Machine Learning Prediction of Treatment Response to Inhaled Corticosteroids in Asthma
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Subjects and Datasets
2.2. Study Outcomes
2.3. Genotyping, Imputation, and Quality Control Procedures
2.4. Development of Predictive Models
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Expert Panel Working Group of the National Heart, Lung, and Blood Institute (NHLBI) Administered and Coordinated National Asthma Education and Prevention Program Coordinating Committee (NAEPPCC); Cloutier, M.M.; Baptist, A.P.; Blake, K.V.; Brooks, E.G.; Bryant-Stephens, T.; DiMango, E.; Dixon, A.E.; Elward, K.S.; Hartert, T.; et al. 2020 Focused Updates to the Asthma Management Guidelines: A Report from the National Asthma Education and Prevention Program Coordinating Committee Expert Panel Working Group. J. Allergy Clin. Immunol. 2002, 146, 1217–1270. [Google Scholar] [CrossRef]
- Childhood Asthma Management Program Research Group; Szefler, S.; Weiss, S.; Tonascia, J.; Adkinson, N.F.; Bender, B.; Cherniack, R.; Donithan, M.; Kelly, H.W.; Reisman, J.; et al. Long-term effects of budesonide or nedocromil in children with asthma. N. Engl. J. Med. 2000, 343, 1054–1063. [Google Scholar]
- Szefler, S.J.; Martin, R.J.; King, T.S.; Boushey, H.A.; Cherniack, R.M.; Chinchilli, V.M.; Craig, T.J.; Dolovich, M.; Drazen, J.M.; Fagan, J.K.; et al. Significant variability in response to inhaled corticosteroids for persistent asthma. J. Allergy Clin. Immunol. 2002, 109, 410–418. [Google Scholar] [CrossRef]
- Szefler, S.J.; Phillips, B.R.; Martinez, F.D.; Chinchilli, V.M.; Lemanske, R.F.; Strunk, R.C.; Zeiger, R.S.; Larsen, G.; Spahn, J.D.; Bacharier, L.B.; et al. Characterization of within-subject responses to fluticasone and montelukast in childhood asthma. J. Allergy Clin. Immunol. 2005, 115, 233–242. [Google Scholar] [CrossRef] [PubMed]
- McDermott, L.; O’Dowd, L. Potential adverse effects of the inhaled corticosteroids. J. Allergy Clin. Immunol. 2004, 112, 469–478. [Google Scholar] [CrossRef]
- Kelly, H.W.; Nelson, H.S. Potential adverse effects of the inhaled corticosteroids. J. Allergy Clin. Immunol. 2003, 112, 469–478. [Google Scholar] [CrossRef]
- Davis, J.S.; Weiss, S.T.; Tantisira, K.G. Asthma pharmacogenomics: 2015 update. Curr. Allergy Asthma Rep. 2015, 15, 42. [Google Scholar] [CrossRef] [PubMed]
- Keskin, O.; Farzan, N.; Birben, E.; Akel, H.; Karaaslan, C.; der Zee, A.H.M.-V.; Wechsler, M.E.; Vijverberg, S.J.; Kalayci, O. Genetic associations of the response to inhaled corticosteroids in asthma: A systematic review. Clin. Transl. Allergy 2019, 9, 2. [Google Scholar] [CrossRef]
- Dijk, F.N.; Vijverberg, S.J.; Hernandez-Pacheco, N.; Repnik, K.; Karimi, L.; Mitratza, M.; Farzan, N.; Nawijn, M.C.; Burchard, E.G.; Engelkes, M.; et al. IL1RL1 gene variations are associated with asthma exacerbations in children and adolescents using inhaled corticosteroids. Allergy 2020, 75, 984–989. [Google Scholar] [CrossRef] [PubMed]
- Tantisira, K.G.; Silverman, E.S.; Mariani, T.J.; Xu, J.; Richter, B.G.; Klanderman, B.J.; Litonjua, A.A.; Lazarus, R.; Rosenwasser, L.J.; Fuhlbrigge, A.L.; et al. FCER2: A pharmacogenetic basis for severe exacerbations in children with asthma. J. Allergy Clin. Immunol. 2007, 120, 1285–1291. [Google Scholar] [CrossRef]
- Tse, S.M.; Krajinovic, M.; Chauhan, B.F.; Zemek, R.; Gravel, J.; Chalut, D.; Poonai, N.; Quach, C.; Laberge, S.; Ducharme, F.M.; et al. Genetic determinants of acute asthma therapy response in children with moderate-to-severe asthma exacerbations. Pediatr. Pulmonol. 2019, 54, 378–385. [Google Scholar] [CrossRef]
- Vijverberg, S.J.H.; Koster, E.S.; Tavendale, R.; Leusink, M.; Koenderman, L.; Raaijmakers, J.A.M.; Postma, D.S.; Koppelman, G.H.; Turner, S.W.; Mukhopadhyay, S.; et al. ST13 polymorphisms and their effect on exacerbations in steroid-treated asthmatic children and young adults. Clin. Exp. Allergy 2015, 45, 1051–1059. [Google Scholar] [CrossRef] [PubMed]
- Edris, A.; de Roos, E.W.; McGeachie, M.J.; Verhamme, K.M.C.; Brusselle, G.G.; Tantisira, K.G.; Iribarren, C.; Lu, M.; Wu, A.C.; Stricker, B.H.; et al. Pharmacogenetics of inhaled corticosteroids and exacerbation risk in adults with asthma. Clin. Exp. Allergy 2021, 52, 33–45. [Google Scholar] [CrossRef] [PubMed]
- Himes, B.E.; Jiang, X.; Wagner, P.; Hu, R.; Wang, Q.; Klanderman, B.; Whitaker, R.M.; Duan, Q.; Lasky-Su, J.; Nikolos, C.; et al. RNA-Seq transcriptome profiling identifies CRISPLD2 as a glucocorticoid responsive gene that modulates cytokine function in airway smooth muscle cells. PLoS ONE 2014, 9, e99625. [Google Scholar] [CrossRef] [PubMed]
- Torkamani, A.; Wineinger, N.E.; Topol, E.J. The personal and clinical utility of polygenic risk scores. Nat. Rev. Genet. 2018, 19, 581–590. [Google Scholar] [CrossRef]
- Childhood Asthma Management Program Research Group. The Childhood Asthma Management Program (CAMP): Design, rationale, and methods. Control. Clin. Trials 1999, 20, 91–120. [Google Scholar] [CrossRef]
- Denlinger, L.C.; Sorkness, C.A.; Chinchilli, V.M.; Lemanske, R.F. Guideline-defining asthma clinical trials of the National Heart, Lung, and Blood Institute’s Asthma Clinical Research Network and Childhood Asthma Research and Education Network. J. Allergy Clin. Immunol. 2007, 119, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Guilbert, T.W.; Morgan, W.J.; Krawiec, M.; Lemanske, R.F.; Sorkness, C.; Szefler, S.J.; Larsen, G.; Spahn, J.D.; Zeiger, R.S.; Heldt, G.; et al. The Prevention of Early Asthma in Kids study: Design, rationale and methods for the Childhood Asthma Research and Education network. Control. Clin. Trials 2004, 25, 286–310. [Google Scholar] [CrossRef] [PubMed]
- McCarty, C.A.; Wilke, R.A.; Giampietro, P.F.; Wesbrook, S.D.; Caldwell, M.D. Marshfield Clinic Personalized Medicine Research Project (PMRP): Design, methods and recruitment for a large population-based biobank. Per. Med. 2005, 2, 49–79. [Google Scholar] [CrossRef]
- Roden, D.M.; Pulley, J.M.; Basford, M.A.; Bernard, G.R.; Clayton, E.W.; Balser, J.R.; Masys, D.R. Development of a large-scale de-identified DNA biobank to enable personalized medicine. Clin. Pharmacol. Ther. 2008, 84, 362–369. [Google Scholar] [CrossRef]
- Wei, W.-Q.; Denny, J.C. Extracting research-quality phenotypes from electronic health records to support precision medicine. Genome Med. 2015, 7, 41. [Google Scholar] [CrossRef]
- Tantisira, K.G.; Damask, A.; Szefler, S.J.; Schuemann, B.; Markezich, A.; Su, J.; Klanderman, B.; Sylvia, J.; Wu, R.; Martinez, F.; et al. Genome-wide association identifies the T gene as a novel asthma pharmacogenetic locus. Am. J. Respir. Crit. Care Med. 2012, 185, 1286–1291. [Google Scholar] [CrossRef]
- Israel, E.; Lasky-Su, J.; Markezich, A.; Damask, A.; Szefler, S.J.; Schuemann, B.; Klanderman, B.; Sylvia, J.; Kazani, S.; Wu, R.; et al. Genome-wide association study of short-acting β2-agonists. A novel genome-wide significant locus on chromosome 2 near ASB3. Am. J. Respir. Crit. Care Med. 2015, 191, 530–537. [Google Scholar] [CrossRef]
- Park, H.-W.; Dahlin, A.; Tse, S.; Duan, Q.L.; Schuemann, B.; Martinez, F.D.; Peters, S.P.; Szefler, S.J.; Lima, J.J.; Kubo, M.; et al. Genetic predictors associated with improvement of asthma symptoms in response to inhaled corticosteroids. J. Allergy Clin. Immunol. 2014, 133, 644–649.e5. [Google Scholar] [CrossRef] [PubMed]
- Himes, B.E.; Jiang, X.; Hu, R.; Wu, A.C.; Lasky-Su, J.A.; Klanderman, B.J.; Ziniti, J.; Senter-Sylvia, J.; Lima, J.J.; Irvin, C.G.; et al. Genome-wide association analysis in asthma subjects identifies SPATS2L as a novel bronchodilator response gene. PLoS Genet. 2012, 8, e1002824. [Google Scholar] [CrossRef]
- Clemmer, G.L.; Wu, A.C.; Rosner, B.; McGeachie, M.J.; Litonjua, A.A.; Tantisira, K.G.; Weiss, S.T. Measuring the corticosteroid responsiveness endophenotype in asthmatic patients. J. Allergy Clin. Immunol. 2015, 136, 274–281.e8. [Google Scholar] [CrossRef][Green Version]
- Tantisira, K.G.; Lasky-Su, J.; Harada, M.; Murphy, A.; Litonjua, A.A.; Himes, B.E.; Lange, C.; Lazarus, R.; Sylvia, J.; Klanderman, B.; et al. Genomewide association between GLCCI1 and response to glucocorticoid therapy in asthma. N. Engl. J. Med. 2011, 365, 1173–1183. [Google Scholar] [CrossRef]
- Agrawal, V.; Manouchehri, A.; Vaitinadin, N.S.; Shi, M.; Bagheri, M.; Gupta, D.K.; Kullo, I.J.; Luo, Y.; McNally, E.M.; Puckelwartz, M.J.; et al. Identification of clinical drivers of left atrial enlargement through genomics of left atrial size. Circ. Heart Fail. 2024, 17, e010557. [Google Scholar] [CrossRef] [PubMed]
- Donahue, J.G.; Weiss, S.T.; Livingston, J.M.; Goetsch, M.A.; Greineder, D.K.; Platt, R. Inhaled steroids and the risk of hospitalization for asthma. JAMA 1997, 277, 887–891. [Google Scholar] [CrossRef] [PubMed]
- Boushey, H.A.; Sorkness, C.A.; King, T.S.; Sullivan, S.D.; Fahy, J.V.; Lazarus, S.C.; Chinchilli, V.M.; Craig, T.J.; Dimango, E.A.; Deykin, A.; et al. Daily versus as-needed corticosteroids for mild persistent asthma. N. Engl. J. Med. 2005, 352, 1519–1528. [Google Scholar] [CrossRef]
- Sorkness, C.A.; Lemanske, R.F.; Mauger, D.T.; Boehmer, S.J.; Chinchilli, V.M.; Martinez, F.D.; Strunk, R.C.; Szefler, S.J.; Zeiger, R.S.; Bacharier, L.B.; et al. Long-term comparison of 3 controller regimens for mild-moderate persistent childhood asthma: The Pediatric Asthma Controller Trial. J. Allergy Clin. Immunol. 2007, 119, 64–72. [Google Scholar] [CrossRef]
- Purcell, S.; Neale, B.; Todd-Brown, K.; Thomas, L.; Ferreira, M.A.R.; Bender, D.; Maller, J.; Sklar, P.; de Bakker, P.I.W.; Daly, M.J.; et al. PLINK: A tool set for whole-genome association and population-based linkage analyses. Am. J. Hum. Genet. 2007, 81, 559–575. [Google Scholar] [CrossRef] [PubMed]
- Delaneau, O.; Coulonges, C.; Zagury, J.-F. Shape-IT: New rapid and accurate algorithm for haplotype inference. BMC Bioinform. 2008, 9, 540. [Google Scholar] [CrossRef] [PubMed]
- Kvale, M.N.; Hesselson, S.; Hoffmann, T.J.; Cao, Y.; Chan, D.; Connell, S.; Croen, L.A.; Dispensa, B.P.; Eshragh, J.; Finn, A.; et al. Genotyping informatics and quality control for 100,000 subjects in the genetic epidemiology research on adult health and aging (GERA) cohort. Genetics 2015, 200, 1051–1060. [Google Scholar] [CrossRef] [PubMed]
- Howie, B.N.; Donnelly, P.; Marchini, J. A flexible and accurate genotype imputation method for the next generation of genome-wide association studies. PLoS Genet. 2009, 5, e1000529. [Google Scholar] [CrossRef] [PubMed]
- Tibshirani, R. Regression shrinkage and selection via the lasso. J. R. Stat. Soc. Ser. B 1996, 58, 267–288. [Google Scholar] [CrossRef]
- Breiman, L. Random forests. Mach. Learn. 2001, 45, 5–32. [Google Scholar] [CrossRef]
- DeLong, E.R.; DeLong, D.M.; Clarke, D.L. Comparing the Areas under Two or More Correlated Receiver Operating Characteristic Curves: A Nonparametric Approach. Biometrics 1988, 44, 837–845. [Google Scholar] [CrossRef]
- Leek, J.T.; Scharpf, R.B.; Bravo, H.C.; Simcha, D.; Langmead, B.; Johnson, W.E.; Geman, D.; Baggerly, K.; Irizarry, R.A. Tackling the widespread and critical impact of batch effects in high-throughput data. Nat. Rev. Genet. 2010, 11, 733–739. [Google Scholar] [CrossRef]
- Mostafa, M.M.; Rider, C.F.; Shah, S.; Traves, S.L.; Gordon, P.M.K.; Miller-Larsson, A.; Leigh, R.; Newton, R. Glucocorticoid-driven transcriptomes in human airway epithelial cells: Commonalities, differences and functional insight from cell lines and primary cells. BMC Med. Genom. 2019, 12, 29. [Google Scholar] [CrossRef]
- Wang, G.; Baines, K.J.; Fu, J.J.; Wood, L.G.; Simpson, J.L.; McDonald, V.M.; Cowan, D.C.; Taylor, D.R.; Cowan, J.O.; Gibson, P.G. Sputum mast cell subtypes relate to eosinophilia and corticosteroid response in asthma. Eur. Respir. J. 2016, 47, 1123–1133. [Google Scholar] [CrossRef] [PubMed]
- Singhania, A.; Wallington, J.C.; Smith, C.G.; Horowitz, D.; Staples, K.J.; Howarth, P.H.; Gadola, S.D.; Djukanović, R.; Woelk, C.H.; Hinks, T.S.C. Multitissue transcriptomics delineates the diversity of airway T cell functions in asthma. Am. J. Respir. Cell Mol. Biol. 2018, 58, 261–270. [Google Scholar] [CrossRef] [PubMed]
- Gaertner, V.D.; Michel, S.; Curtin, J.A.; Pulkkinen, V.; Acevedo, N.; Söderhäll, C.; von Berg, A.; Bufe, A.; Laub, O.; Rietschel, E.; et al. Nocturnal asthma is affected by genetic interactions between RORA and NPSR1. Pediatr. Pulmonol. 2019, 54, 847–857. [Google Scholar] [CrossRef] [PubMed]
- Gwinn, W.M.; Damsker, J.M.; Falahati, R.; Okwumabua, I.; Kelly-Welch, A.; Keegan, A.D.; Vanpouille, C.; Lee, J.J.; Dent, L.A.; Leitenberg, D.; et al. Novel approach to inhibit asthma-mediated lung inflammation using anti-CD147 intervention. J. Immunol. 2006, 177, 4870–4879. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Mao, Z.-D.; Shi, Y.-J.; Qian, Y.; Liu, Z.-G.; Yin, X.-W.; Zhang, Q. Comprehensive analysis of miRNA-mRNA-lncRNA networks in severe asthma. Epigenomics 2019, 11, 115–131. [Google Scholar] [CrossRef] [PubMed]
- Kasaian, M.T.; Lee, J.; Brennan, A.; Danto, S.I.; Black, K.E.; Fitz, L.; Dixon, A.E. Proteomic analysis of serum and sputum analytes distinguishes controlled and poorly controlled asthmatics. Clin. Exp. Allergy 2018, 48, 814–824. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Zhou, X.-D.; Xu, R.; Du, X.-Z.; Li, Q.; Li, B.; Zhang, G.-Y.; Chen, L.-X.; Perelman, J.M.; Kolosov, V.P. The Degradation of Airway Epithelial Tight Junctions in Asthma Under High Airway Pressure Is Probably Mediated by Piezo-1. Front. Physiol. 2021, 12, 637790. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, H.; Azuma, M.; Tsunematsu, T.; Morimoto, Y.; Kondo, M.; Tezuka, T.; Nishioka, Y.; Tsuneyama, K. Neutrophils induce smooth muscle hyperplasia via neutrophil elastase-induced FGF-2 in a mouse model of asthma with mixed inflammation. Clin. Exp. Allergy 2018, 48, 1715–1725. [Google Scholar] [CrossRef]
- James, R.G.; Reeves, S.R.; Barrow, K.A.; White, M.P.; Glukhova, V.A.; Haghighi, C.; Seyoum, D.; Debley, J.S. Deficient Follistatin-like 3 Secretion by Asthmatic Airway Epithelium Impairs Fibroblast Regulation and Fibroblast-to-Myofibroblast Transition. Am. J. Respir. Cell Mol. Biol. 2018, 59, 104–113. [Google Scholar] [CrossRef]
- Rastogi, D.; Nico, J.; Johnston, A.D.; Tobias, T.A.M.; Jorge, Y.; Macian, F.; Greally, J.M. CDC42-related genes are upregulated in helper T cells from obese asthmatic children. J. Allergy Clin. Immunol. 2018, 141, 539–548.e7. [Google Scholar] [CrossRef] [PubMed]
- Sasanuma, H.; Ozawa, M.; Yoshida, N. RNA-binding protein Ptbp1 is essential for BCR-mediated antibody production. Int. Immunol. 2019, 31, 157–166. [Google Scholar] [CrossRef] [PubMed]
- Geng, G.; Xu, C.; Peng, N.; Li, Y.; Liu, J.; Wu, J.; Liang, J.; Zhu, Y.; Shi, L. PTBP1 is necessary for dendritic cells to regulate T-cell homeostasis and antitumour immunity. Immunology 2021, 163, 74–85. [Google Scholar] [CrossRef]
- Figueroa-Lozano, S.; Ren, C.; Yin, H.; Pham, H.; van Leeuwen, S.; Dijkhuizen, L.; de Vos, P. The impact of oligosaccharide content, glycosidic linkages and lactose content of galacto-oligosaccharides (GOS) on the expression of mucus-related genes in goblet cells. Food Funct. 2020, 11, 3506–3515. [Google Scholar] [CrossRef]
- Brigelius-Flohé, R.; Flohé, L. Regulatory phenomena in the glutathione peroxidase superfamily. Antioxid. Redox Signal. 2020, 33, 498–516. [Google Scholar] [CrossRef] [PubMed]
- Jahreis, S.; Trump, S.; Bauer, M.; Bauer, T.; Thürmann, L.; Feltens, R.; Wang, Q.; Gu, L.; Grützmann, K.; Röder, S.; et al. Maternal phthalate exposure promotes allergic airway inflammation over 2 generations through epigenetic modifications. J. Allergy Clin. Immunol. 2018, 141, 741–753. [Google Scholar] [CrossRef]
- Ferreira, M.A.R.; Mathur, R.; Vonk, J.M.; Szwajda, A.; Brumpton, B.; Granell, R.; Brew, B.K.; Ullemar, V.; Lu, Y.; Jiang, Y.; et al. Genetic Architectures of Childhood- and Adult-Onset Asthma Are Partly Distinct. Am. J. Hum. Genet. 2019, 104, 665–684. [Google Scholar] [CrossRef]
- Abbas, A.R.; Jackman, J.K.; Bullens, S.L.; Davis, S.M.; Choy, D.F.; Fedorowicz, G.; Tan, M.; Truong, B.-T.; Meng, Y.G.; Diehl, L.; et al. Lung gene expression in a rhesus allergic asthma model correlates with physiologic parameters of disease and exhibits common and distinct pathways with human asthma and a mouse asthma model. Am. J. Pathol. 2011, 179, 1667–1680. [Google Scholar] [CrossRef]
- Kosoff, R.; Chow, H.Y.; Radu, M.; Chernoff, J. Pak2 kinase restrains mast cell FcϵRI receptor signaling through modulation of Rho protein guanine nucleotide exchange factor (GEF) activity. J. Bio. Chem. 2013, 288, 974–983. [Google Scholar] [CrossRef]
- Chhabra, D.; Sharma, S.; Kho, A.T.; Gaedigk, R.; Vyhlidal, C.A.; Leeder, J.S.; Morrow, J.; Carey, V.J.; Weiss, S.T.; Tantisira, K.G.; et al. Fetal lung and placental methylation is associated with in utero nicotine exposure. Epigenetics 2014, 9, 1473–1484. [Google Scholar] [CrossRef]
- Cohen, R.T.; Raby, B.A.; Van Steen, K.; Fuhlbrigge, A.L.; Celedón, J.C.; Rosner, B.A.; Strunk, R.C.; Zeiger, R.S.; Weiss, S.T.; Childhood Asthma Management Program Research Group. In utero smoke exposure and impaired response to inhaled corticosteroids in children with asthma. J. Allergy Clin. Immunol. 2010, 126, 491–497. [Google Scholar] [CrossRef] [PubMed]
- O’Beirne, S.L.; Shenoy, S.A.; Salit, J.; Strulovici-Barel, Y.; Kaner, R.J.; Visvanathan, S.; Fine, J.S.; Mezey, J.G.; Crystal, R.G. Ambient Pollution-related Reprogramming of the Human Small Airway Epithelial Transcriptome. Am. J. Respir. Crit. Care Med. 2018, 198, 1413–1422. [Google Scholar] [CrossRef] [PubMed]
- Melbourne, C.A.; Erzurumluoglu, A.M.; Shrine, N.; Chen, J.; Tobin, M.D.; Hansell, A.; Wain, L.V. Genome-wide gene-air pollution interaction analysis of lung function in 300,000 individuals. Environ. Int. 2022, 159, 107041. [Google Scholar] [CrossRef] [PubMed]
- Sayols-Baixeras, S.; Fernández-Sanlés, A.; Prats-Uribe, A.; Subirana, I.; Plusquin, M.; Künzli, N.; Marrugat, J.; Basagaña, X.; Elosua, R. Association between long-term air pollution exposure and DNA methylation: The REGICOR study. Environ. Res. 2019, 176, 108550. [Google Scholar] [CrossRef]
- Gruzieva, O.; Xu, C.-J.; Yousefi, P.; Relton, C.; Merid, S.K.; Breton, C.V.; Gao, L.; Volk, H.E.; Feinberg, J.I.; Ladd-Acosta, C.; et al. Prenatal Particulate Air Pollution and DNA Methylation in Newborns: An Epigenome-Wide Meta-Analysis. Environ. Health Perspect. 2019, 127, 57012. [Google Scholar] [CrossRef]
- Pickett, G.; Seagrave, J.; Boggs, S.; Polzin, G.; Richter, P.; Tesfaigzi, Y. Effects of 10 cigarette smoke condensates on primary human airway epithelial cells by comparative gene and cytokine expression studies. Toxicol. Sci. 2010, 114, 79–89. [Google Scholar] [CrossRef]

| Variable | Training Cohort (n = 823) | Test Cohort (n = 548) |
|---|---|---|
| Sex | ||
| Female, n (%) | 500 (60.1) | 331 (60.4) |
| Male, n (%) | 323 (39.2) | 217 (39.6) |
| Age, years, mean (SD) | 25.5 (13.0) | 25.9 (13.1) |
| BMI, kg/m2, mean (SD) | 26.8 (8.3) | 26.2 (7.5) |
| Exacerbation while on ICS, n (%) | 323 (39.2) | 199 (36.3) |
| Performance Measure | LASSO Regression Model | Random Forest Model | ||
|---|---|---|---|---|
| Training Cohort (n = 823) | Test Cohort (n = 548) | Training Cohort (n = 818) | Test Cohort (n = 546) | |
| Models including SNPs only | ||||
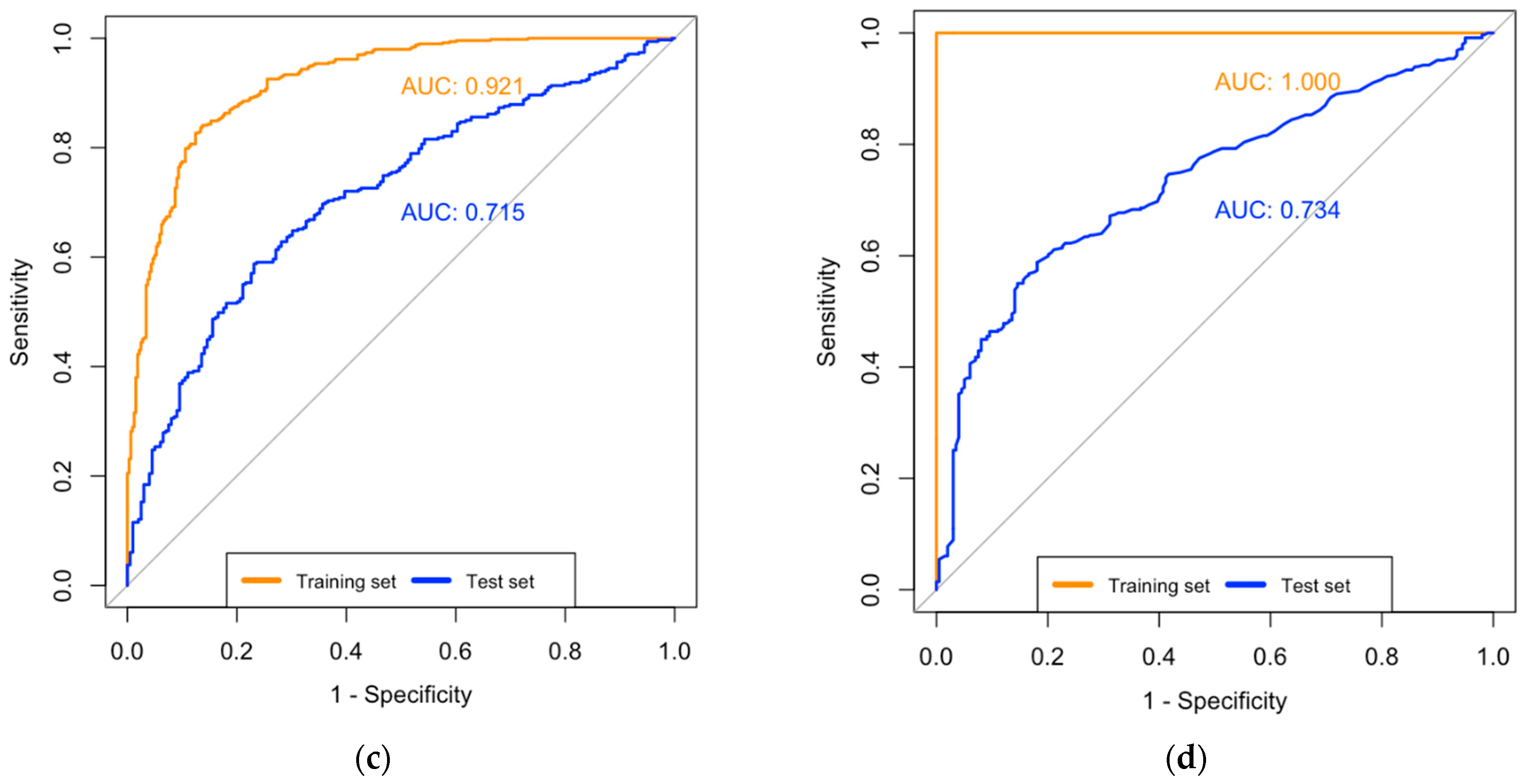
| AUC | 0.94 (0.93–0.96) | 0.71 (0.67–0.76) | 1.00 (1.00–1.00) | 0.74 (0.70–0.78) |
| Sensitivity | 0.87 | 0.57 | 1.00 | 0.70 |
| Specificity | 0.86 | 0.75 | 1.00 | 0.68 |
| Models including SNPs and phenotype data | ||||
| AUC | 0.92 (0.90–0.94) | 0.71 (0.67–0.76) * | 1.00 (1.00–1.00) | 0.73 (0.69–0.78) ^ |
| Sensitivity | 0.87 | 0.59 | 1.00 | 0.69 |
| Specificity | 0.83 | 0.72 | 1.00 | 0.63 |
| Chr:Postion | Rs Number | Nearest Gene | Reference Allele | Effect Allele | Effect Allele Frequency | Variable Importance * |
|---|---|---|---|---|---|---|
| (a) | ||||||
| 21:20856221 | rs4818452 | RPL37P4/TMPRSS15 | G | C | 0.44 | 100 |
| 10:37228865 | rs1852484 | ANKRD30A | G | A | 0.27 | 91.9 |
| 3:196520050 | rs79390411 | PAK2 | C | T | 0.13 | 76.8 |
| 4:77473026 | rs114847105 | SHROOM3 | T | A | 0.28 | 62.1 |
| 13:69770987 | rs9541819 | KLHL1 | G | A | 0.07 | 61.8 |
| 8:51961588 | rs10093174 | PXDNL | G | T | 0.07 | 54.4 |
| 4:111218700 | rs75800589 | ZBED1P1 | G | A | 0.14 | 51.4 |
| 19:339675 | rs878685 | MIER2 | C | T | 0.39 | 49.9 |
| 11:6718704 | rs1466977 | MRPL17 | T | G | 0.29 | 48.7 |
| 2:215660643 | rs6747962 | BARD1 | C | A | 0.16 | 47.9 |
| 4:118248606 | rs75774008 | TRAM1L1 | T | G | 0.05 | 47.2 |
| 19:654968 | rs4594371 | RNF126 | G | A | 0.05 | 46.5 |
| 16:88731011 | rs752843 | RNF166 | A | G | 0.14 | 42.4 |
| 9:139502019 | rs55892012 | EGFL7 | G | A | 0.10 | 42.1 |
| 7:334719 | rs36177169 | C | T | 0.09 | 39.1 | |
| 17:18584142 | rs116808485 | ZNF286B | A | G | 0.08 | 38.1 |
| 16:88826073 | rs2278053 | PIEZO1 | G | C | 0.31 | 35.1 |
| 16:88555879 | rs34319485 | ZFPM1 | G | A | 0.25 | 35.1 |
| 16:870711 | rs2382764 | PRR25 | T | C | 0.07 | 34.3 |
| 15:20587599 | rs1846765 | GOLGA6L6 | G | C | 0.12 | 33.1 |
| 19:2012477 | rs4405674 | BTBD2 | T | G | 0.36 | 29.1 |
| 22:17164773 | rs361799 | TPTEP1 | C | T | 0.05 | 28.9 |
| 1:1065296 | rs4072537 | C1orf159 | T | C | 0.25 | 28.4 |
| 16:1194047 | rs4288998 | CACNA1H | A | G | 0.22 | 26.6 |
| 9:140304779 | rs9414736 | EXD3 | A | G | 0.27 | 26.6 |
| 8:51478714 | rs17709272 | SNTG1 | G | T | 0.37 | 26.2 |
| 17:80214198 | rs12601586 | CSNK1D | A | G | 0.19 | 26.0 |
| (b) | ||||||
| 19:1086211 | rs1061233 | HMHA1 | G | A | 0.31 | 100 |
| 21:20856221 | rs4818452 | RPL37P4/TMPRSS15 | G | C | 0.44 | 97.7 |
| 19:780209 | rs7343137 | PTBP1 | T | C | 0.38 | 95.3 |
| 1:1097291 | rs61768478 | MIR200B | C | A | 0.17 | 95.2 |
| 19:840090 | rs351109 | PRTN3 | T | C | 0.34 | 94.5 |
| 19:710050 | rs8109226 | PALM | T | G | 0.22 | 90.6 |
| 16:1184532 | rs34056718 | CACNA1H | C | T | 0.39 | 90.3 |
| 19:1773999 | rs4807140 | ONECUT3 | C | T | 0.33 | 88.0 |
| 1:1053385 | rs4970408 | C1orf159 | C | T | 0.38 | 85.9 |
| 11:6718704 | rs1466977 | MRPL17 | T | G | 0.29 | 85.7 |
| 8:144987934 | rs6999129 | MIR661/EPPK1 | A | T | 0.37 | 85.4 |
| 10:37228865 | rs1852484 | ANKRD30A | G | A | 0.27 | 84.9 |
| 19:1723463 | rs10413694 | ONECUT3 | A | G | 0.38 | 84.1 |
| 16:798229 | rs8050465 | NARFL | G | A | 0.34 | 84.0 |
| 8:51478714 | rs17709272 | SNTG1 | G | T | 0.37 | 83.1 |
| 15:20303075 | rs76044586 | T | C | 0.19 | 83.4 | |
| 19:702286 | rs8106722 | PALM | G | C | 0.24 | 83.3 |
| 1:949608 | rs1921 | ISG15 | G | A | 0.29 | 81.6 |
| 19:1063930 | rs4807499 | ABCA7 | C | T | 0.26 | 80.6 |
| 19:646891 | rs10403235 | FGF22 | G | A | 0.28 | 80.5 |
| 19:539279 | rs2288956 | CDC34 | C | T | 0.19 | 80.1 |
| 16:877334 | rs28541981 | PRR25 | C | T | 0.34 | 79.7 |
| 19:1766737 | rs12978813 | ONECUT3 | C | A | 0.26 | 79.6 |
| 16:32603025 | rs28887512 | A | G | 0.41 | 79.2 | |
| 3:196520050 | rs79390411 | PAK2 | C | T | 0.13 | 79.2 |
| 19:2012477 | rs4405674 | BTBD2 | T | G | 0.36 | 79.2 |
| 9:140304779 | rs9414736 | EXD3 | A | G | 0.27 | 78.9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ong, M.-S.; Sordillo, J.E.; Dahlin, A.; McGeachie, M.; Tantisira, K.; Wang, A.L.; Lasky-Su, J.; Brilliant, M.; Kitchner, T.; Roden, D.M.; et al. Machine Learning Prediction of Treatment Response to Inhaled Corticosteroids in Asthma. J. Pers. Med. 2024, 14, 246. https://doi.org/10.3390/jpm14030246
Ong M-S, Sordillo JE, Dahlin A, McGeachie M, Tantisira K, Wang AL, Lasky-Su J, Brilliant M, Kitchner T, Roden DM, et al. Machine Learning Prediction of Treatment Response to Inhaled Corticosteroids in Asthma. Journal of Personalized Medicine. 2024; 14(3):246. https://doi.org/10.3390/jpm14030246
Chicago/Turabian StyleOng, Mei-Sing, Joanne E. Sordillo, Amber Dahlin, Michael McGeachie, Kelan Tantisira, Alberta L. Wang, Jessica Lasky-Su, Murray Brilliant, Terrie Kitchner, Dan M. Roden, and et al. 2024. "Machine Learning Prediction of Treatment Response to Inhaled Corticosteroids in Asthma" Journal of Personalized Medicine 14, no. 3: 246. https://doi.org/10.3390/jpm14030246
APA StyleOng, M.-S., Sordillo, J. E., Dahlin, A., McGeachie, M., Tantisira, K., Wang, A. L., Lasky-Su, J., Brilliant, M., Kitchner, T., Roden, D. M., Weiss, S. T., & Wu, A. C. (2024). Machine Learning Prediction of Treatment Response to Inhaled Corticosteroids in Asthma. Journal of Personalized Medicine, 14(3), 246. https://doi.org/10.3390/jpm14030246










