Tailored Therapy vs. Empirical Therapy for Helicobacter pylori Eradication: An Umbrella Review of Systematic Reviews and Meta-Analyses
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Strategy
2.2. Eligibility Criteria and Quality Assessment
2.3. Risk of Bias Evaluation
2.4. Overlap of Primary Studies
2.5. Data Extraction
3. Results
3.1. Study Selection
3.2. Robis Assessment
3.3. Groove Analysis
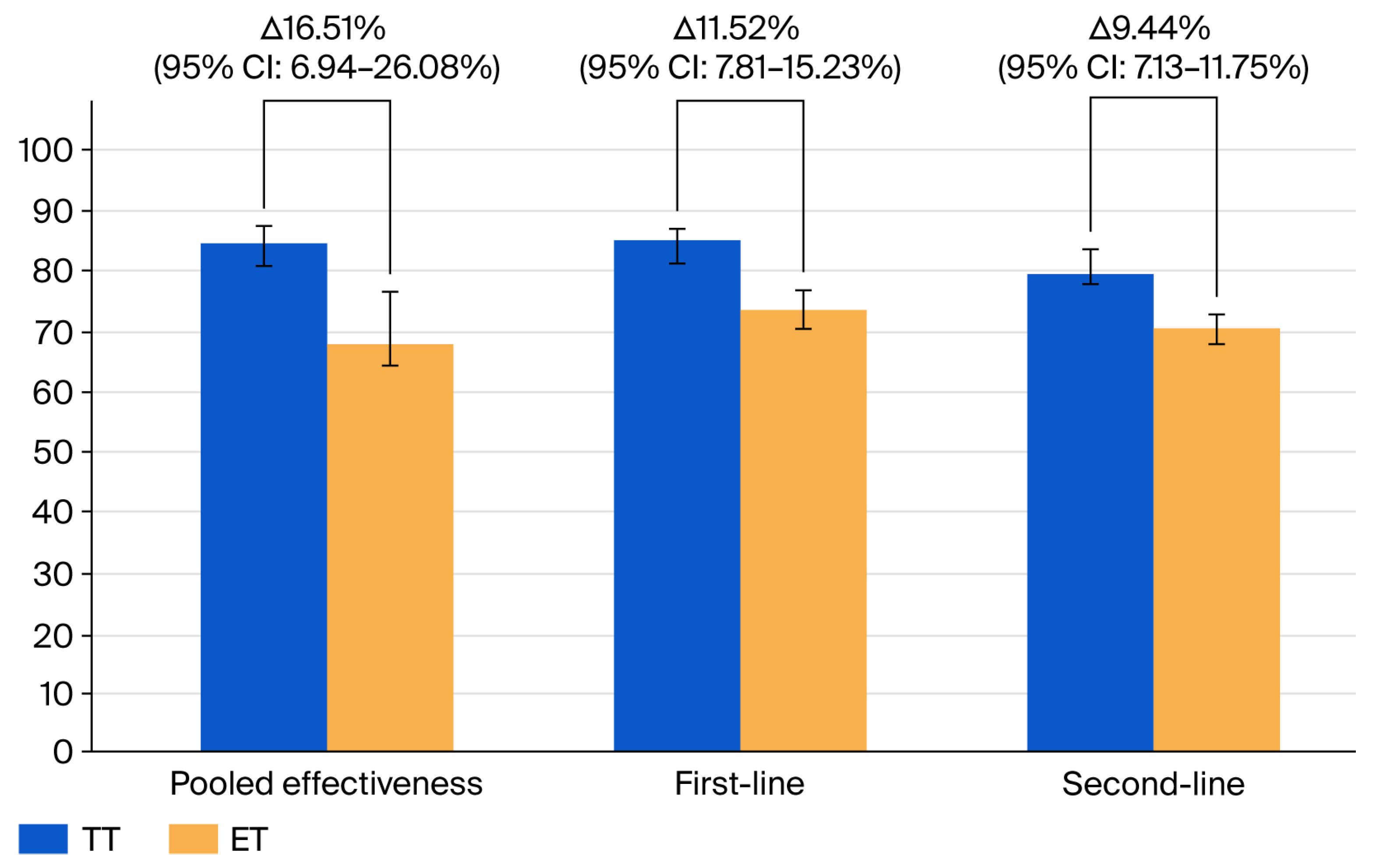
3.4. Effectiveness of TT Compared to ET
3.5. Sensitivity Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Katelaris, P.; Hunt, R.; Bazzoli, F.; Cohen, H.; Fock, K.M.; Gemilyan, M.; Malfertheiner, P.; Mégraud, F.; Piscoya, A.; Quach, D.; et al. Helicobacter pylori World Gastroenterology Organization Global Guideline. J. Clin. Gastroenterol. 2023, 57, 111–126. [Google Scholar] [CrossRef]
- Malfertheiner, P.; Megraud, F.; Rokkas, T.; Gisbert, J.P.; Liou, J.M.; Schulz, C.; Gasbarrini, A.; Hunt, R.H.; Leja, M.; O’Morain, C.; et al. Management of Helicobacter pylori infection: The Maastricht VI/Florence consensus report. Gut 2022, 71, 1724–1762. [Google Scholar] [CrossRef]
- Hooi, J.K.Y.; Lai, W.Y.; Ng, W.K.; Suen, M.M.Y.; Underwood, F.E.; Tanyingoh, D.; Malfertheiner, P.; Graham, D.Y.; Wong, V.W.S.; Wu, J.C.Y.; et al. Global Prevalence of Helicobacter pylori Infection: Systematic Review and Meta-Analysis. Gastroenterology 2017, 153, 420–429. [Google Scholar] [CrossRef]
- Yuan, C.; Adeloye, D.; Luk, T.T.; Huang, L.; He, Y.; Xu, Y.; Ye, X.; Yi, Q.; Song, P.; Rudan, I. The global prevalence of and factors associated with Helicobacter pylori infection in children: A systematic review and meta-analysis. Lancet Child. Adolesc. Health 2022, 6, 185–194. [Google Scholar] [CrossRef]
- Wu, Z.; Tang, Y.; Tang, M.; Wu, Z.; Xu, Y. The relationship between the eradication of Helicobacter pylori and the occurrence of stomach cancer: An updated meta-analysis and systemic review. BMC Gastroenterol. 2025, 25, 278. [Google Scholar] [CrossRef]
- Yu, Y.; Xue, J.; Lin, F.; Liu, D.; Zhang, W.; Ru, S.; Jiang, F. Global Primary Antibiotic Resistance Rate of Helicobacter pylori in Recent 10 years: A Systematic Review and Meta-Analysis. Helicobacter 2024, 29, e13103. [Google Scholar] [CrossRef]
- Venerito, M.; Krieger, T.; Ecker, T.; Leandro, G.; Malfertheiner, P. Meta-Analysis of Bismuth Quadruple Therapy versus Clarithromycin Triple Therapy for Empiric Primary Treatment of Helicobacter pylori Infection. Digestion 2013, 88, 33–45. [Google Scholar] [CrossRef]
- Zhang, C.; Zhang, J.; Cheng, Y.J. High-dose dual therapy versus bismuth-containing quadruple therapy for the treatment of Helicobacter pylori infection: A meta-analysis of randomized controlled trials. Saudi J. Gastroenterol. 2023, 29, 88–94. [Google Scholar] [CrossRef]
- Chey, W.D.; Howden, C.W.; Moss, S.F.; Morgan, D.R.; Greer, K.B.; Grover, S.; Shah, S.C. ACG Clinical Guideline: Treatment of Helicobacter pylori Infection. Am. J. Gastroenterol. 2024, 119, 1730–1753. [Google Scholar] [CrossRef]
- Graham, D.Y.; Lew, G.M.; Malaty, H.M.; Evans, D.G.; Evans, D.J.; Klein, P.D.; Alpert, L.C.; Genta, R.M. Factors influencing the eradication of Helicobacter pylori with triple therapy. Gastroenterology 1992, 102, 493–496. [Google Scholar] [CrossRef]
- Glupczynski, Y.; Labbé, M.; Hansen, W.; Crokaert, F.; Yourassowsky, E. Evaluation of the E test for quantitative antimicrobial susceptibility testing of Helicobacter pylori. J. Clin. Microbiol. 1991, 29, 2072–2075. [Google Scholar] [CrossRef] [PubMed]
- Nyssen, O.P.; Espada, M.; Gisbert, J.P. Empirical vs. Susceptibility-Guided Treatment of Helicobacter pylori Infection: A Systematic Review and Meta-Analysis. Front. Microbiol. 2022, 13, 913436. [Google Scholar] [CrossRef]
- Gingold-Belfer, R.; Niv, Y.; Schmilovitz-Weiss, H.; Levi, Z.; Boltin, D. Susceptibility-guided versus empirical treatment for Helicobacter pylori infection: A systematic review and meta-analysis. J. Gastroenterol. Hepatol. 2021, 36, 2649–2658. [Google Scholar] [CrossRef]
- Aromataris, E.; Fernandez, R.; Godfrey, C.M.; Holly, C.; Khalil, H.; Tungpunkom, P. Summarizing systematic reviews. Int. J. Evid. Based Healthc. 2015, 13, 132–140. [Google Scholar]
- Ioannidis, J.P.A. Integration of evidence from multiple meta-analyses: A primer on umbrella reviews, treatment networks and multiple treatments meta-analyses. Can. Med. Assoc. J. 2009, 181, 488–493. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 88, n71. [Google Scholar]
- Shea, B.J.; Reeves, B.C.; Wells, G.; Thuku, M.; Hamel, C.; Moran, J.; Moher, D.; Tugwell, P.; Welch, V.; Kristjansson, E.; et al. AMSTAR 2: A critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. BMJ 2017, 358, j4008. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Morgan, R.L.; Rooney, A.A.; Taylor, K.W.; Thayer, K.A.; Silva, R.A.; Lemeris, C.; Akl, E.A.; Bateson, T.F.; Berkman, N.D.; et al. A tool to assess risk of bias in non-randomized follow-up studies of exposure effects (ROBINS-E). Environ. Int. 2024, 186, 108602. [Google Scholar]
- Schünemann, H.J.; Cuello, C.; Akl, E.A.; Mustafa, R.A.; Meerpohl, J.J.; Thayer, K.; Morgan, R.L.; Gartlehner, G.; Kunz, R.; Katikireddi, S.V.; et al. GRADE guidelines: 18. How ROBINS-I and other tools to assess risk of bias in nonrandomized studies should be used to rate the certainty of a body of evidence. J. Clin. Epidemiol. 2019, 111, 105–114. [Google Scholar]
- Bracchiglione, J.; Meza, N.; Bangdiwala, S.I.; Niño de Guzmán, E.; Urrútia, G.; Bonfill, X.; Madrid, E. Graphical Representation of Overlap for OVErviews: GROOVE tool. Res. Synth. Methods 2022, 13, 381–388. [Google Scholar] [CrossRef]
- Li, M.; Wang, X.; Meng, W.; Dai, Y.; Wang, W. Empirical versus tailored therapy based on genotypic resistance detection for Helicobacter pylori eradication: A systematic review and meta-analysis. Ther. Adv. Gastroenterol. 2023, 16, 17562848231196357. [Google Scholar] [CrossRef]
- Rokkas, T.; Ekmektzoglou, K.; Graham, D.Y. Current role of tailored therapy in treating Helicobacter pylori infections. A systematic review, meta-analysis and critical analysis. Helicobacter 2023, 28, e12936. [Google Scholar] [CrossRef]
- Ma, Q.; Li, H.; Liao, J.; Cai, Z.; Zhang, B. Tailored therapy for Helicobacter pylori eradication: A systematic review and meta-analysis. Front. Pharmacol. 2022, 13, 908202. [Google Scholar] [CrossRef]
- López-Góngora, S.; Puig, I.; Calvet, X.; Villoria, A.; Baylina, M.; Muñoz, N.; Sanchez-Delgado, J.; Suarez, D.; García-Hernando, V.; Gisbert, J.P. Systematic review and meta-analysis: Susceptibility-guided versus empirical antibiotic treatment for Helicobacter pylori infection. J. Antimicrob. Chemother. 2015, 70, 2447–2455. [Google Scholar] [CrossRef]
- Chen, H.; Dang, Y.; Zhou, X.; Liu, B.; Liu, S.; Zhang, G. Tailored Therapy Versus Empiric Chosen Treatment for Helicobacter pylori Eradication. Medicine 2016, 95, e2750. [Google Scholar] [CrossRef]
- Graham, D.Y. Efficient Identification and Evaluation of Effective Helicobacter pylori Therapies. Clin. Gastroenterol. Hepatol. 2009, 7, 145–148. [Google Scholar] [CrossRef]
- Graham, D.Y.; Lee, Y.; Wu, M. Rational Helicobacter pylori Therapy: Evidence-Based Medicine Rather Than Medicine-Based Evidence. Clin. Gastroenterol. Hepatol. 2014, 12, 177–186.e3. [Google Scholar] [CrossRef]
- Herardi, R.; Syam, A.F.; Simadibrata, M.; Setiati, S.; Darnindro, N.; Abdullah, M.; Makmun, D. Comparison of 10-Day Course of Triple Therapy Versus 14-Day Course for Eradication of Helicobacter pylori Infection in an Indonesian Population: Double-Blinded Randomized Clinical Trial. Asian Pac. J. Cancer Prev. 2020, 21, 19–24. [Google Scholar] [CrossRef]
- Huguet, J.M.; Ferrer-Barceló, L.; Suárez, P.; Barcelo-Cerda, S.; Sempere, J.; Saracino, I.M.; Fiorini, G.; Vaira, D.; Pérez-Aísa, Á.; Jonaitis, L.; et al. Role of compliance in Helicobacter pylori eradication treatment: Results of the European Registry on H. pylori management. United Eur. Gastroenterol. J. 2024, 12, 691–704. [Google Scholar] [CrossRef]
- Zeng, R.; Li, X.; Wang, F.; Xie, J.; Song, C.; Xie, Y. Reinforced medication adherence improves Helicobacter pylori eradication rate in developing countries: A systematic review and meta-analysis of randomized controlled trials. Helicobacter 2023, 28, e12989. [Google Scholar] [CrossRef]
- Baradaran, A.; Dehghanbanadaki, H.; Naderpour, S.; Pirkashani, L.M.; Rajabi, A.; Rashti, R.; Riahifar, S.; Moradi, Y. The association between Helicobacter pylori and obesity: A systematic review and meta-analysis of case–control studies. Clin. Diabetes Endocrinol. 2021, 7, 15. [Google Scholar] [CrossRef]
- Horikawa, C.; Kodama, S.; Fujihara, K.; Hirasawa, R.; Yachi, Y.; Suzuki, A.; Hanyu, O.; Shimano, H.; Sone, H. High risk of failing eradication of Helicobacter pylori in patients with diabetes: A meta-analysis. Diabetes Res. Clin. Pract. 2014, 106, 81–87. [Google Scholar] [CrossRef]
- Choe, A.R.; Shim, K.N.; Park, Y.; Song, E.M.; Tae, C.H.; Jung, S.A. Cost-Effectiveness, Efficacy, and Safety Analysis of Tailored Therapy in Patients with Helicobacter pylori Infection. J. Clin. Med. 2021, 10, 2619. [Google Scholar] [CrossRef]
- Lee, J.W.; Kim, S.J.; Choi, C.W.; Kim, H.J.; Kang, D.H.; Kim, H.W.; Park, S.B.; Nam, H.S.; Ryu, D.G. Seven-day triple therapy is sufficient to eradicate infection caused by Helicobacter pylori without 23S rRNA point mutation. Medicine 2021, 100, e26133. [Google Scholar] [CrossRef]
- Ishibashi, F.; Suzuki, S.; Nagai, M.; Mochida, K.; Morishita, T. Optimizing Helicobacter pylori Treatment: An Updated Review of Empirical and Susceptibility Test-Based Treatments. Gut Liver 2023, 17, 684–697. [Google Scholar] [CrossRef]
- Maev, I.V.; Andreev, D.N.; Kucheryavyi, Y.u.A.; Dicheva, D.T. Host factors influencing the eradication rate of Helicobacter pylori. World Appl. Sci. J. 2014, 30, 134–140. [Google Scholar]
- Cho, J.H.; Jin, S.Y. Efficacy and Safety of Modified Bismuth Quadruple Therapy for First-Line Helicobacter pylori Eradication: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Microorganisms 2025, 13, 519. [Google Scholar] [CrossRef]
- Zagari, R.M.; Dajti, E.; Cominardi, A.; Frazzoni, L.; Fuccio, L.; Eusebi, L.H.; Vestito, A.; Lisotti, A.; Galloro, G.; Romano, M.; et al. Standard Bismuth Quadruple Therapy versus Concomitant Therapy for the First-Line Treatment of Helicobacter pylori Infection: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. J. Clin. Med. 2023, 12, 3258. [Google Scholar] [CrossRef]
- Chang, Y.W.; Shin, G.Y.; Kim, J.W.; Moon, J.C.; Chang, E.J.; Oh, C.H.; Jang, J.Y. Cost-Effectiveness of Empirical Bismuth-Based Quadruple Therapy and Tailored Therapy After Clarithromycin Resistance Tests for Helicobacter pylori Eradication. Dig. Dis. Sci. 2022, 67, 1222–1230. [Google Scholar] [CrossRef]
- Gisbert, J.P. Empirical or susceptibility-guided treatment for Helicobacter pylori infection? A comprehensive review. Ther. Adv. Gastroenterol. 2020, 13, 1756284820968736. [Google Scholar] [CrossRef]
- Graham, D.Y.; Shiotani, A. New concepts of resistance in the treatment of Helicobacter pylori infections. Nat. Clin. Pract. Gastroenterol. Hepatol. 2008, 5, 321–331. [Google Scholar] [CrossRef]

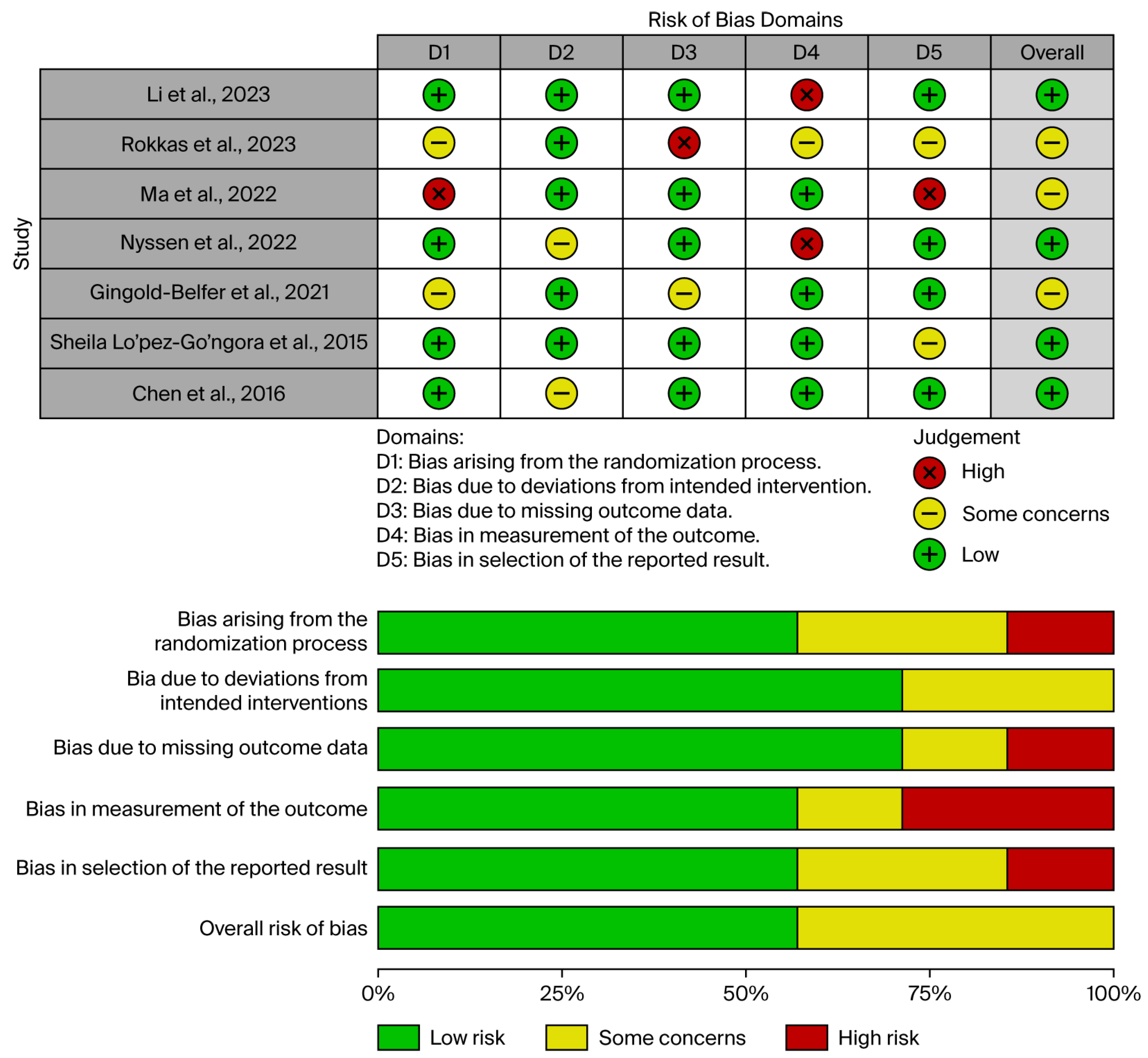
| Study, Year | Total Number of Included Studies | Methodology for TT | ET Type | Total Population, n | TT Total, n | TT Effective, n | ET Total, n | ET Effective, n | Heterogeneity, I2 | Types of Included Studies | Quality Evaluation, AMSTAR-2 | Quality Evaluation, GRADE |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Li et al., 2023 [21] | 12 | Molecular methods | First and second line | 3940 | 1780 | 1414 | 2160 | 1599 | 84% | RCT | High | High |
| Rokkas et al., 2023 [22] | 34 | Culture + PCR (gastric biopsy) | First and second line | 9613 | 4875 | 3770 | 4738 | 2419 | 83.87% | RCT + non-RCT | Moderate | Low |
| Ma et al., 2022 [23] | 21 | 18: cultural; 3: molecular | First line; | 6005 | 2948 | 2462 | 2277 | 1701 | 72.2% | RCT | Low | Low |
| second line | 436 | 345 | 435 | 333 | 80.6% | |||||||
| Nyssen et al., 2022 [12] | 54 | 36: cultural; 16: molecular | First line | 14,600 | 6705 | 5771 | 7895 | 6006 | 83% | 27 RCT; 9 non-RCT | High | High |
| Second line | 78% | |||||||||||
| Gingold-Belfer et al., 2021 [13] | 16 | 14: cultural; 2: genetic | First line | 4825 | 2374 | 2009 | 2451 | 1814 | 75% | 11 RCT; 5 non-RCT | Moderate | Moderate |
| Second line | 342 | 274 | 468 | 305 | ||||||||
| Sheila Lo’pez-Go’ngora et al., 2015 [24] | 12 | n/s | First line | 1958 | 860 | 767 | 1098 | 849 | 33% | 10 RCT; 3 quasi-RCT | Moderate | High |
| Second line | 455 | 207 | 169 | 248 | 150 | 87% | ||||||
| Chen et al., 2016 [25] | 13 | 10: cultural; 3: genetic | First line | 3512 | 1085 | 904 | 1930 | 1317 | 57% | 10 RCT; 3 non-RCT | High | Moderate |
| Second line | 161 | 127 | 300 | 234 |
| Type of Comparison, n of Included Meta-Analyses | RR (95% CI) | Pooled TT Efficiency in Percentages and 95% CI | Pooled ET Efficiency in Percentages and 95% CI | Heterogeneity for RR | p-Value for RR |
|---|---|---|---|---|---|
| All studies, seven | 1.27 (95% CI: 1.14–1.41) | 84.31% (95% CI: 80.94–87.41) | 67.80% (95% CI: 58.48–76.46) | 99% (95% CI: 98.66–99.25) | p < 0.0001 |
| First-line, four | 1.16 (95% CI: 1.12–1.20) | 85.09% (95% CI: 82.81–87.23) | 73.57% (95% CI: 70.03–76.96) | 75.61% (95% CI: 32.67–91.16) | p = 0.0064 |
| Second-line, four | 1.29 (95% CI: 0.83–2.0) | 79.75% (95% CI: 77.38–82.02) | 70.31% (95% CI: 62.03–77.97) | 99.31% (95% CI: 99.02–99.52) | p = 0.252 |
| Only high-quality meta-analyses, three | 1.29 (95% CI: 1.02–1.62) | 84.14% (95% CI: 79.79–88.06) | 66.85% (95% CI: 50.33–81.47) | 99.40% (99.10–99.61) | p < 0.0001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Andreev, D.N.; Khurmatullina, A.R.; Maev, I.V.; Bordin, D.S.; Zaborovsky, A.V.; Kucheryavyy, Y.A.; Sokolov, P.S.; Beliy, P.A. Tailored Therapy vs. Empirical Therapy for Helicobacter pylori Eradication: An Umbrella Review of Systematic Reviews and Meta-Analyses. J. Pers. Med. 2025, 15, 458. https://doi.org/10.3390/jpm15100458
Andreev DN, Khurmatullina AR, Maev IV, Bordin DS, Zaborovsky AV, Kucheryavyy YA, Sokolov PS, Beliy PA. Tailored Therapy vs. Empirical Therapy for Helicobacter pylori Eradication: An Umbrella Review of Systematic Reviews and Meta-Analyses. Journal of Personalized Medicine. 2025; 15(10):458. https://doi.org/10.3390/jpm15100458
Chicago/Turabian StyleAndreev, Dmitrii N., Alsu R. Khurmatullina, Igor V. Maev, Dmitry S. Bordin, Andrey V. Zaborovsky, Yury A. Kucheryavyy, Philipp S. Sokolov, and Petr A. Beliy. 2025. "Tailored Therapy vs. Empirical Therapy for Helicobacter pylori Eradication: An Umbrella Review of Systematic Reviews and Meta-Analyses" Journal of Personalized Medicine 15, no. 10: 458. https://doi.org/10.3390/jpm15100458
APA StyleAndreev, D. N., Khurmatullina, A. R., Maev, I. V., Bordin, D. S., Zaborovsky, A. V., Kucheryavyy, Y. A., Sokolov, P. S., & Beliy, P. A. (2025). Tailored Therapy vs. Empirical Therapy for Helicobacter pylori Eradication: An Umbrella Review of Systematic Reviews and Meta-Analyses. Journal of Personalized Medicine, 15(10), 458. https://doi.org/10.3390/jpm15100458








