1. J. Clin. Pharmacol. 2016, 56, 1570–1581. [Google Scholar] [CrossRef] [PubMed]
- Gaedigk, A.; Sangkuhl, K.; Whirl-Carrillo, M.; Twist, G.P.; Klein, T.E.; Miller, N.A. The Evolution of PharmVar. Clin. Pharmacol. Ther. 2019, 105, 29–32. [Google Scholar] [CrossRef]
- Gaedigk, A.; Simon, S.; Pearce, R.; Bradford, L.; Kennedy, M.; Leeder, J. The CYP2D6 Activity Score: Translating Genotype Information into a Qualitative Measure of Phenotype. Clin. Pharmacol. Ther. 2008, 83, 234–242. [Google Scholar] [CrossRef]
- Saladores, P.; Mürdter, T.; Eccles, D.; Chowbay, B.; Zgheib, N.K.; Winter, S.; Ganchev, B.; Eccles, B.; Gerty, S.; Tfayli, A.; et al. Tamoxifen Metabolism Predicts Drug Concentrations and Outcome in Premenopausal Patients with Early Breast Cancer. Pharmacogenom. J. 2015, 15, 84–94. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Li, Z.; Li, L.; Liu, T.; Qian, C.; Ren, Y.; Li, Z.; Chen, K.; Ji, D.; Zhang, M.; et al. Toremifene, an Alternative Adjuvant Endocrine Therapy, Is Better Than Tamoxifen in Breast Cancer Patients with CYP2D6*10 Mutant Genotypes. Cancer Res. Treat. 2024, 56, 134–142. [Google Scholar] [CrossRef]
- Goetz, M.P.; Suman, V.J.; Hoskin, T.L.; Gnant, M.; Filipits, M.; Safgren, S.L.; Kuffel, M.; Jakesz, R.; Rudas, M.; Greil, R.; et al. CYP2D6 Metabolism and Patient Outcome in the Austrian Breast and Colorectal Cancer Study Group Trial (ABCSG) 8. Clin. Cancer Res. 2013, 19, 500–507. [Google Scholar] [CrossRef] [PubMed]
- Karle, J.; Bolbrinker, J.; Vogl, S.; Kreutz, R.; Denkert, C.; Eucker, J.; Wischnewsky, M.; Possinger, K.; Regierer, A.C. Influence of CYP2D6-Genotype on Tamoxifen Efficacy in Advanced Breast Cancer. Breast Cancer Res. Treat. 2013, 139, 553–560. [Google Scholar] [CrossRef][Green Version]
- Lammers, L.A.; Mathijssen, R.H.J.; van Gelder, T.; Bijl, M.J.; de Graan, A.-J.M.; Seynaeve, C.; van Fessem, M.A.; Berns, E.M.; Vulto, A.G.; van Schaik, R.H.N. The Impact of CYP2D6-Predicted Phenotype on Tamoxifen Treatment Outcome in Patients with Metastatic Breast Cancer. Br. J. Cancer 2010, 103, 765–771. [Google Scholar] [CrossRef]
- Malash, I.; Mansour, O.; Shaarawy, S.; Abdellateif, M.S.; Omar, A.; Gaafar, R.; Zekri, A.-R.N.; Ahmed, O.S.; Bahnassy, A. The Role of CYP2D6 Polymorphisms in Determining Response to Tamoxifen in Metastatic Breast Cancer Patients: Review and Egyptian Experience. Asian Pac. J. Cancer Prev. APJCP 2020, 21, 3619–3625. [Google Scholar] [CrossRef] [PubMed]
- Newman, W.G.; Hadfield, K.D.; Latif, A.; Roberts, S.A.; Shenton, A.; McHague, C.; Lalloo, F.; Howell, S.; Evans, D.G. Impaired Tamoxifen Metabolism Reduces Survival in Familial Breast Cancer Patients. Clin. Cancer Res. 2008, 14, 5913–5918. [Google Scholar] [CrossRef]
- He, W.; Eriksson, M.; Eliasson, E.; Grassmann, F.; Bäcklund, M.; Gabrielson, M.; Hammarström, M.; Margolin, S.; Thorén, L.; Wengström, Y.; et al. CYP2D6 Genotype Predicts Tamoxifen Discontinuation and Drug Response: A Secondary Analysis of the KARISMA Trial. Ann. Oncol. 2021, 32, 1286–1293. [Google Scholar] [CrossRef] [PubMed]
- Henry, N.L.; Rae, J.M.; Li, L.; Azzouz, F.; Skaar, T.C.; Desta, Z.; Sikora, M.J.; Philips, S.; Nguyen, A.; Storniolo, A.M.; et al. Association between CYP2D6 Genotype and Tamoxifen-Induced Hot Flashes in a Prospective Cohort. Breast Cancer Res. Treat. 2009, 117, 571–575. [Google Scholar] [CrossRef] [PubMed]
- Ramón y Cajal, T.; Altés, A.; Paré, L.; del Rio, E.; Alonso, C.; Barnadas, A.; Baiget, M. Impact of CYP2D6 Polymorphisms in Tamoxifen Adjuvant Breast Cancer Treatment. Breast Cancer Res. Treat. 2010, 119, 33–38. [Google Scholar] [CrossRef]
- Wickramage, I.; Tennekoon, K.H.; Ariyaratne, M.A.Y.; Hewage, A.S.; Sundralingam, T. CYP2D6 Polymorphisms May Predict Occurrence of Adverse Effects to Tamoxifen: A Preliminary Retrospective Study. Breast Cancer Targets Ther. 2017, 9, 111–120. [Google Scholar] [CrossRef] [PubMed]
- Goetz, M.; Kamal, A.; Ames, M. Tamoxifen Pharmacogenomics: The Role of CYP2D6 as a Predictor of Drug Response. Clin. Pharmacol. Ther. 2008, 83, 160–166. [Google Scholar] [CrossRef]
- Hwang, G.S.; Bhat, R.; Crutchley, R.D.; Trivedi, M.V. Impact of CYP2D6 Polymorphisms on Endoxifen Concentrations and Breast Cancer Outcomes. Pharmacogenom. J. 2018, 18, 201–208. [Google Scholar] [CrossRef]
- Madlensky, L.; Natarajan, L.; Tchu, S.; Pu, M.; Mortimer, J.; Flatt, S.W.; Nikoloff, D.M.; Hillman, G.; Fontecha, M.R.; Lawrence, H.J.; et al. Tamoxifen Metabolite Concentrations, CYP2D6 Genotype, and Breast Cancer Outcomes. Clin. Pharmacol. Ther. 2011, 89, 718–725. [Google Scholar] [CrossRef]
- Braal, C.L.; Westenberg, J.D.; Buijs, S.M.; Abrams, S.; Mulder, T.A.M.; van Schaik, R.H.N.; Koolen, S.L.W.; Jager, A.; Mathijssen, R.H.J. Factors Affecting Inter-Individual Variability in Endoxifen Concentrations in Patients with Breast Cancer: Results from the Prospective TOTAM Trial. Breast Cancer Res. Treat. 2022, 195, 65–74. [Google Scholar] [CrossRef] [PubMed]
- Ximenez, J.P.B.; de Andrade, J.M.; Marques, M.P.; Coelho, E.B.; Suarez-Kurtz, G.; Lanchote, V.L. Hormonal Status Affects Plasma Exposure of Tamoxifen and Its Main Metabolites in Tamoxifen-Treated Breast Cancer Patients. BMC Pharmacol. Toxicol. 2019, 20, 81. [Google Scholar] [CrossRef]
- Sanchez-Spitman, A.B.; Böhringer, S.; Dezentjé, V.O.; Gelderblom, H.; Swen, J.J.; Guchelaar, H.-J. A Genome-Wide Association Study of Endoxifen Serum Concentrations and Adjuvant Tamoxifen Efficacy in Early-Stage Breast Cancer Patients. Clin. Pharmacol. Ther. 2024, 116, 155–164. [Google Scholar] [CrossRef] [PubMed]
- Khor, C.C.; Winter, S.; Sutiman, N.; Mürdter, T.E.; Chen, S.; Lim, J.S.L.; Li, Z.; Li, J.; Sim, K.S.; Ganchev, B.; et al. Cross-Ancestry Genome-Wide Association Study Defines the Extended CYP2D6 Locus as the Principal Genetic Determinant of Endoxifen Plasma Concentrations. Clin. Pharmacol. Ther. 2023, 113, 712–723. [Google Scholar] [CrossRef]
- Sanchez-Spitman, A.; Dezentjé, V.; Swen, J.; Moes, D.J.A.R.; Böhringer, S.; Batman, E.; van Druten, E.; Smorenburg, C.; van Bochove, A.; Zeillemaker, A.; et al. Tamoxifen Pharmacogenetics and Metabolism: Results From the Prospective CYPTAM Study. J. Clin. Oncol. 2019, 37, 636–646. [Google Scholar] [CrossRef] [PubMed]
- Stearns, V.; ONeill, A.; Schneider, B.P.; Skaar, T.C.; Liu, M.C.; Lohrisch, C.; Goetz, M.P.; Vallejos, C.S.; Sparano, J.A.; Villa, D.; et al. CYP2D6 activity in patients with metastatic breast cancer treated with single agent tamoxifen: Results from ECOG-ACRIN E3108. Breast Cancer Res. Treat. 2025, 209, 595–602. [Google Scholar] [CrossRef]
- Thorén, L.; Lindh, J.D.; Molden, E.; Kristiansen Kringen, M.; Bergh, J.; Eliasson, E.; Margolin, S. CYP2D6 genotype and outcome in tamoxifen treated early breast cancer. Acta Oncol. 2025, 64, 848–856. [Google Scholar] [CrossRef]
- Ahern, T.P.; Hertz, D.L.; Damkier, P.; Ejlertsen, B.; Hamilton-Dutoit, S.J.; Rae, J.M.; Regan, M.M.; Thompson, A.M.; Lash, T.L.; Cronin-Fenton, D.P. Cytochrome P-450 2D6 (CYP2D6) Genotype and Breast Cancer Recurrence in Tamoxifen-Treated Patients: Evaluating the Importance of Loss of Heterozygosity. Am. J. Epidemiol. 2017, 185, 75–85. [Google Scholar] [CrossRef]
- Dezentjé, V.O.; van Schaik, R.H.N.; Vletter-Bogaartz, J.M.; van der Straaten, T.; Wessels, J.a.M.; Kranenbarg, E.M.-K.; Berns, E.M.; Seynaeve, C.; Putter, H.; van de Velde, C.J.H.; et al. CYP2D6 Genotype in Relation to Tamoxifen Efficacy in a Dutch Cohort of the Tamoxifen Exemestane Adjuvant Multinational (TEAM) Trial. Breast Cancer Res. Treat. 2013, 140, 363–373. [Google Scholar] [CrossRef]
- Hertz, D.L.; Kidwell, K.M.; Hilsenbeck, S.G.; Oesterreich, S.; Osborne, C.K.; Philips, S.; Chenault, C.; Hartmaier, R.J.; Skaar, T.C.; Sikora, M.J.; et al. CYP2D6 genotype is not associated with survival in breast cancer patients treated with tamoxifen: Results from a population-based study. Breast Cancer Res. Treat. 2017, 166, 277–287. [Google Scholar] [CrossRef]
- Province, M.A.; Goetz, M.P.; Brauch, H.; Flockhart, D.A.; Hebert, J.M.; Whaley, R.; Suman, V.J.; Schroth, W.; Winter, S.; Zembutsu, H.; et al. CYP2D6 genotype and adjuvant tamoxifen: Meta-analysis of heterogeneous study populations. Clin. Pharmacol. Ther. 2014, 95, 216–227. [Google Scholar] [CrossRef]
- Goetz, M.P.; Suman, V.J.; Nakamura, Y.; Kiyotani, K.; Jordan, V.C.; Ingle, J.N. Tamoxifen Metabolism and Breast Cancer Recurrence: A Question Unanswered by CYPTAM. J. Clin. Oncol. 2019, 37, 1982–1983. [Google Scholar] [CrossRef] [PubMed]
- Baatjes, K.J.; Conradie, M.; Apffelstaedt, J.P.; Kotze, M.J. Pharmacogenetics of Aromatase Inhibitors in Endocrine Responsive Breast Cancer: Lessons Learnt from Tamoxifen and CYP2D6 Genotyping. Anti-Cancer Agents Med. Chem. Former. Curr. Med. Chem. Anti-Cancer Agents 2017, 17, 1805–1813. [Google Scholar] [CrossRef]
- Turner, A.J.; Aggarwal, P.; Boone, E.C.; Haidar, C.-E.; Relling, M.V.; Derezinski, A.D.; Broeckel, U.; Gaedigk, A. Identification of CYP2D6 Haplotypes That Interfere with Commonly Used Assays for Copy Number Variation Characterization. J. Mol. Diagn. 2021, 23, 577–588. [Google Scholar] [CrossRef]
- MacLehose, R.F.; Ahern, T.P.; Collin, L.J.; Li, A.; Lash, T.L. CYP2D6 Phenotype and Breast Cancer Outcomes: A Bias Analysis and Meta-Analysis. Cancer Epidemiol. Biomark. Prev. 2025, 34, 224–233. [Google Scholar] [CrossRef] [PubMed]
- Puszkiel, A.; Arellano, C.; Vachoux, C.; Evrard, A.; Le Morvan, V.; Boyer, J.-C.; Robert, J.; Delmas, C.; Dalenc, F.; Debled, M.; et al. Model-Based Quantification of Impact of Genetic Polymorphisms and Co-Medications on Pharmacokinetics of Tamoxifen and Six Metabolites in Breast Cancer. Clin. Pharmacol. Ther. 2021, 109, 1244–1255. [Google Scholar] [CrossRef]
- Hertz, D.L.; Deal, A.; Ibrahim, J.G.; Walko, C.M.; Weck, K.E.; Anderson, S.; Magrinat, G.; Olajide, O.; Moore, S.; Raab, R.; et al. Tamoxifen Dose Escalation in Patients with Diminished CYP2D6 Activity Normalizes Endoxifen Concentrations Without Increasing Toxicity. Oncologist 2016, 21, 795–803. [Google Scholar] [CrossRef]
- Khalaj, Z.; Baratieh, Z.; Nikpour, P.; Schwab, M.; Schaeffeler, E.; Mokarian, F.; Khanahmad, H.; Salehi, R.; Mürdter, T.E.; Salehi, M. Clinical Trial: CYP2D6 Related Dose Escalation of Tamoxifen in Breast Cancer Patients with Iranian Ethnic Background Resulted in Increased Concentrations of Tamoxifen and Its Metabolites. Front. Pharmacol. 2019, 10, 530. [Google Scholar] [CrossRef]
- Tamoxifen. Available online: https://www.clinpgx.org/chemical/PA451581/guidelineAnnotation/PA166104966 (accessed on 30 September 2025).
- Tamura, K.; Imamura, C.K.; Takano, T.; Saji, S.; Yamanaka, T.; Yonemori, K.; Takahashi, M.; Tsurutani, J.; Nishimura, R.; Sato, K.; et al. CYP2D6 Genotype–Guided Tamoxifen Dosing in Hormone Receptor–Positive Metastatic Breast Cancer (TARGET-1): A Randomized, Open-Label, Phase II Study. J. Clin. Oncol. 2020, 38, 558–566. [Google Scholar] [CrossRef] [PubMed]
- Buijs, S.M.; Koolen, S.L.W.; Mathijssen, R.H.J.; Jager, A. Tamoxifen Dose De-Escalation: An Effective Strategy for Reducing Adverse Effects? Drugs 2024, 84, 385–401. [Google Scholar] [CrossRef]
- Gene-Specific Information Tables for CYP3A5. Available online: https://www.pharmgkb.org/page/cyp3a5RefMaterials (accessed on 16 April 2024).
- Zhang, Y.; Wang, Z.; Wang, Y.; Jin, W.; Zhang, Z.; Jin, L.; Qian, J.; Zheng, L. CYP3A4 and CYP3A5: The Crucial Roles in Clinical Drug Metabolism and the Significant Implications of Genetic Polymorphisms. PeerJ 2024, 12, e18636. [Google Scholar] [CrossRef]
- Wegman, P.; Elingarami, S.; Carstensen, J.; Stål, O.; Nordenskjöld, B.; Wingren, S. Genetic Variants of CYP3A5, CYP2D6, SULT1A1, UGT2B15 and Tamoxifen Response in Postmenopausal Patients with Breast Cancer. Breast Cancer Res. BCR 2007, 9, R7. [Google Scholar] [CrossRef]
- Tucker, A.N.; Tkaczuk, K.A.; Lewis, L.M.; Tomic, D.; Lim, C.K.; Flaws, J.A. Polymorphisms in Cytochrome P4503A5 (CYP3A5) May Be Associated with Race and Tumor Characteristics, but Not Metabolism and Side Effects of Tamoxifen in Breast Cancer Patients. Cancer Lett. 2005, 217, 61–72. [Google Scholar] [CrossRef]
- Collins, J.M.; Wang, D. Cytochrome P450 3A4 (CYP3A4) protein quantification using capillary western blot technology and total protein normalization. J. Pharmacol. Toxicol. Methods 2021, 112, 107117. [Google Scholar] [CrossRef] [PubMed]
- Sanchez Spitman, A.B.; Moes, D.J.A.R.; Gelderblom, H.; Dezentje, V.O.; Swen, J.J.; Guchelaar, H.J. Effect of CYP3A4*22, CYP3A5*3, and CYP3A Combined Genotypes on Tamoxifen Metabolism. Eur. J. Clin. Pharmacol. 2017, 73, 1589–1598. [Google Scholar] [CrossRef] [PubMed]
- Teft, W.A.; Gong, I.Y.; Dingle, B.; Potvin, K.; Younus, J.; Vandenberg, T.A.; Brackstone, M.; Perera, F.E.; Choi, Y.-H.; Zou, G.; et al. CYP3A4 and Seasonal Variation in Vitamin D Status in Addition to CYP2D6 Contribute to Therapeutic Endoxifen Level during Tamoxifen Therapy. Breast Cancer Res. Treat. 2013, 139, 95–105. [Google Scholar] [CrossRef]
- Baxter, S.D.; Teft, W.A.; Choi, Y.-H.; Winquist, E.; Kim, R.B. Tamoxifen-Associated Hot Flash Severity Is Inversely Correlated with Endoxifen Concentration and CYP3A4*22. Breast Cancer Res. Treat. 2014, 145, 419–428. [Google Scholar] [CrossRef]
- Lim, J.S.L.; Sutiman, N.; Muerdter, T.E.; Singh, O.; Cheung, Y.B.; Ng, R.C.H.; Yap, Y.S.; Wong, N.S.; Ang, P.C.S.; Dent, R.; et al. Association of CYP2C19*2 and Associated Haplotypes with Lower Norendoxifen Concentrations in Tamoxifen-treated Asian Breast Cancer Patients. Br. J. Clin. Pharmacol. 2016, 81, 1142–1152. [Google Scholar] [CrossRef] [PubMed]
- Ruiter, R.; Bijl, M.J.; van Schaik, R.H.N.; Berns, E.M.J.J.; Hofman, A.; Coebergh, J.-W.W.; van Noord, C.; Visser, L.E.; Stricker, B.H.C. CYP2C19*2 Polymorphism Is Associated with Increased Survival in Breast Cancer Patients Using Tamoxifen. Pharmacogenomics 2010, 11, 1367–1375. [Google Scholar] [CrossRef]
- van Schaik, R.H.N.; Kok, M.; Sweep, F.C.G.J.; van Vliet, M.; van Fessem, M.; Meijer-van Gelder, M.E.; Seynaeve, C.; Lindemans, J.; Wesseling, J.; Van ’t Veer, L.J.; et al. The CYP2C19*2 Genotype Predicts Tamoxifen Treatment Outcome in Advanced Breast Cancer Patients. Pharmacogenomics 2011, 12, 1137–1146. [Google Scholar] [CrossRef]
- Schroth, W.; Antoniadou, L.; Fritz, P.; Schwab, M.; Muerdter, T.; Zanger, U.M.; Simon, W.; Eichelbaum, M.; Brauch, H. Breast Cancer Treatment Outcome with Adjuvant Tamoxifen Relative to Patient CYP2D6 and CYP2C19 Genotypes. J. Clin. Oncol. 2007, 25, 5187–5193. [Google Scholar] [CrossRef]
- Justenhoven, C.; Hamann, U.; Pierl, C.B.; Baisch, C.; Harth, V.; Rabstein, S.; Spickenheuer, A.; Pesch, B.; Brüning, T.; Winter, S.; et al. CYP2C19*17 Is Associated with Decreased Breast Cancer Risk. Breast Cancer Res. Treat. 2009, 115, 391–396. [Google Scholar] [CrossRef]
- Bai, L.; He, J.; He, G.-H.; He, J.-C.; Xu, F.; Xu, G.-L. Association of CYP2C19 Polymorphisms with Survival of Breast Cancer Patients Using Tamoxifen: Results of a Meta- Analysis. Asian Pac. J. Cancer Prev. APJCP 2014, 15, 8331–8335. [Google Scholar] [CrossRef]
- Sanchez-Spitman, A.B.; Swen, J.J.; Dezentjé, V.O.; Moes, D.J.A.R.; Gelderblom, H.; Guchelaar, H.J. Effect of CYP2C19 Genotypes on Tamoxifen Metabolism and Early-Breast Cancer Relapse. Sci. Rep. 2021, 11, 415. [Google Scholar] [CrossRef] [PubMed]
- Mwinyi, J.; Vokinger, K.; Jetter, A.; Breitenstein, U.; Hiller, C.; Kullak-Ublick, G.A.; Trojan, A. Impact of Variable CYP Genotypes on Breast Cancer Relapse in Patients Undergoing Adjuvant Tamoxifen Therapy. Cancer Chemother. Pharmacol. 2014, 73, 1181–1188. [Google Scholar] [CrossRef]
- Marcath, L.; Deal, A.M.; Van Wieren, E.; Danko, W.; Walko, C.M.; Ibrahim, J.G.; Weck, K.E.; Jones, D.R.; Desta, Z.; McLeod, H.L.; et al. Comprehensive Assessment of Cytochromes P450 and Transporter Genetics with Endoxifen Concentration during Tamoxifen Treatment. Pharmacogenet. Genom. 2017, 27, 402–409. [Google Scholar] [CrossRef]
- Jernström, H.; Bågeman, E.; Rose, C.; Jönsson, P.-E.; Ingvar, C. CYP2C8 and CYP2C9 Polymorphisms in Relation to Tumour Characteristics and Early Breast Cancer Related Events among 652 Breast Cancer Patients. Br. J. Cancer 2009, 101, 1817–1823. [Google Scholar] [CrossRef] [PubMed]
- Haeggström, S.; Ingelman-Sundberg, M.; Pääbo, S.; Zeberg, H. The Clinically Relevant CYP2C8*3 and CYP2C9*2 Haplotype Is Inherited from Neandertals. Pharmacogenom. J. 2022, 22, 247–249. [Google Scholar] [CrossRef]
- Woo, H.I.; Lee, S.K.; Kim, J.; Kim, S.W.; Yu, J.; Bae, S.Y.; Lee, J.E.; Nam, S.J.; Lee, S.-Y. Variations in Plasma Concentrations of Tamoxifen Metabolites and the Effects of Genetic Polymorphisms on Tamoxifen Metabolism in Korean Patients with Breast Cancer. Oncotarget 2017, 8, 100296–100311. [Google Scholar] [CrossRef] [PubMed]
- Kuo, S.-H.; Yang, S.-Y.; You, S.-L.; Lien, H.-C.; Lin, C.-H.; Lin, P.-H.; Huang, C.-S. Polymorphisms of ESR1, UGT1A1, HCN1, MAP3K1 and CYP2B6 Are Associated with the Prognosis of Hormone Receptor-Positive Early Breast Cancer. Oncotarget 2017, 8, 20925–20938. [Google Scholar] [CrossRef]
- Nowell, S.A.; Ahn, J.; Rae, J.M.; Scheys, J.O.; Trovato, A.; Sweeney, C.; MacLeod, S.L.; Kadlubar, F.F.; Ambrosone, C.B. Association of Genetic Variation in Tamoxifen-Metabolizing Enzymes with Overall Survival and Recurrence of Disease in Breast Cancer Patients. Breast Cancer Res. Treat. 2005, 91, 249–258. [Google Scholar] [CrossRef]
- Nowell, S.; Sweeney, C.; Winters, M.; Stone, A.; Lang, N.P.; Hutchins, L.F.; Kadlubar, F.F.; Ambrosone, C.B. Association between Sulfotransferase 1A1 Genotype and Survival of Breast Cancer Patients Receiving Tamoxifen Therapy. J. Natl. Cancer Inst. 2002, 94, 1635–1640. [Google Scholar] [CrossRef]
- Fernández-Santander, A.; Gaibar, M.; Novillo, A.; Romero-Lorca, A.; Rubio, M.; Chicharro, L.M.; Tejerina, A.; Bandrés, F. Relationship between Genotypes Sult1a2 and Cyp2d6 and Tamoxifen Metabolism in Breast Cancer Patients. PLoS ONE 2013, 8, e70183. [Google Scholar] [CrossRef] [PubMed]
- Miranda, C.; Galleguillos, M.; Torres, R.; Tardón, K.; Cáceres, D.D.; Lee, K.; Redal, M.A.; Varela, N.M.; Quiñones, L.A. Preliminary Pharmacogenomic-Based Predictive Models of Tamoxifen Response in Hormone-Dependent Chilean Breast Cancer Patients. Front. Pharmacol. 2021, 12, 661443. [Google Scholar] [CrossRef] [PubMed]
- Hoffmeyer, S.; Burk, O.; von Richter, O.; Arnold, H.P.; Brockmöller, J.; Johne, A.; Cascorbi, I.; Gerloff, T.; Roots, I.; Eichelbaum, M.; et al. Functional Polymorphisms of the Human Multidrug-Resistance Gene: Multiple Sequence Variations and Correlation of One Allele with P-Glycoprotein Expression and Activity in Vivo. Proc. Natl. Acad. Sci. USA 2000, 97, 3473–3478. [Google Scholar] [CrossRef]
- Taheri, M.; Mahjoubi, F.; Omranipour, R. Effect of MDR1 Polymorphism on Multidrug Resistance Expression in Breast Cancer Patients. Genet. Mol. Res. GMR 2010, 9, 34–40. [Google Scholar] [CrossRef] [PubMed]
- Sensorn, I.; Sirachainan, E.; Chamnanphon, M.; Pasomsub, E.; Trachu, N.; Supavilai, P.; Sukasem, C.; Pinthong, D. Association of CYP3A4/5, ABCB1 and ABCC2 Polymorphisms and Clinical Outcomes of Thai Breast Cancer Patients Treated with Ta-moxifen. Pharmacogenom. Pers. Med. 2013, 6, 93–98. [Google Scholar] [CrossRef]
- Zhang, X.; Pu, Z.; Ge, J.; Shen, J.; Yuan, X.; Xie, H. Association of CYP2D6*10, OATP1B1 A388G, and OATP1B1 T521C Pol-ymorphisms and Overall Survival of Breast Cancer Patients after Tamoxifen Therapy. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2015, 21, 563–569. [Google Scholar] [CrossRef]
- Gao, C.-M.; Pu, Z.; He, C.; Liang, D.; Jia, Y.; Yuan, X.; Wang, G.; Xie, H. Effect of OATP1B1 Genetic Polymorphism on the Uptake of Tamoxifen and Its Metabolite, Endoxifen. Oncol. Rep. 2017, 38, 1124–1132. [Google Scholar] [CrossRef]
- Pu, Z.; Zhang, X.; Chen, Q.; Yuan, X.; Xie, H. Establishment of an Expression Platform of OATP1B1 388GG and 521CC Genetic Polymorphism and the Therapeutic Effect of Tamoxifen in MCF-7 Cells. Oncol. Rep. 2015, 33, 2420–2428. [Google Scholar] [CrossRef][Green Version]
- Keller, D.N.; Medwid, S.J.; Ross, C.D.; Wigle, T.J.; Kim, R.B. Impact of Organic Anion Transporting Polypeptide, P-Glycoprotein, and Breast Cancer Resistance Protein Transporters on Observed Tamoxifen and Endoxifen Concentration and Adverse Effects. Pharmacogenet. Genom. 2023, 33, 10–18. [Google Scholar] [CrossRef]
- Sanchez-Spitman, A.B.; Dezentjé, V.O.; Swen, J.J.; Moes, D.J.A.R.; Gelderblom, H.; Guchelaar, H.-J. Genetic Polymorphisms of 3′-Untranslated Region of SULT1A1 and Their Impact on Tamoxifen Metabolism and Efficacy. Breast Cancer Res. Treat. 2018, 172, 401–411. [Google Scholar] [CrossRef]
- Forat-Yazdi, M.; Jafari, M.; Kargar, S.; Abolbaghaei, S.M.; Nasiri, R.; Farahnak, S.; Foroughi, E.; Neamatzadeh, H. Association between SULT1A1 Arg213His (rs9282861) Polymorphism and Risk of Breast Cancer: A Systematic Review and Meta-Analysis. J. Res. Health Sci. 2017, 17, 396. [Google Scholar] [PubMed Central]
- El Daibani, A.A.; Alherz, F.A.; Abunnaja, M.S.; Bairam, A.F.; Rasool, M.I.; Kurogi, K.; Liu, M.-C. Impact of Human SULT1E1 Polymorphisms on the Sulfation of 17β-Estradiol, 4-Hydroxytamoxifen, and Diethylstilbestrol by SULT1E1 Allozymes. Eur. J. Drug Metab. Pharmacokinet. 2021, 46, 105–118. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Zheng, Z.; Xu, C.; Wang, J.; Min, M.; Zhao, Y.; Wang, X.; Gong, Y.; Yin, J.; Guo, M.; et al. Disturbance of mammary UDP-glucuronosyltransferase represses estrogen metabolism and exacerbates experimental breast Cancer. J. Pharm. Sci. 2017, 106, 2152–2162. [Google Scholar] [CrossRef] [PubMed]
- Romero-Lorca, A.; Novillo, A.; Gaibar, M.; Bandrés, F.; Fernández-Santander, A. Impacts of the Glucuronidase Genotypes UGT1A4, UGT2B7, UGT2B15 and UGT2B17 on Tamoxifen Metabolism in Breast Cancer Patients. PLoS ONE 2015, 10, e0132269. [Google Scholar] [CrossRef]
- Ahmed, J.H.; Makonnen, E.; Fotoohi, A.; Aseffa, A.; Howe, R.; Aklillu, E. CYP2D6 Genotype Predicts Plasma Concentrations of Tamoxifen Metabolites in Ethiopian Breast Cancer Patients. Cancers 2019, 11, 1353. [Google Scholar] [CrossRef]
- Lan, B.; Ma, F.; Han, M.; Chen, S.; Wang, W.; Li, Q.; Fan, Y.; Luo, Y.; Cai, R.; Wang, J.; et al. The Effect of Polymorphism in UGT1A4 on Clinical Outcomes of Adjuvant Tamoxifen Therapy for Patients With Breast Cancer in China. Clin. Breast Cancer 2019, 19, e370–e375. [Google Scholar] [CrossRef]
- Li, J.; Bluth, M.H. Pharmacogenomics of Drug Metabolizing Enzymes and Transporters: Implications for Cancer Therapy. Pharmacogenom. Pers. Med. 2011, 4, 11–33. [Google Scholar] [CrossRef]
- Teft, W.A.; Mansell, S.E.; Kim, R.B. Endoxifen, the Active Metabolite of Tamoxifen, Is a Substrate of the Efflux Transporter P-Glycoprotein (Multidrug Resistance 1). Drug Metab. Dispos. Biol. Fate Chem. 2011, 39, 558–562. [Google Scholar] [CrossRef]
- Iusuf, D.; Teunissen, S.F.; Wagenaar, E.; Rosing, H.; Beijnen, J.H.; Schinkel, A.H. P-Glycoprotein (ABCB1) Transports the Primary Active Tamoxifen Metabolites Endoxifen and 4-Hydroxytamoxifen and Restricts Their Brain Penetration. J. Pharmacol. Exp. Ther. 2011, 337, 710–717. [Google Scholar] [CrossRef] [PubMed]
- McLaughlin, A.M.; Helland, T.; Klima, F.; Koolen, S.L.W.; van Schaik, R.H.N.; Mathijssen, R.H.J.; Neven, P.; Swen, J.J.; Guchelaar, H.-J.; Dalenc, F.; et al. CYP2D6 Endoxifen Percentage Activity Model (CEPAM) Consortium. Nonlinear Mixed-Effects Model of Z-Endoxifen Concentrations in Tamoxifen-Treated Patients from the CEPAM Cohort. Clin. Pharmacol. Ther. 2024, 116, 690–702. [Google Scholar] [CrossRef] [PubMed]
- Sim, S.; Mueller-Schoell, A.; Klopp-Schulze, L.; Schroth, W.; Mürdter, T.; Michelet, R.; Brauch, H.; Huisinga, W.; Joerger, M.; Neven, P.; et al. Obesity Alters Endoxifen Plasma Levels in Young Breast Cancer Patients: A Pharmacometric Simulation Approach. Clin. Pharmacol. Ther. 2020, 108, 661–670. [Google Scholar] [CrossRef]
- Smith, D.A.; Sadler, M.C.; Altman, R.B. Promises and Challenges in Pharmacoepigenetics. Camb. Prism. Precis. Med. 2023, 1, e18. [Google Scholar] [CrossRef]
- Woolpert, K.M.; Ahern, T.P.; Baurley, J.W.; Maliniak, M.L.; Damkier, P.; Kjærsgaard, A.; Collin, L.J.; Hamilton-Dutoit, S.; Tramm, T.; Ejlertsen, B.; et al. Genetic variants in tamoxifen metabolism and early treatment discontinuation among premenopausal breast cancer patients. Breast Cancer Res. Treat. 2025, 212, 251–260. [Google Scholar] [CrossRef]
- Antunes, M.V.; da Fontoura Timm, T.A.; de Oliveira, V.; Staudt, D.E.; Raymundo, S.; Gössling, G.; Biazús, J.V.; Cavalheiro, J.A.; Rosa, D.D.; Wallemacq, P.; et al. Influence of CYP2D6 and CYP3A4 Phenotypes, Drug Interactions, and Vitamin D Status on Tamoxifen Biotransformation. Ther. Drug Monit. 2015, 37, 733–744. [Google Scholar] [CrossRef]
- Wegman, P.; Vainikka, L.; Stål, O.; Nordenskjöld, B.; Skoog, L.; Rutqvist, L.-E.; Wingren, S. Genotype of Metabolic Enzymes and the Benefit of Tamoxifen in Postmenopausal Breast Cancer Patients. Breast Cancer Res. 2005, 7, R284–R290. [Google Scholar] [CrossRef]
- Sim, S.; Lövrot, J.; Lindh, J.D.; Bergh, J.; Xie, H. Effect of CYP2C19 and CYP2D6 Genotype on Tamoxifen Treatment Outcome Indicates Endogenous and Exogenous Interplay. Pharmacogenomics 2018, 19, 1027–1037. [Google Scholar] [CrossRef]
- Teh, L.K.; Mohamed, N.I.; Salleh, M.Z.; Rohaizak, M.; Shahrun, N.S.; Saladina, J.J.; Shia, J.K.S.; Roslan, H.; Sood, S.; Rajoo, T.S.; et al. The Risk of Recurrence in Breast Cancer Patients Treated with Tamoxifen: Polymorphisms of CYP2D6 and ABCB1. AAPS J. 2012, 14, 52–59. [Google Scholar] [CrossRef] [PubMed]
- Sensorn, I.; Sukasem, C.; Sirachainan, E.; Chamnanphon, M.; Pasomsub, E.; Trachu, N.; Supavilai, P.; Pinthong, D.; Wongwaisayawan, S. ABCB1 and ABCC2 and the Risk of Distant Metastasis in Thai Breast Cancer Patients Treated with Tamoxifen. OncoTargets Ther. 2016, 9, 2121–2129. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ashcraft, K.; Grande, K.; Bristow, S.L.; Moyer, N.; Schmidlen, T.; Moretz, C.; Wick, J.A.; Blaxall, B.C. Validation of Pharmacogenomic Interaction Probability (PIP) Scores in Predicting Drug–Gene, Drug–Drug–Gene, and Drug–Gene–Gene Interaction Risks in a Large Patient Population. J. Pers. Med. 2022, 12, 1972. [Google Scholar] [CrossRef]
- Javan Biparva, A.; Raoofi, S.; Rafiei, S.; Masoumi, M.; Doustmehraban, M.; Bagheribayati, F.; Vaziri Shahrebabak, E.S.; Noorani Mejareh, Z.; Khani, S.; Abdollahi, B.; et al. Global Depression in Breast Cancer Patients: Systematic Review and Meta-Analysis. PLoS ONE 2023, 18, e0287372. [Google Scholar] [CrossRef] [PubMed]
- Klomp, S.D.; Manson, M.L.; Guchelaar, H.-J.; Swen, J.J. Phenoconversion of Cytochrome P450 Metabolism: A Systematic Review. J. Clin. Med. 2020, 9, 2890. [Google Scholar] [CrossRef]
- Mostafa, S.; Kirkpatrick, C.M.J.; Byron, K.; Sheffield, L. An Analysis of Allele, Genotype and Phenotype Frequencies, Actionable Pharmacogenomic (PGx) Variants and Phenoconversion in 5408 Australian Patients Genotyped for CYP2D6, CYP2C19, CYP2C9 and VKORC1 Genes. J. Neural Transm. Vienna Austria 1996 2019, 126, 5–18. [Google Scholar] [CrossRef]
- Bousman, C.A.; Stevenson, J.M.; Ramsey, L.B.; Sangkuhl, K.; Hicks, J.K.; Strawn, J.R.; Singh, A.B.; Ruaño, G.; Mueller, D.J.; Tsermpini, E.E.; et al. Clinical Pharmacogenetics Implementation Consortium (CPIC) Guideline for CYP2D6, CYP2C19, CYP2B6, SLC6A4, and HTR2A Genotypes and Serotonin Reuptake Inhibitor Antidepressants. Clin. Pharmacol. Ther. 2023, 114, 51–68. [Google Scholar] [CrossRef]
- Borges, S.; Desta, Z.; Li, L.; Skaar, T.C.; Ward, B.A.; Nguyen, A.; Jin, Y.; Storniolo, A.M.; Nikoloff, D.M.; Wu, L.; et al. Quantitative Effect of CYP2D6 Genotype and Inhibitors on Tamoxifen Metabolism: Implication for Optimization of Breast Cancer Treatment. Clin. Pharmacol. Ther. 2006, 80, 61–74. [Google Scholar] [CrossRef] [PubMed]
- Klopp-Schulze, L.; Mueller-Schoell, A.; Neven, P.; Koolen, S.L.W.; Mathijssen, R.H.J.; Joerger, M.; Kloft, C. Integrated Data Analysis of Six Clinical Studies Points Toward Model-Informed Precision Dosing of Tamoxifen. Front. Pharmacol. 2020, 11, 283. [Google Scholar] [CrossRef]
- Binkhorst, L.; Bannink, M.; de Bruijn, P.; Ruit, J.; Droogendijk, H.; van Alphen, R.J.; den Boer, T.D.; Lam, M.H.; Jager, A.; van Gelder, T.; et al. Augmentation of Endoxifen Exposure in Tamoxifen-Treated Women Following SSRI Switch. Clin. Pharmacokinet. 2016, 55, 249–255. [Google Scholar] [CrossRef][Green Version]
- Borges, S.; Desta, Z.; Jin, Y.; Faouzi, A.; Robarge, J.D.; Philip, S.; Nguyen, A.; Stearns, V.; Hayes, D.; Rae, J.M.; et al. A Composite Functional Genetic and Co-Medication CYP2D6 Activity Score in Predicting Tamoxifen Drug Exposure Among Breast Cancer Patients. J. Clin. Pharmacol. 2010, 50, 450–458. [Google Scholar] [CrossRef]
- Buck, S.A.J.; Braal, C.L.; Hofman, M.M.; Oomen-de Hoop, E.; de Bruijn, P.; Ghobadi Moghaddam-Helmantel, I.M.; Hussaarts, K.G.A.M.; Vastbinder, M.B.; van Rossum-Schornagel, Q.C.; van Schaik, R.H.N.; et al. Influence of Probenecid on Endoxifen Systemic Exposure in Breast Cancer Patients on Adjuvant Tamoxifen Treatment. Ther. Adv. Med. Oncol. 2022, 14, 17588359221081075. [Google Scholar] [CrossRef]
- Henderson, S.L.; Teft, W.A.; Kim, R.B. Profound Reduction in Tamoxifen Active Metabolite Endoxifen in a Breast Cancer Patient Treated with Rifampin Prior to Initiation of an Anti-TNFα Biologic for Ulcerative Colitis: A Case Report. BMC Cancer 2016, 16, 304. [Google Scholar] [CrossRef]
- Chiwambutsa, S.M.; Ayeni, O.; Kapungu, N.; Kanji, C.; Thelingwani, R.; Chen, W.C.; Mokone, D.H.; O’Neil, D.S.; Neugut, A.I.; Jacobson, J.S.; et al. Effects of Genetic Polymorphisms of Drug Metabolizing Enzymes and Co-Medications on Tamoxifen Metabolism in Black South African Women with Breast Cancer. Clin. Pharmacol. Ther. 2023, 114, 127–136. [Google Scholar] [CrossRef] [PubMed]
- Puszkiel, A.; Arellano, C.; Vachoux, C.; Evrard, A.; Le Morvan, V.; Boyer, J.-C.; Robert, J.; Delmas, C.; Dalenc, F.; Debled, M.; et al. Factors Affecting Tamoxifen Metabolism in Patients with Breast Cancer: Preliminary Results of the French PHACS Study. Clin. Pharmacol. Ther. 2019, 106, 585–595. [Google Scholar] [CrossRef]
- Abdullah-Koolmees, H.; van Keulen, A.M.; Nijenhuis, M.; Deneer, V.H.M. Pharmacogenetics Guidelines: Overview and Comparison of the DPWG, CPIC, CPNDS, and RNPGx Guidelines. Front. Pharmacol. 2021, 11, 595219. [Google Scholar] [CrossRef] [PubMed]
- Drug Label Information and Legend. Available online: https://www.pharmgkb.org/page/drugLabelLegend (accessed on 3 January 2025).
- Koutsilieri, S.; Tzioufa, F.; Sismanoglou, D.-C.; Patrinos, G.P. Unveiling the Guidance Heterogeneity for Genome-Informed Drug Treatment Interventions among Regulatory Bodies and Research Consortia. Pharmacol. Res. 2020, 153, 104590. [Google Scholar] [CrossRef] [PubMed]
- Shekhani, R.; Steinacher, L.; Swen, J.J.; Ingelman-Sundberg, M. Evaluation of Current Regulation and Guidelines of Pharmacogenomic Drug Labels: Opportunities for Improvements. Clin. Pharmacol. Ther. 2020, 107, 1240–1255. [Google Scholar] [CrossRef]
- Ingelman-Sundberg, M. Pharmacogenomic Prescribing Guidelines: Are They Always Useful? Clin. Pharmacol. Ther. 2024, 116, 899–901. [Google Scholar] [CrossRef]
- Kaur, G.; Nwabufo, C.K. Healthcare Provider and Patient Perspectives on the Implementation of Pharmacogenetic-Guided Treatment in Routine Clinical Practice. Pharmacogenet. Genom. 2024, 34, 236. [Google Scholar] [CrossRef]
- Klein, C.J.; Gong, L.; Caudle, K.E.; Naik, H.; Empey, P.E.; Hoffman, J.M.; Scherer, S.; Iwuchukwu, O.F.; Gregornik, D.; Monte, A.A.; et al. Toward an Integrated Resource for Pharmacogenomics (PGx): Survey Findings from the Genomic Medicine Communities. Genet. Med. 2025, 27, 101529. [Google Scholar] [CrossRef]
- Morris, S.A.; Alsaidi, A.T.; Verbyla, A.; Cruz, A.; Macfarlane, C.; Bauer, J.; Patel, J.N. Cost Effectiveness of Pharmacogenetic Testing for Drugs with Clinical Pharmacogenetics Implementation Consortium (CPIC) Guidelines: A Systematic Review. Clin. Pharmacol. Ther. 2022, 112, 1318–1328. [Google Scholar] [CrossRef] [PubMed]
- Matey, E.T.; Ragan, A.K.; Oyen, L.J.; Vitek, C.R.; Aoudia, S.L.; Ragab, A.K.; Fee-Schroeder, K.C.; Black, J.L.; Moyer, A.M.; Nicholson, W.T.; et al. Nine-Gene Pharmacogenomics Profile Service: The Mayo Clinic Experience. Pharmacogenom. J. 2022, 22, 69–74. [Google Scholar] [CrossRef]
- Swen, J.J.; van der Wouden, C.H.; Manson, L.E.; Abdullah-Koolmees, H.; Blagec, K.; Blagus, T.; Böhringer, S.; Cambon-Thomsen, A.; Cecchin, E.; Cheung, K.-C.; et al. A 12-Gene Pharmacogenetic Panel to Prevent Adverse Drug Reactions: An Open-Label, Multicentre, Controlled, Cluster-Randomised Crossover Implementation Study. Lancet 2023, 401, 347–356. [Google Scholar] [CrossRef]
- Lerena, A.; Peñas-LLedó, E.; de Andrés, F.; Mata-Martín, C.; Sánchez, C.L.; Pijierro, A.; Cobaleda, J. Clinical Implementation of Pharmacogenetics and Personalized Drug Prescription Based on E-Health: The MedeA Initiative. Drug Metab. Pers. Ther. 2020, 35, 20200143. [Google Scholar] [CrossRef]
- McDermott, J.H.; Tsakiroglou, M.; Newman, W.G.; Pirmohamed, M. Pharmacogenomics in the UK National Health Service: Progress towards Implementation. Br. J. Clin. Pharmacol. 2025, 91, 2241–2250. [Google Scholar] [CrossRef] [PubMed]
- van der Wouden, C.H.; van Rhenen, M.H.; Jama, W.O.M.; Ingelman-Sundberg, M.; Lauschke, V.M.; Konta, L.; Schwab, M.; Swen, J.J.; Guchelaar, H. Development of the PGx-Passport: A Panel of Actionable Germline Genetic Variants for Pre-Emptive Pharmacogenetic Testing. Clin. Pharmacol. Ther. 2019, 106, 866–873. [Google Scholar] [CrossRef]
- Storelli, F.; Matthey, A.; Lenglet, S.; Thomas, A.; Desmeules, J.; Daali, Y. Impact of CYP2D6 Functional Allelic Variations on Phenoconversion and Drug-Drug Interactions. Clin. Pharmacol. Ther. 2018, 104, 148–157. [Google Scholar] [CrossRef]
- Storelli, F.; Samer, C.; Reny, J.-L.; Desmeules, J.; Daali, Y. Complex Drug-Drug-Gene-Disease Interactions Involving Cytochromes P450: Systematic Review of Published Case Reports and Clinical Perspectives. Clin. Pharmacokinet. 2018, 57, 1267–1293. [Google Scholar] [CrossRef]
- Lenoir, C.; Rollason, V.; Desmeules, J.A.; Samer, C.F. Influence of Inflammation on Cytochromes P450 Activity in Adults: A Systematic Review of the Literature. Front. Pharmacol. 2021, 12, 733935. [Google Scholar] [CrossRef] [PubMed]
- Braal, C.L.; Jager, A.; Hoop, E.O.; Westenberg, J.D.; Lommen, K.M.W.T.; de Bruijn, P.; Vastbinder, M.B.; van Rossum-Schornagel, Q.C.; Thijs-Visser, M.F.; van Alphen, R.J.; et al. Therapeutic Drug Monitoring of Endoxifen for Tamoxifen Precision Dosing: Feasible in Patients with Hormone-Sensitive Breast Cancer. Clin. Pharmacokinet. 2022, 61, 527–537. [Google Scholar] [CrossRef]
- Sanchez-Spitman, A.B.; Moes, D.-J.A.R.; Swen, J.J.; Dezentjé, V.O.; Lambrechts, D.; Neven, P.; Gelderblom, H.; Guchelaar, H.-J. Exposure-Response Analysis of Endoxifen Serum Concentrations in Early-Breast Cancer. Cancer Chemother. Pharmacol. 2020, 85, 1141–1152. [Google Scholar] [CrossRef]
- Braal, C.L.; Kleijburg, A.; Jager, A.; Koolen, S.L.W.; Mathijssen, R.H.J.; Corro Ramos, I.; Wetzelaer, P.; Uyl-de Groot, C.A. Therapeutic Drug Monitoring-Guided Adjuvant Tamoxifen Dosing in Patients with Early Breast Cancer: A Cost-Effectiveness Analysis from the Prospective TOTAM Trial. Clin. Drug Investig. 2022, 42, 163–175. [Google Scholar] [CrossRef]
- Lee, C.I.; Low, S.K.; Maldonado, R.; Fox, P.; Balakrishnar, B.; Coulter, S.; de Bruijn, P.; Koolen, S.L.W.; Gao, B.; Lynch, J.; et al. Simplified Phenotyping of CYP2D6 for Tamoxifen Treatment Using the N-Desmethyl-Tamoxifen/Endoxifen Ratio. Breast 2020, 54, 229–234. [Google Scholar] [CrossRef]
- Gusella, M.; Pasini, F.; Corso, B.; Bertolaso, L.; De Rosa, G.; Falci, C.; Modena, Y.; Barile, C.; Da Corte, Z.D.; Fraccon, A.; et al. Predicting steady-state endoxifen plasma concentrations in breast cancer patients by CYP2D6 genotyping or phenotyping. Which approach is more reliable? Pharmacol. Res. Perspect. 2020, 8, e00646. [Google Scholar] [CrossRef]
- Opdam, F.L.; Dezentje, V.O.; den Hartigh, J.; Modak, A.S.; Vree, R.; Batman, E.; Smorenburg, C.H.; Nortier, J.W.R.; Gelderblom, H.; Guchelaar, H.-J. The Use of the 13C-Dextromethorphan Breath Test for Phenotyping CYP2D6 in Breast Cancer Patients Using Tamoxifen: Association with CYP2D6 Genotype and Serum Endoxifen Levels. Cancer Chemother. Pharmacol. 2013, 71, 593–601. [Google Scholar] [CrossRef] [PubMed]
- Frye, R.F.; Matzke, G.R.; Adedoyin, A.; Porter, J.A.; Branch, R.A. Validation of the Five-Drug “Pittsburgh Cocktail” Approach for Assessment of Selective Regulation of Drug-Metabolizing Enzymes. Clin. Pharmacol. Ther. 1997, 62, 365–376. [Google Scholar] [CrossRef] [PubMed]
- Ing Lorenzini, K.; Desmeules, J.; Rollason, V.; Bertin, S.; Besson, M.; Daali, Y.; Samer, C.F. CYP450 Genotype—Phenotype Concordance Using the Geneva Micrococktail in a Clinical Setting. Front. Pharmacol. 2021, 12, 730637. [Google Scholar] [CrossRef]
- Suenderhauf, C.; Berger, B.; Puchkov, M.; Schmid, Y.; Müller, S.; Huwyler, J.; Haschke, M.; Krähenbühl, S.; Duthaler, U. Pharmacokinetics and phenotyping properties of the Basel phenotyping cocktail combination capsule in healthy male adults. Br. J. Clin. Pharmacol. 2020, 86, 352–361. [Google Scholar] [CrossRef]
- Magliocco, G.; Desmeules, J.; Matthey, A.; Quirós-Guerrero, L.M.; Bararpour, N.; Joye, T.; Marcourt, L.; Queiroz, E.F.; Wolfender, J.-L.; Gloor, Y.; et al. Metabolomics Reveals Biomarkers in Human Urine and Plasma to Predict Cytochrome P450 2D6 (CYP2D6) Activity. Br. J. Pharmacol. 2021, 178, 4708–4725. [Google Scholar] [CrossRef]
- Wollmann, B.M.; Størset, E.; Kringen, M.K.; Molden, E.; Smith, R.L. Prediction of CYP2D6 Poor Metabolizers by Measurements of Solanidine and Metabolites—A Study in 839 Patients with Known CYP2D6 Genotype. Eur. J. Clin. Pharmacol. 2023, 79, 523–531. [Google Scholar] [CrossRef] [PubMed]
- Oda, A.; Suzuki, Y.; Sato, H.; Koyama, T.; Nakatochi, M.; Momozawa, Y.; Tanaka, R.; Ono, H.; Tatsuta, R.; Ando, T.; et al. Evaluation of the Usefulness of Plasma 4β-Hydroxycholesterol Concentration Normalized by 4α-Hydroxycholesterol for Accurate CYP3A Phenotyping. Clin. Transl. Sci. 2024, 17, e13768. [Google Scholar] [CrossRef] [PubMed]
- Taya, Y.; Mizunaga, M.; Nakao, S.; Jutanom, M.; Shimizu, N.; Nomura, Y.; Nakagawa, K. Clinical Evaluation Based on a New Approach to Improve the Accuracy of 4β-Hydroxycholesterol Measurement as a Biomarker of CYP3A4 Activity. Molecules 2023, 28, 1576. [Google Scholar] [CrossRef] [PubMed]
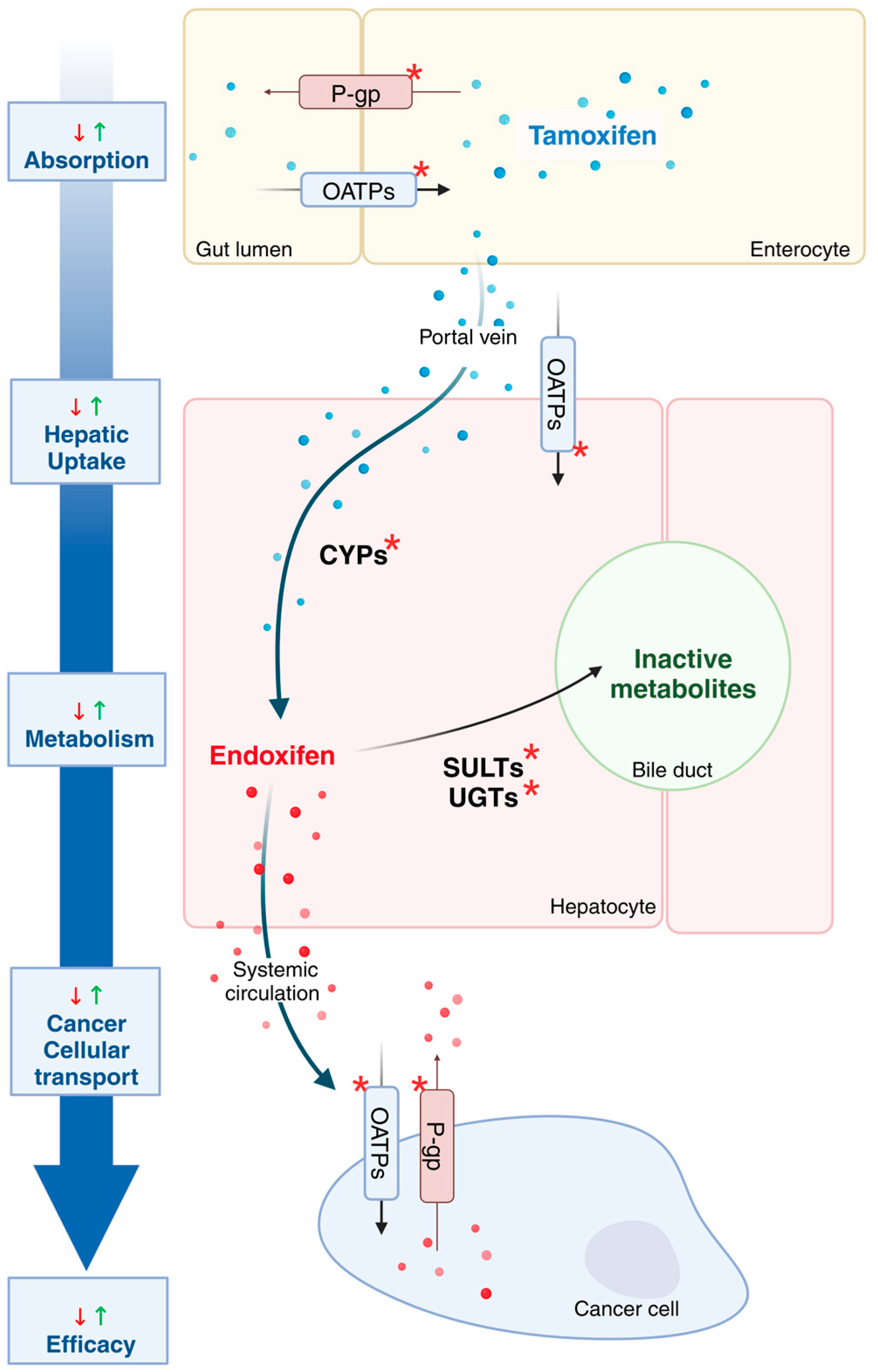
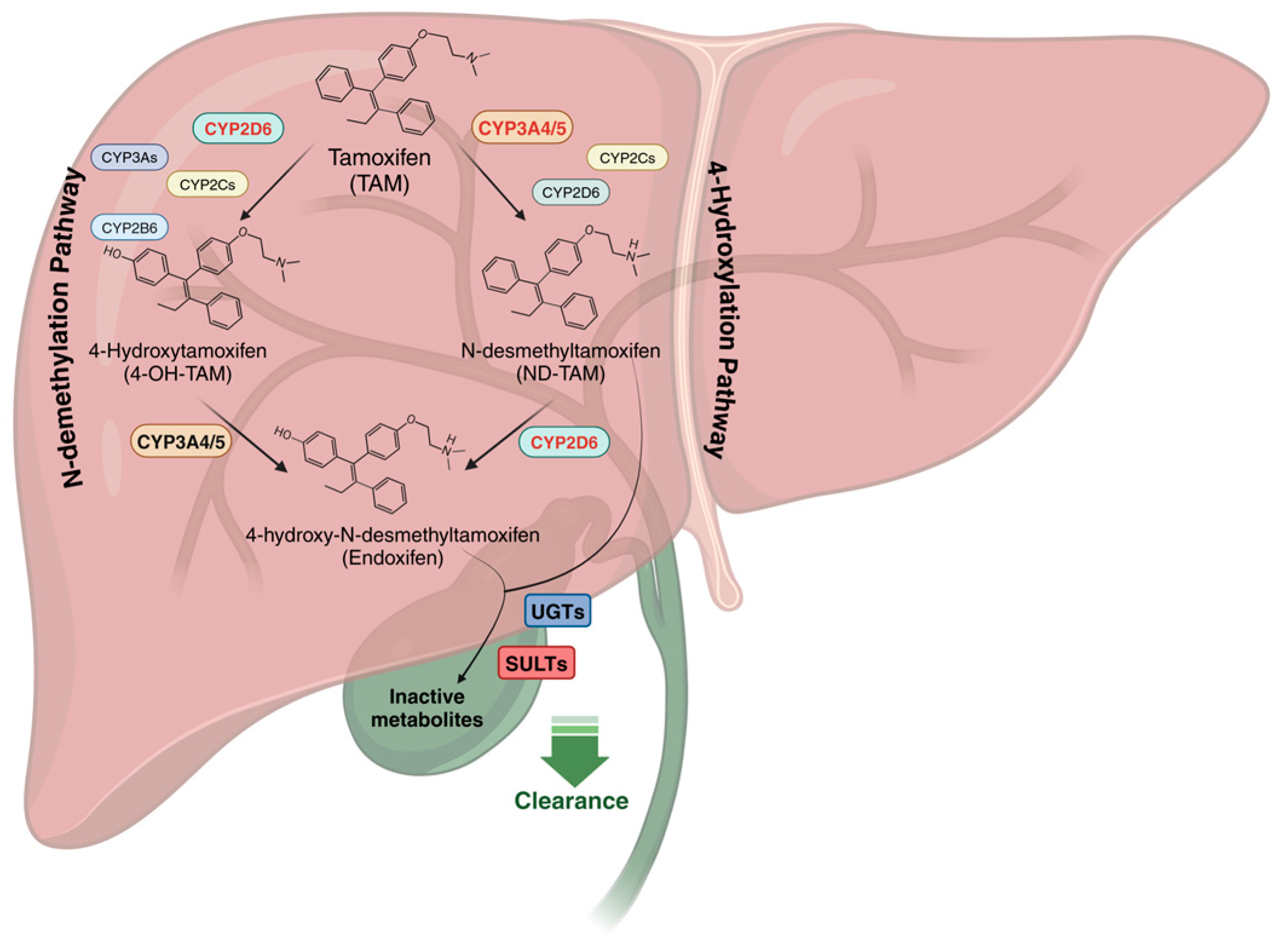
| Gene | Variant (s) | Functional Effect | Effect on Metabolism | Clinical Outcome | Clinical Recommendations | References |
|---|---|---|---|---|---|---|
| CYP2D6 | PM/IM alleles (*4, *3, *41, *10…) | Reduced activity | ↓ Endoxifen levels | Higher recurrence risk, poorer survival | Consider higher tamoxifen dose or switch to aromatase inhibitor | [19,20,21,22,23,24,25] |
| Copy number variations | Increased activity | ↑ Endoxifen levels | Better efficacy, but more side effects | Monitor for toxicity | [26] | |
| CYP3A5 | *3 | Reduced activity | ↓ DM-TAM and endoxifen levels | Mixed evidence on survival | Limited clinical impact; more studies needed | [55,56] |
| CYP3A4 | *22 | Reduced activity | ↓ First-pass metabolism; ↑ Tamoxifen/metabolites levels | Potentially improved efficacy; less ADRs | No routine action; consider in polygenic context | [15,59,60,61] |
| CYP2C19 | *2 | Reduced activity | ↓ NorEND levels | Conflicting results | More evidence needed before clinical use | [62,63,64,67] |
| *17 | Increased activity | ↑ 4-OH-TAM levels | Lower risk for relapse | Consider as potential protective variant | [65,66,67] | |
| CYP2C8/9 | *2, *3 | Reduced activity | ↓ Endoxifen levels | Mixed evidence on survival | Insufficient evidence for clinical action | [7,70,71,72] |
| SULT1A1 | *2 | Reduced activity | ↑ Endoxifen and 4-OH-TAM levels | Higher relapse risk and mortality | Potential predictor of tamoxifen response | [75,76] |
| SULT1A2 | *2, *3 | Reduced activity | ↑ Endoxifen and 4-OH-TAM levels | No clinical studies | May help maintain optimal metabolite levels; needs validation | [77] |
| UGT2B15 | *2 | Reduced activity | ↓ Tamoxifen plasma levels | Lower relapse risk | Potential prognostic marker; may act as protective variant | [78] |
| ABCB1 | C3435T | Reduced activity | ↓ Endoxifen intracellular concentration | Worse survival with CC/CT genotype | Variant allele carriers may respond better | [79,80,81] |
| SLCO1B1 | T521C | Reduced activity | ↓ Endoxifen in target tissue | Worse outcomes | Consider in multigene models | [82,83,84] |
| SlCO1A2 | c.935G > A | Reduced activity | ↑ Endoxifen | Reduction in ADRs | Potential predictor of tamoxifen ADRs | [85] |
| Variant A | Variant B | Impact on Metabolism and/or Outcome | Clinical Implication | References |
|---|---|---|---|---|
| CYP2D6 PM | CYP3A4 PM | ↑ active metabolites levels compared to CYP2D6 NM | Potential compensatory effect via CYP3A4 | [100] |
| SULT1A1 NM | ↑ risk of disease recurrence compared to SULT1A1 PM | Possible adverse impact on treatment outcomes | [101] | |
| CYP2C19 UM | ↓ recurrence-free survival and breast cancer-specific survival | Potentially linked to more aggressive tumors; validation required | [102] | |
| CYP2D6 IM | ABCB1 C3435T | ↑ risk of disease recurrence | Potential risk factor for treatment failure | [16,103] |
| CYP2D6 NM | SULT1A2 PM | Maintain optimal endoxifen and OH-TAM levels | Potential protective effect | [77] |
| CYP2C19 UM | ↑ relapse-free survival and disease-free survival | Potential protective effect | [65] | |
| ABCC2 −24C > T | ABCB1 C3435T | ↑ risk of disease recurrence | May identify patients at higher recurrence risk | [104] |
| DDIs | Effect on DMEs | Effect on Metabolism | Recommendations | References |
|---|---|---|---|---|
| Strong SSRIs | Strong CYP2D6 inhibition | ↓ Endoxifen levels; phenoconversion: UM → PM and NM → PM | Avoid strong/moderate inhibitors; consider switching to AIs | [11,110,111,112,113] |
| Weak SSRIs | Weak CYP2D6 inhibition | ↓ Endoxifen levels; phenoconversion: UM → NM/IM | No specific recommendations | |
| probenecid | CYP3A4 Inducer and pan-UGT inhibitor | ↑ Endoxifen levels; ↑ Endoxifen/tamoxifen ratio | If validated, may serve as corrective therapy for CYP2D6 PMs | [114] |
| rifampin | CYP3A inducer | ↓ Endoxifen levels | Potential DDIs; monitor endoxifen if co-administration unavoidable | [111,115] |
| Antiretroviral therapy (efavirenz) | CYP3A, CYP2B6 and UGT inducer | ↓ DM-TAM; ↑ DM/TAM and ↑ DM/endoxifen ratios | Requires validation in larger cohorts | [116] |
| amiodarone, clarithromycin, ciprofloxacin, diltiazem, fluconazole, and fusidic acid | Strong CYP3A inhibitors and UGT inducer | ↓ Endoxifen levels | Requires validation in larger cohorts | [117] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saad, I.; Bentayebi, K.; Ettoury, S.; Zarrik, O.; Bourais, I.; Boutayeb, S.; Samer, C.; Daali, Y.; Eljaoudi, R.; Louati, S. Impact of Complex Genetic and Drug–Drug Interactions on Tamoxifen Metabolism and Efficacy. J. Pers. Med. 2025, 15, 505. https://doi.org/10.3390/jpm15110505
Saad I, Bentayebi K, Ettoury S, Zarrik O, Bourais I, Boutayeb S, Samer C, Daali Y, Eljaoudi R, Louati S. Impact of Complex Genetic and Drug–Drug Interactions on Tamoxifen Metabolism and Efficacy. Journal of Personalized Medicine. 2025; 15(11):505. https://doi.org/10.3390/jpm15110505
Chicago/Turabian StyleSaad, Ibtissam, Kaoutar Bentayebi, Soukaina Ettoury, Oumaima Zarrik, Ilhame Bourais, Saber Boutayeb, Caroline Samer, Youssef Daali, Rachid Eljaoudi, and Sara Louati. 2025. "Impact of Complex Genetic and Drug–Drug Interactions on Tamoxifen Metabolism and Efficacy" Journal of Personalized Medicine 15, no. 11: 505. https://doi.org/10.3390/jpm15110505
APA StyleSaad, I., Bentayebi, K., Ettoury, S., Zarrik, O., Bourais, I., Boutayeb, S., Samer, C., Daali, Y., Eljaoudi, R., & Louati, S. (2025). Impact of Complex Genetic and Drug–Drug Interactions on Tamoxifen Metabolism and Efficacy. Journal of Personalized Medicine, 15(11), 505. https://doi.org/10.3390/jpm15110505








