Safety and Efficacy of the Use of Supraglottic Airway Devices in Children and Adolescents Undergoing Adenotonsillectomy—A Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Strategy
2.2. Eligibility Criteria
2.3. Data Extraction
2.4. Data Collection
2.5. Methodological Assessment
2.6. Strength of Quality across All the Trials
2.7. Quantitative Meta-Analysis
2.8. Publication Bias
2.9. Sensitivity Analysis
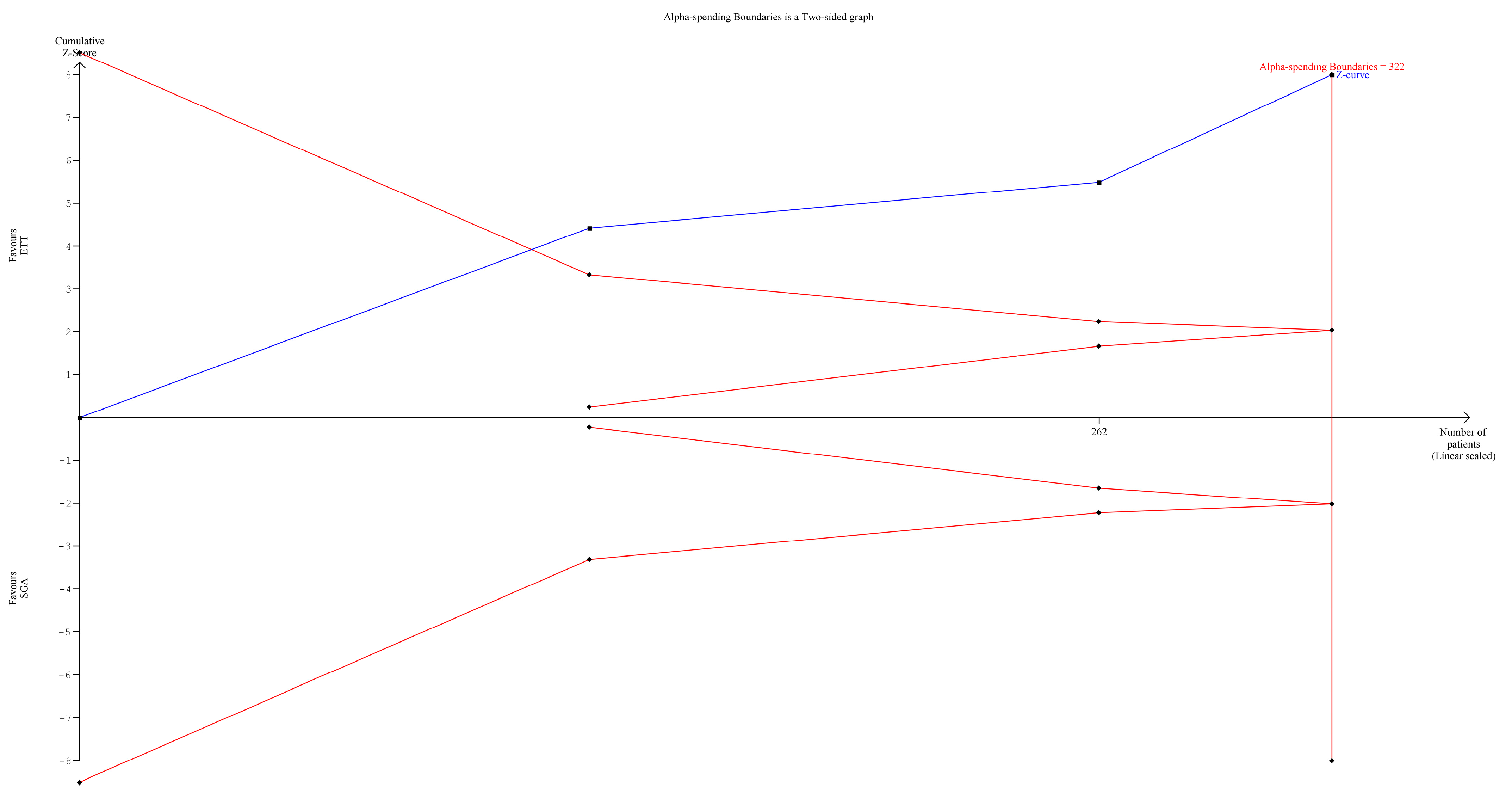
2.10. Trial Sequential Analysis (TSA)
3. Results
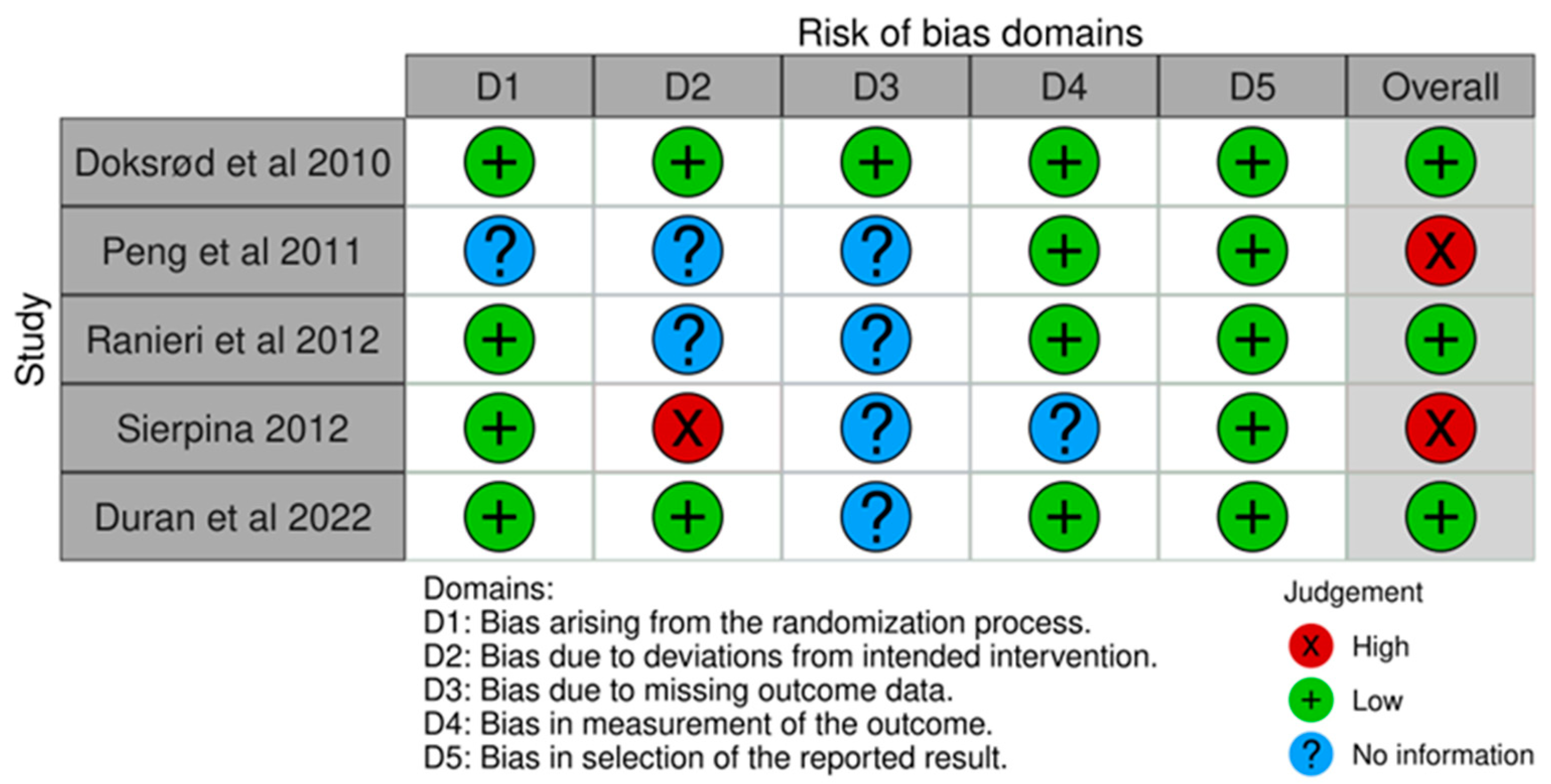
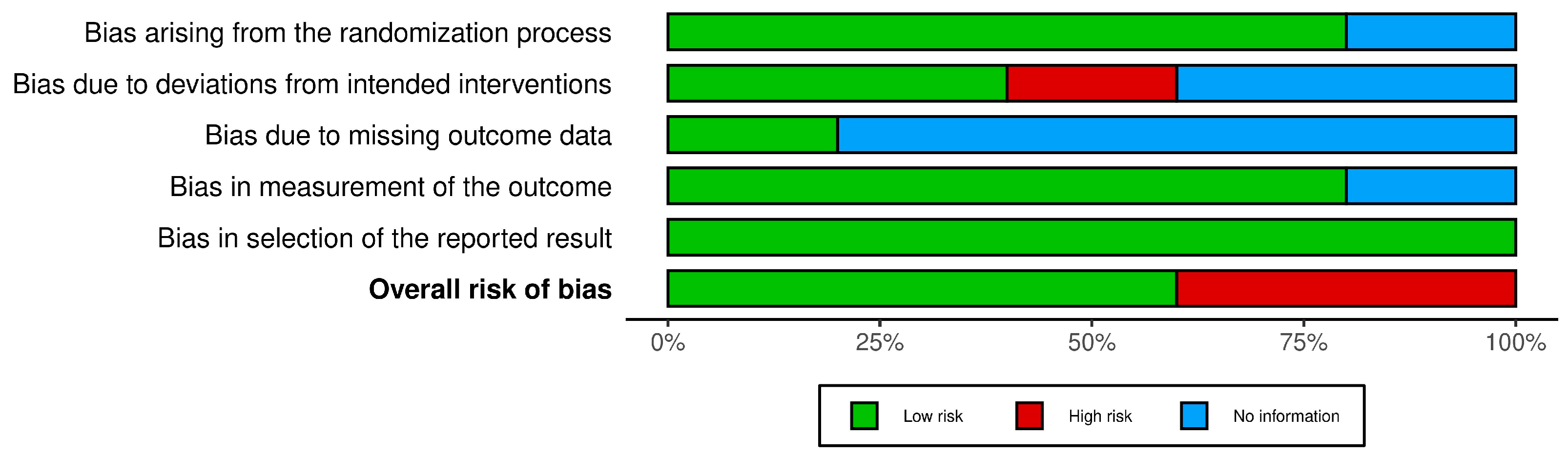
3.1. Risk-of-Bias Assessment
3.2. Quality of Evidence
3.3. Primary Outcome Meta-Analysis
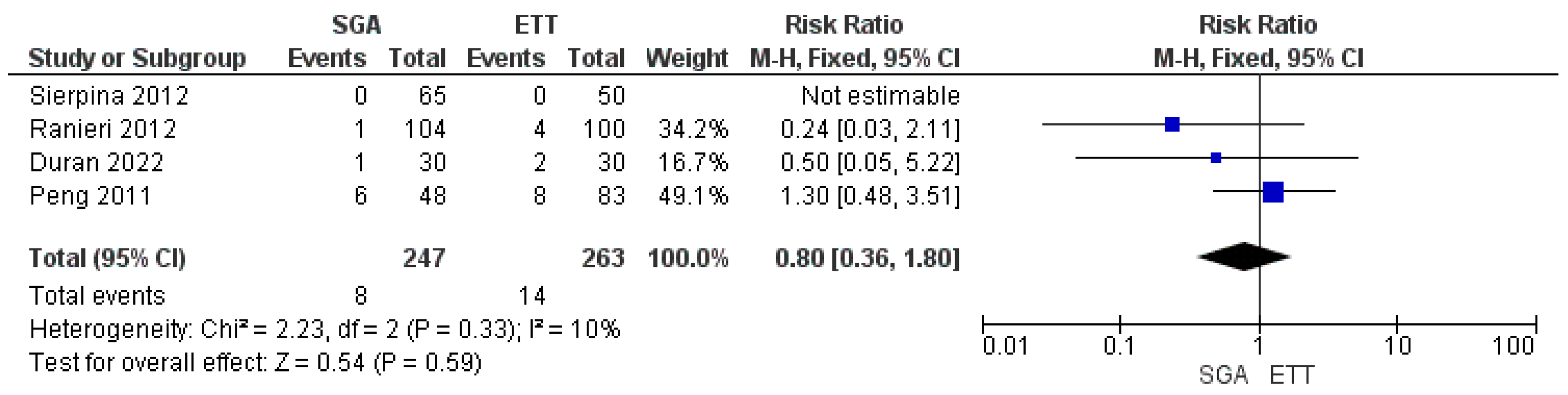
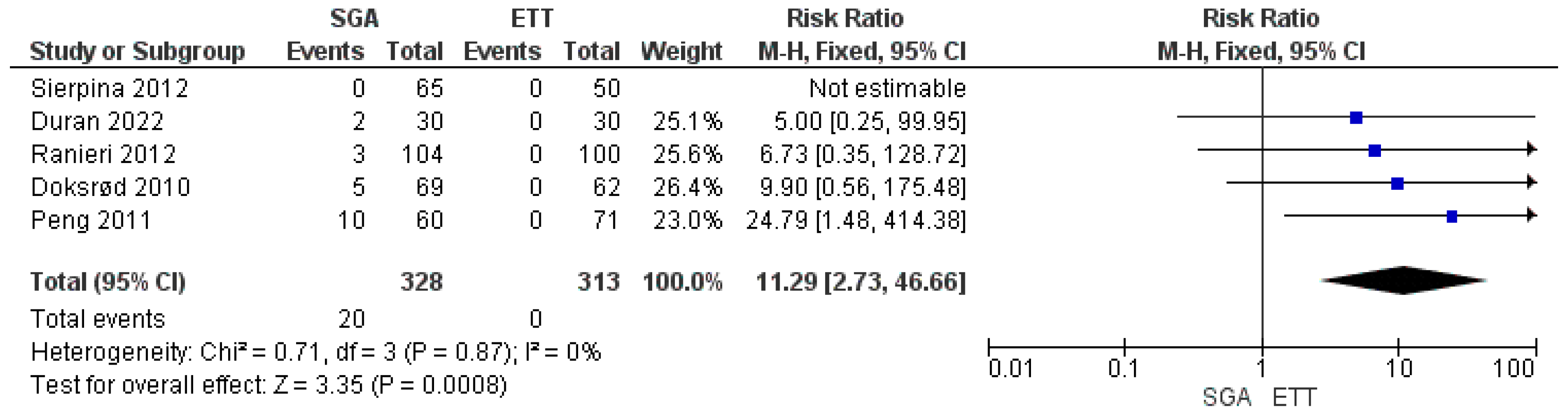
3.4. Meta-Analysis of Airway Device Failure
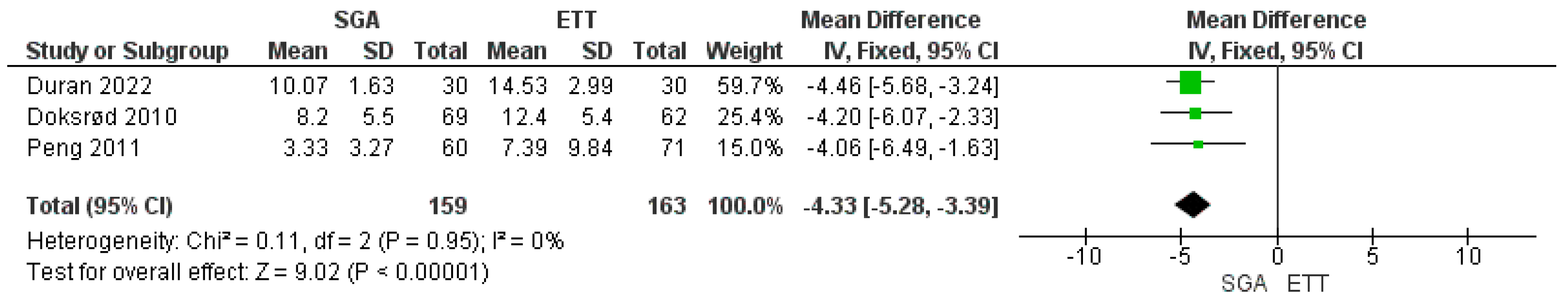
3.5. Meta-Analysis of Postoperative Recovery Time
3.6. Other Outcomes
3.7. TSA
4. Discussion
Summary of Results
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bangera, A. Anaesthesia for adenotonsillectomy: An update. Indian J. Anaesth. 2017, 61, 103–109. [Google Scholar] [CrossRef]
- Zalan, J.; Vaccani, J.-P.; Murto, K. Paediatric adenotonsillectomy, part 2: Considerations for anaesthesia. BJA Educ. 2020, 20, 193–200. [Google Scholar] [CrossRef]
- McGuire, S.R.; Doyle, N.M. Update on the safety of anesthesia in young children presenting for adenotonsillectomy. World J. Otorhinolaryngol. Head Neck Surg. 2021, 7, 179–185. [Google Scholar] [CrossRef]
- Kleine-Brueggeney, M.; Gottfried, A.; Nabecker, S.; Greif, R.; Book, M.; Theiler, L. Pediatric supraglottic airway devices in clinical practice: A prospective observational study. BMC Anesthesiol. 2017, 17, 119. [Google Scholar] [CrossRef]
- Huang, A.S.; Hajduk, J.; Jagannathan, N. Advances in supraglottic airway devices for the management of difficult airways in children. Expert. Rev. Med. Devices 2016, 13, 157–169. [Google Scholar] [CrossRef]
- Luce, V.; Harkouk, H.; Brasher, C.; Michelet, D.; Hilly, J.; Maesani, M.; Diallo, T.; Mangalsuren, N.; Nivoche, Y.; Dahmani, S. Supraglottic airway devices vs tracheal intubation in children: A quantitative meta-analysis of respiratory complications. Pediatr. Anesth. 2014, 24, 1088–1098. [Google Scholar] [CrossRef] [PubMed]
- Gravningsbråten, R.; Nicklasson, B.; Raeder, J. Safety of laryngeal mask airway and short-stay practice in office-based adenotonsillectomy. Acta Anaesthesiol. Scand. 2009, 53, 218–222. [Google Scholar] [CrossRef] [PubMed]
- Ozmete, O.; Sener, M.; Caliskan, E.; Kipri, M.; Aribogan, A. The use of flexible laryngeal mask airway for Adenoidectomies: An experience of 814 Paediatric patients. Pak. J. Med. Sci. 2017, 33, 823–828. [Google Scholar] [CrossRef] [PubMed]
- Mandel, J.E. Laryngeal mask airways in ear, nose, and throat procedures. Anesthesiol. Clin. 2010, 28, 469–483. [Google Scholar] [CrossRef]
- Turnbull, J.; Patel, A. The use of the laryngeal mask airway in ENT surgery: Facts and fiction. Trends Anaesth. Crit. Care 2013, 3, 346–350. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Thomas, J.; Chandler, J.; Cumpston, M.; Li, T.; Page, M.J.; Welch, V.A. (Eds.) Cochrane Handbook for Systematic Reviews of Interventions Version 6.3 (Updated February 2022). Cochrane. 2022. Available online: www.training.cochrane.org/handbook (accessed on 14 January 2024).
- GRADEpro GDT: GRADEpro Guideline Development Tool [Software]. McMaster University, 2015 (Developed by Evidence Prime, Inc.). Available online: https://www.gradepro.org (accessed on 14 January 2024).
- Review Manager (RevMan) [Computer Program]. Version 5.4.; The Cochrane Collaboration. 2020. Available online: http://revman.cochrane.org (accessed on 14 January 2024).
- Sterne, J.A.; Egger, M. Funnel plots for detecting bias in meta-analysis: Guidelines on choice of axis. J. Clin. Epidemiol. 2001, 54, 1046–1055. [Google Scholar] [CrossRef]
- Egger, M.; Smith, G.D.; Schneider, M.; Minder, C. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997, 315, 629–634. [Google Scholar] [CrossRef]
- Kang, H. Trial sequential analysis: Novel approach for meta-analysis. Anesth. Pain. Med. 2021, 16, 138–150. [Google Scholar] [CrossRef]
- De Cassai, A.; Pasin, L.; Boscolo, A.; Salvagno, M.; Navalesi, P. Trial sequential analysis: Plain and simple. Korean J. Anesthesiol. 2021, 74, 363–365. [Google Scholar] [CrossRef] [PubMed]
- Doksrød, S.; Løfgren, B.; Nordhammer, A.; Svendsen, M.V.; Gisselsson, L.; Ræder, J. Reinforced laryngeal mask airway compared with endotracheal tube for adenotonsillectomies. Eur. J. Anaesthesiol. 2010, 27, 941–946. [Google Scholar] [CrossRef] [PubMed]
- Peng, A.; Dodson, K.M.; Thacker, L.R.; Kierce, J.; Shapiro, J.; Baldassari, C.M. Use of laryngeal mask airway in pediatric adenotonsillectomy. Arch. Otolaryngol. Head Neck Surg. 2011, 137, 42–46. [Google Scholar] [CrossRef]
- Ranieri, D., Jr.; Neubauer, A.G.; Ranieri, D.M.; do Nascimento, P., Jr. The use of disposable laryngeal mask airway for adenotonsillectomies. Rev. Bras. Anestesiol. 2012, 62, 788–797. [Google Scholar] [CrossRef] [PubMed]
- Sierpina, D.I.; Chaudhary, H.; Walner, D.L.; Villines, D.; Schneider, K.; Lowenthal, M.; Aronov, Y. Laryngeal mask airway versus endotracheal tube in pediatric adenotonsillectomy. Laryngoscope 2012, 122, 429–435. [Google Scholar] [CrossRef] [PubMed]
- Duran, M.; Kuşderci, H.; Uludağ, Ö. Comparison of flexible laryngeal mask insertion and endotracheal intubation in adenotonsillectomy surgery. Acta Clin. Croat. 2022, 61, 613–619. [Google Scholar] [CrossRef] [PubMed]
- Hsu, G.; von Ungern-Sternberg, B.S.; Engelhardt, T. Pediatric airway management. Curr. Opin. Anaesthesiol. 2021, 34, 276–283. [Google Scholar] [CrossRef]
- Harless, J.; Ramaiah, R.; Bhananker, S.M. Pediatric airway management. Int. J. Crit. Illn. Inj. Sci. 2014, 4, 65–70. [Google Scholar] [PubMed]
- Bradley, J.; Lee, G.S.; Peyton, J. Anesthesia for shared airway surgery in children. Pediatr. Anesth. 2020, 30, 288–295. [Google Scholar] [CrossRef] [PubMed]
- Kundra, P.; Supraja, N.; Agrawal, K.; Ravishankar, M. Flexible laryngeal mask airway for cleft palate surgery in children: A randomized clinical trial on efficacy and safety. Cleft Palate-Craniofacial J. 2009, 46, 368–373. [Google Scholar] [CrossRef] [PubMed]
- Patel, M.G.; Swadia, V.; Bansal, G. Prospective randomized comparative study of use of PLMA and ET tube for airway management in children under general anaesthesia. Indian J. Anaesth. 2010, 54, 109–115. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.-J.; Cheng, P.-L.; Huang, Y.-J.; Huang, F.-H. Laryngeal Mask Airway as an Appropriate Option in Pediatric Laparoscopic Inguinal Hernia Repair: A Systematic Review and Meta-Analysis. J. Pediatr. Surg. 2023. [Google Scholar] [CrossRef]
- Totonchi, Z.; Siamdoust, S.A.S.; Zaman, B.; Rokhtabnak, F.; Alavi, S.A. Comparison of laryngeal mask airway (LMA) insertion with and without muscle relaxant in pediatric anesthesia; a randomized clinical trial. Heliyon 2022, 8, e11504. [Google Scholar] [CrossRef]
- Mir, S.A.; Dhar, R.; Sofi, K.; Jehangir, M.; Wazir, M. Comparison of Spontaneous Ventilation, Pressure Control Ventilation and Pressure Support Ventilation in Pediatric Patients Undergoing Infraumbilical Surgery Using ProSeal Laryngeal Mask Airway. Anesth. Essays Res. 2021, 15, 321–326. [Google Scholar] [CrossRef]
- Wu, L.; Wei, S.W.; Xiang, Z.; Yu, E.Y.; Qu, S.Q.; Du, Z. Effect of neuromuscular block on surgical conditions during short-duration paediatric laparoscopic surgery involving a supraglottic airway. Br. J. Anaesth. 2021, 127, 281–288. [Google Scholar] [CrossRef]
- Lalwani, K.; Richins, S.; Aliason, I.; Milczuk, H.; Fu, R. The laryngeal mask airway for pediatric adenotonsillectomy: Predictors of failure and complications. Int. J. Pediatr. Otorhinolaryngol. 2013, 77, 25–28. [Google Scholar] [CrossRef]
- Aziz, L.; Bashir, K. Comparison of armoured laryngeal mask airway with endotracheal tube for adenotonsillectomy. J. Coll. Physicians Surg. Pak. 2006, 16, 685–688. [Google Scholar]
- Thorning, G.; Robb, P.; Ewah, B.; Shylaja, V. The laryngeal mask airway in elective paediatric day case ENT surgery: A prospective audit. Eur. J. Anaesthesiol. 2016, 33, 872–873. [Google Scholar] [CrossRef] [PubMed]
- Boroda, N.; Malesinska, M.; Kars, M.S.; Smith, L.P. The use of laryngeal mask airway for adenoidectomy. Int. J. Pediatr. Otorhinolaryngol. 2018, 107, 42–44. [Google Scholar] [CrossRef] [PubMed]
- Ramgolam, A.; Hall, G.; Zhang, G.; Hegarty, M.; von Ungern-Sternberg, B. Deep or awake removal of laryngeal mask airway in children at risk of respiratory adverse events undergoing tonsillectomy—A randomised controlled trial. Br. J. Anaesth. 2018, 120, 571–580. [Google Scholar] [CrossRef] [PubMed]
- Eguia, A.; Jiang, Z.Y.; Brollier, L.; Matuszczak, M.; Yuksel, S.; Roy, S.; Huang, Z. Reducing intraoperative time with laryngeal mask airway and stretcher in pediatric adenotonsillectomy. Am. J. Otolaryngol. 2022, 43, 103195. [Google Scholar] [CrossRef]
- Gehrke, T.; Hackenberg, S.; Steinhübel, B.; Hagen, R.; Scherzad, A. Laryngeal mask versus intubation for adenoidectomies in children: Analysis of 1500 operations. Laryngoscope 2019, 129, E383–E388. [Google Scholar] [CrossRef] [PubMed]

| PubMed search details: (100) | ((((“supraglottal” [All Fields] OR “supraglottic” [All Fields]) AND (“airway” [All Fields] OR “airway s” [All Fields] OR “airways” [All Fields]) AND (“laryngeal masks” [MeSH Terms] OR (“laryngeal” [All Fields] AND “masks” [All Fields]) OR “laryngeal masks” [All Fields] OR (“laryngeal” [All Fields] AND “mask” [All Fields] AND “airway” [All Fields]) OR “laryngeal mask airway” [All Fields])) OR (“adenoidectomy” [MeSH Terms] OR “adenoidectomy” [All Fields] OR “adenoidectomies” [All Fields]) OR (“tonsillectomy” [MeSH Terms] OR “tonsillectomy” [All Fields] OR “tonsillectomies” [All Fields])) AND (“adenotonsillectomies” [All Fields] OR “adenotonsillectomy” [All Fields]) AND (“anaesthesia” [All Fields] OR “anesthesia” [MeSH Terms] OR “anesthesia” [All Fields] OR “anaesthesias” [All Fields] OR “anesthesias” [All Fields])) AND ((clinicaltrial[Filter] OR randomizedcontrolledtrial[Filter]) AND (2000/1/1:2024/1/10 [pdat]) AND (allinfant[Filter] OR infant[Filter] OR child[Filter] OR adolescent[Filter] OR preschoolchild[Filter])) |
| Scopus search details: (37) | TITLE-ABS-KEY “Supraglottic Airway” OR “Laryngeal mask airway” AND “Adenoidectomy” AND “Tonsillectomy” OR “Adenotonsillectomy” AND “Anesthesia” |
| Cochrane library: (7) | “laryngeal mask airway” in Title Abstract Keyword AND “adenoidectomies” in Title Abstract Keyword AND “tonsillectomies” in Title Abstract Keyword AND “anesthesia” in Title Abstract Keyword AND “pediatric” in Title Abstract Keyword (Word variations have been searched) “laryngeal mask airway”:ti,ab,kw AND “adenoidectomies”:ti,ab,kw AND “tonsillectomies”:ti,ab,kw AND “anesthesia”:ti,ab,kw AND “pediatric”:ti,ab,kw (Word variations have been searched) |
| CINAHL: (36) | Search terms: supraglottic airway OR laryngeal mask airway AND (adenoidectomy or tonsillectomy or adenotonsillectomy) Limiters—Publication Date: 20000101–20241231 Expanders—Apply equivalent subjects Narrow by SubjectAge:—adolescent: 13–18 years Narrow by SubjectAge:—infant: 1–23 months Narrow by SubjectAge:—child: 6–12 years Narrow by SubjectAge:—child, preschool: 2–5 years Narrow by SubjectAge:—all child Search modes—Boolean/Phrase |
| OViD: (20) | Supraglottic airway* laryngeal mask airway* adenoidectomy*tonsillectomy*pediatric*adolescent.mp. [mp = title, abstract, full text, caption text] |
| Author/Year | Study Design | Study Population | Group I (SGA) | Group II (ETT) | Sample Size | Age Group (Years) | Type of SGA Used | Outcomes | Findings |
|---|---|---|---|---|---|---|---|---|---|
| Doksrød et al./2010 [18] | RCT | Pediatric and adolescent | 69 | 62 | 131 | 3–16 | R-LMA | To compare pain, nausea, and respiratory irritation with R-LMA and ETT. Time spent in the operating room after surgery. | Significantly lower pain scores with R-LMA at 4 h (p = 0.015) only and not significant at other times till 24 h Lesser time spent after surgery in the operating room (4.2 min lesser) with R-LMA (p = 0.001) Nausea was comparable in both groups. In patients in ETT group and five patients in whom R-LMA was changed to ETT, airway irritation was higher (p < 0.02). |
| Peng et al./2011 [19] | RCT | Pediatric | 60 | 71 | 131 | 2–12 | F-LMA | Primary outcome: to compare the incidence of laryngospasm. Secondary outcomes: anesthesia, operative and recovery time. | Incidence of postoperative laryngospasm between LMA and ETT (12.5% and 9.6%) was similar (p = 0.77). Time from surgery end to extubation was significant with LMA (p = 0.01) by 4.06 min. Mean surgical time and postoperative recovery times were comparable (p = 0.15 and p = 0.49). |
| Ranieri et al./2012 [20] | RCT | Pediatric | 104 | 100 | 204 | 2–10 | LMA Unique | To evaluate the levels of blood oxygenation and the occurrence of respiratory complications during adenotonsillectomies. | The use of LMA resulted in a lower intraoperative SpO2, compared to using an ETT (at induction: p > 0.25, after establishing operative field: p < 0.001, at end of surgical procedure: p = 0.037, 3 min after extubation: p < 0.001 On admission to recovery room: p < 0.001). Respiratory complications (bronchospasm, laryngospasm, stridor, breathing noise, regurgitation) were comparable. |
| Sierpina et al./2012 [21] | RCT | Pediatric and adolescent | 65 | 50 | 115 | 2–18 | F-LMA | Safety, duration of surgery, and patient comfort | Less coughing and gagging with LMA (p = 0.002). Safety and various postoperative outcomes were comparable. |
| Duran et al./2022 [22] | Comparative study | Pediatric | 30 | 30 | 60 | 2–12 | F-LMA | Intubation and recovery time, view of the surgical field, SpO2, ETCO2, heart rate, and airway pressure. | Intubation and recovery time were shorter in the F-LMA group than in the ETT group, in minutes (16.93 ± 4.84 s vs. 23.93 ± 8.74 s; and 10 ± 2 vs. 14.5 ± 3; p < 0.001). The airway pressures were significantly lower in the F-LMA group than in ETT (p < 0.001). |
| S. No. | Study | Airway Device Used | Total Patients | Laryngospasm | SGA Failure | Recovery Time (Minutes) |
|---|---|---|---|---|---|---|
| 1 | Doksrød et al./2010 [18] | SAD | 69 | -- | 5 | 8.2 ± 5.5 |
| ETT | 62 | -- | 0 | 12.4 ± 5.4 | ||
| 2 | Peng et al./2012 [19] | SAD | 48 | 6 | 10 | 3.33 ± 3.27 |
| ETT | 83 | 8 | 0 | 7.39 ± 9.84 | ||
| 3 | Ranieri et al./2012 [20] | SAD | 104 | 1 | 3 | -- |
| ETT | 100 | 4 | 0 | -- | ||
| 4 | Sierpina et al./2012 [21] | SAD | 65 | 0 | 0 | -- |
| ETT | 50 | 0 | 0 | -- | ||
| 5 | Duran et al./2022 [22] | SAD | 30 | 1 | 2 | 10.07 ± 1.63 |
| ETT | 30 | 2 | 0 | 14.53 ± 2.99 |
| Certainty Assessment | № of Patients | Effect | Certainty | Importance | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| № of Studies | Study Design | Risk of Bias | Inconsistency | Indirectness | Imprecision | Other Considerations | Supraglottic Airway | Endotracheal Tube | Relative (95% CI) | Absolute (95% CI) | ||
| Laryngospasm | ||||||||||||
| 4 | randomized trials | Serious a | Serious b | not serious | not serious | all plausible residual confounding would reduce the demonstrated effect | 8/259 (3.1%) | 14/251 (5.6%) | RR 0.00 (0.32 to 1.92) | -- per 100 (from 4 fewer to 5 more) | ⨁⨁⨁◯ Moderate | IMPORTANT |
| Airway device failure | ||||||||||||
| 5 | randomized trials | Serious a | Serious b | not serious | Serious b | publication bias strongly suspected all plausible residual confounding would reduce the demonstrated effect c | 10/328 (3.0%) | 0/313 (0.0%) | RR 0.00 (2.73 to 46.66) | -- per 100 (from 0 fewer to 0 fewer) | ⨁◯◯◯ Very low | IMPORTANT |
| Time spent in OR | ||||||||||||
| 3 | randomized trials | Serious a | Serious d | Serious d | Serious d | strong association all plausible residual confounding would reduce the demonstrated effect | 159 | 163 | - | 0 (0 to 0) | ⨁⨁◯◯ Low | IMPORTANT |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nair, A.; Borkar, N.; Murke, S.S.; Dudhedia, U. Safety and Efficacy of the Use of Supraglottic Airway Devices in Children and Adolescents Undergoing Adenotonsillectomy—A Systematic Review and Meta-Analysis. J. Pers. Med. 2024, 14, 311. https://doi.org/10.3390/jpm14030311
Nair A, Borkar N, Murke SS, Dudhedia U. Safety and Efficacy of the Use of Supraglottic Airway Devices in Children and Adolescents Undergoing Adenotonsillectomy—A Systematic Review and Meta-Analysis. Journal of Personalized Medicine. 2024; 14(3):311. https://doi.org/10.3390/jpm14030311
Chicago/Turabian StyleNair, Abhijit, Nitinkumar Borkar, Sunil Subhash Murke, and Ujjwalraj Dudhedia. 2024. "Safety and Efficacy of the Use of Supraglottic Airway Devices in Children and Adolescents Undergoing Adenotonsillectomy—A Systematic Review and Meta-Analysis" Journal of Personalized Medicine 14, no. 3: 311. https://doi.org/10.3390/jpm14030311
APA StyleNair, A., Borkar, N., Murke, S. S., & Dudhedia, U. (2024). Safety and Efficacy of the Use of Supraglottic Airway Devices in Children and Adolescents Undergoing Adenotonsillectomy—A Systematic Review and Meta-Analysis. Journal of Personalized Medicine, 14(3), 311. https://doi.org/10.3390/jpm14030311