Efficacity and Safety of the Fluocinolone Acetonide Implant in Uveitic Macular Edema: A Real-Life Study from the French Uveitis Network
Abstract
1. Introduction
2. Methods
3. Results
3.1. Patients’ Characteristics at Baseline
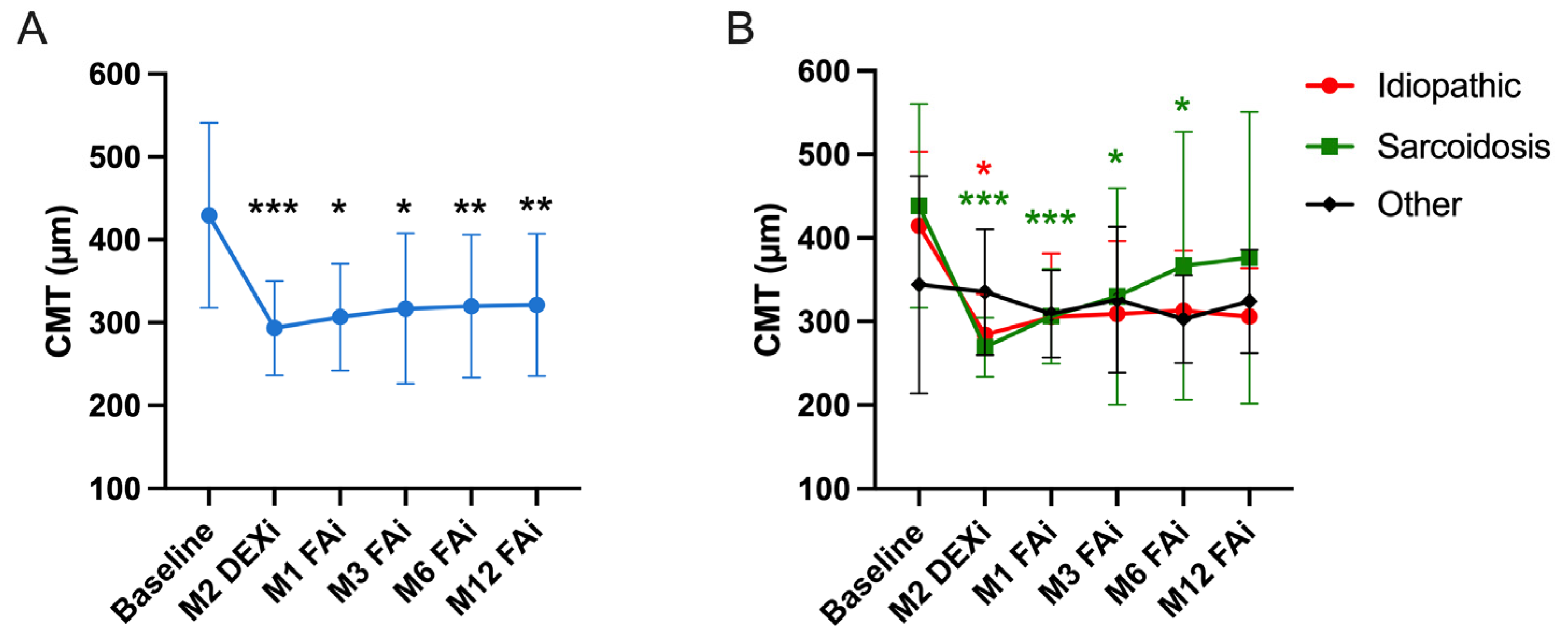
3.2. Functional and Anatomical Outcomes of FAi
3.3. Systemic Treatments
3.4. Exploratory Predictive Factors of Functional and Anatomical Response to FAi
3.5. Predictive Factors of Rescue DEXi Injections
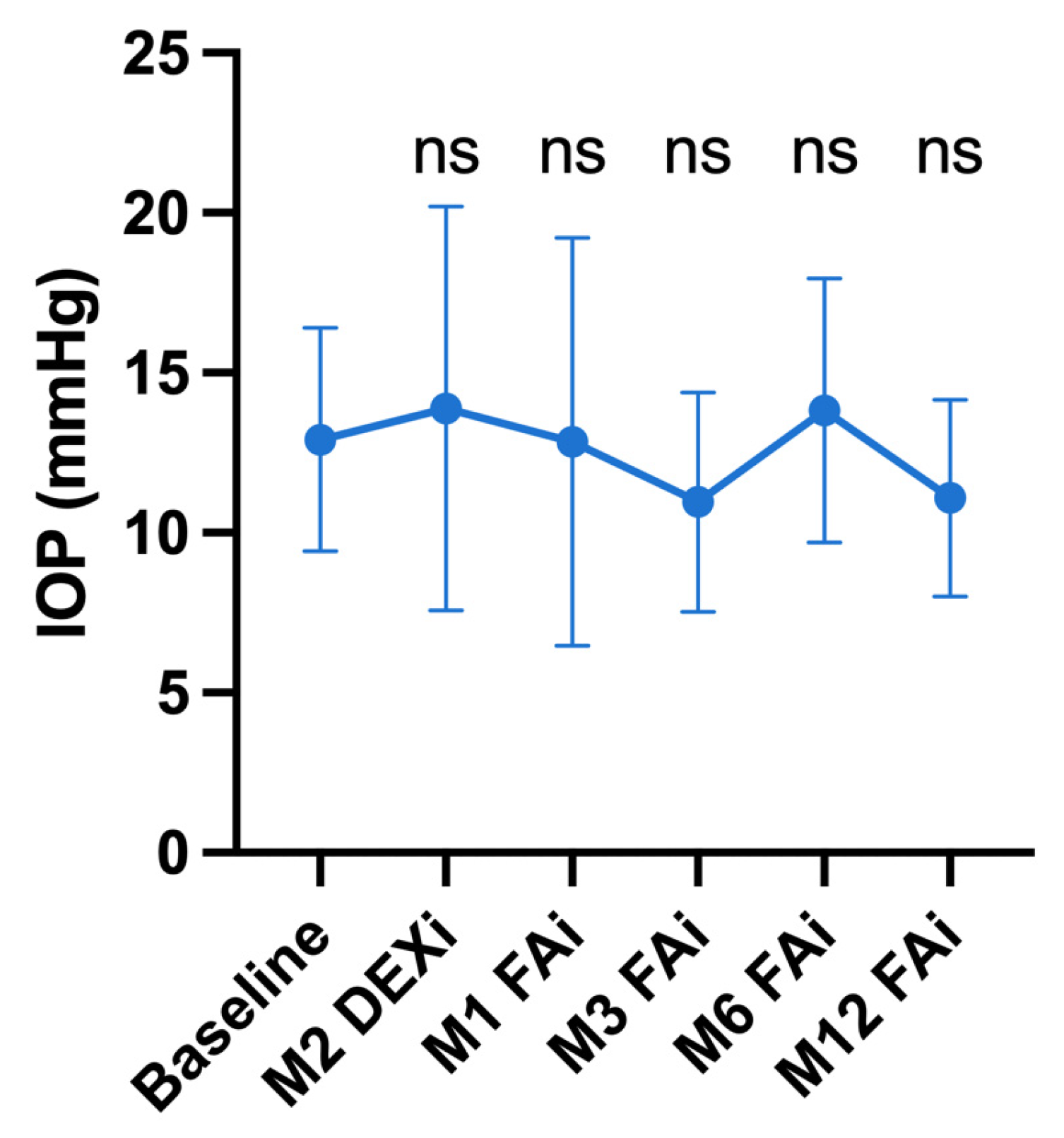
3.6. Adverse Events
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lardenoye, C.W.; van Kooij, B.; Rothova, A. Impact of Macular Edema on Visual Acuity in Uveitis. Ophthalmology 2006, 113, 1446–1449. [Google Scholar] [CrossRef]
- Thurau, S.R. Zystoides Makulaödem bei Uveitis. Der Ophthalmol. 2005, 102, 485–490. [Google Scholar] [CrossRef]
- Jabs, D.A.; Rosenbaum, J.T.; Foster, C.S.; Holland, G.N.; Jaffe, G.J.; Louie, J.S.; Nussenblatt, R.B.; Stiehm, E.R.; Tessler, H.; Van Gelder, R.N.V.; et al. Guidelines for the use of immunosuppressive drugs in patients with ocular inflammatory disorders: Recommendations of an expert panel. Am. J. Ophthalmol. 2000, 130, 492–513. [Google Scholar] [CrossRef]
- Paire, V.; Lebreton, O.; Weber, M. Efficacité de l’interféron alpha dans le traitement des œdèmes maculaires uvéitiques réfractaires au traitement corticoïde et/ou immunosupresseur. J. Fr. Ophtalmol. 2010, 33, 152–162. [Google Scholar] [CrossRef]
- Vegas-Revenga, N.; Calvo-Río, V.; Mesquida, M.; Adán, A.; Hernández, M.V.; Beltrán, E.; Pascual, E.V.; Díaz-Valle, D.; Díaz-Cordovés, G.; Hernandez-Garfella, M.; et al. Anti-IL6-Receptor Tocilizumab in Refractory and Noninfectious Uveitic Cystoid Macular Edema: Multicenter Study of 25 Patients. Am. J. Ophthalmol. 2019, 200, 85–94. [Google Scholar] [CrossRef]
- Multicenter Uveitis Steroid Treatment (MUST) Trial Research Group; Kempen, J.H.; Altaweel, M.M.; Drye, L.T.; Holbrook, J.T.; Jabs, D.A.; Sugar, E.A.; Thorne, J.E. Benefits of Systemic Anti-inflammatory Therapy versus Fluocinolone Acetonide Intraocular Implant for Intermediate Uveitis, Posterior Uveitis, and Panuveitis. Ophthalmology 2015, 122, 1967–1975. [Google Scholar] [CrossRef]
- Boyer, D.S.; Yoon, Y.H.; Belfort, R.; Bandello, F.; Maturi, R.K.; Augustin, A.J.; Li, X.-Y.; Cui, H.; Hashad, Y.; Whitcup, S.M. Three-Year, Randomized, Sham-Controlled Trial of Dexamethasone Intravitreal Implant in Patients with Diabetic Macular Edema. Ophthalmology 2014, 121, 1904–1914. [Google Scholar] [CrossRef]
- Garweg, J.G.; Zandi, S. Retinal vein occlusion and the use of a dexamethasone intravitreal implant (Ozurdex®) in its treatment. Graefe’s Arch. Clin. Exp. Ophthalmol. 2016, 254, 1257–1265. [Google Scholar] [CrossRef] [PubMed]
- Khurana, R.N.; Porco, T.C. Efficacy and safety of dexamethasone intravitreal implant for persistent uveitic cystoid macular edema. Retina 2015, 35, 1640–1646. [Google Scholar] [CrossRef] [PubMed]
- Lowder, C.; Belfort, R., Jr.; Lightman, S.; Foster, C.S.; Robinson, M.R.; Schiffman, R.M.; Li, X.-Y.; Cui, H.; Whitcup, S.M.; Ozurdex HURON Study Group. Dexamethasone Intravitreal Implant for Noninfectious Intermediate or Posterior Uveitis. Arch. Ophthalmol. 2011, 129, 545–553. [Google Scholar] [CrossRef] [PubMed]
- Mathis, T.; Cerquaglia, A.; Weber, M.; Guillarme-Sallit, R.; Malclès, A.; Voirin, N.; Servant, M.; Sudhalkar, A.; Bilgic, A.; Denis, P.; et al. Uveitis treated with dexamethasone implant. Retina 2021, 41, 620–629. [Google Scholar] [CrossRef] [PubMed]
- Tomkins-Netzer, O.; Taylor, S.R.; Bar, A.; Lula, A.; Yaganti, S.; Talat, L.; Lightman, S. Treatment with Repeat Dexamethasone Implants Results in Long-Term Disease Control in Eyes with Noninfectious Uveitis. Ophthalmology 2014, 121, 1649–1654. [Google Scholar] [CrossRef] [PubMed]
- Tsang, A.C.; Virgili, G.; Abtahi, M.; Gottlieb, C.C. Intravitreal Dexamethasone Implant for the Treatment of Macular Edema in Chronic Non-infectious Uveitis. Ocul. Immunol. Inflamm. 2017, 25, 690–697. [Google Scholar] [CrossRef] [PubMed]
- Pohlmann, D.; Brocke, G.A.V.; Winterhalter, S.; Steurer, T.; Thees, S.; Pleyer, U. Dexamethasone Inserts in Noninfectious Uveitis. Ophthalmology 2018, 125, 1088–1099. [Google Scholar] [CrossRef]
- Kang, E.Y.-C.; Garg, S.J.; Chen, H.-F.; Wu, W.-C.; Chen, L.Y.-H.; Chou, H.-D.; Liu, L.; Chen, K.-J.; Hwang, Y.-S. Intravitreal Dexamethasone Implants for Refractory Macular Edema in Eyes with Noninfectious Uveitis. J. Clin. Med. 2021, 10, 3762. [Google Scholar] [CrossRef]
- Jaffe, G.J.; Yang, C.H.; Guo, H.; Denny, J.P.; Lima, C.; Ashton, P. Safety and Pharmacokinetics of an Intraocular Fluocinolone Acetonide Sustained Delivery Device. Investig. Ophthalmol. Vis. Sci. 2000, 41, 3569–3575. [Google Scholar]
- Lim, L.L.; Smith, J.R.; Rosenbaum, J.T. Retisert (Bausch & Lomb/Control Delivery Systems). Curr. Opin. Investig. Drugs 2005, 6, 1159–1167. [Google Scholar]
- Jaffe, G.J.; Foster, C.S.; Pavesio, C.E.; Paggiarino, D.A.; Riedel, G.E. Effect of an Injectable Fluocinolone Acetonide Insert on Recurrence Rates in Chronic Noninfectious Uveitis Affecting the Posterior Segment. Ophthalmology 2019, 126, 601–610. [Google Scholar] [CrossRef]
- Jaffe, G.J.; Martin, D.; Callanan, D.; Pearson, P.A.; Levy, B.; Comstock, T. Fluocinolone Acetonide Implant (Retisert) for Noninfectious Posterior Uveitis: Thirty-Four–Week Results of a Multicenter Randomized Clinical Study. Ophthalmology 2006, 113, 1020–1027. [Google Scholar] [CrossRef] [PubMed]
- Jaffe, G.J.; Pavesio, C.E. Effect of a Fluocinolone Acetonide Insert on Recurrence Rates in Noninfectious Intermediate, Posterior, or Panuveitis. Ophthalmology 2020, 127, 1395–1404. [Google Scholar] [CrossRef] [PubMed]
- Jabs, D.A.; Nussenblatt, R.B.; Rosenbaum, J.T. Standardization of Uveitis Nomenclature for Reporting Clinical Data. Results of the First International Workshop. Am. J. Ophthalmol. 2005, 140, 509–516. [Google Scholar] [CrossRef]
- Pockar, S.; Leal, I.; Chhabra, R.; Jones, N.P.; Steeples, L.R. Intravitreal Fluocinolone 0.19mg Implant in the Management of Chronic Non-Infectious Uveitis: 12-Month Outcomes from a Single Tertiary Centre. Ocul. Immunol. Inflamm. 2023, 31, 1572–1578. [Google Scholar] [CrossRef] [PubMed]
- Buhl, L.; Thurau, S.; Kern, C. Fluocinolone acetonide 0.19-mg implant for the treatment of noninfectious uveitis with involvement of the posterior segment: A real-world study. Graefe’s Arch. Clin. Exp. Ophthalmol. 2023, 261, 1101–1108. [Google Scholar] [CrossRef]
- Kessler, L.J.; Auffarth, G.U.; Khoramnia, R. Functional and Morphological Responses to Fluocinolone Acetonide 0.19 mg in Noninfectious Uveitic Macular Edema Evaluated as the Area-Under-the-Curve. J. Ocul. Pharmacol. Ther. 2023, 39, 449–455. [Google Scholar] [CrossRef]
- Buhl, L.; Schmelter, V.; Schworm, B.; Thurau, S.; Kern, C. Long-Term Results of 0.19 mg Fluocinolone Acetonide Insert for Treatment of Non-Infectious Uveitis in Clinical Practice. Ocul. Immunol. Inflamm. 2023, 1–5. [Google Scholar] [CrossRef]
- Roth, D.B.; Eichenbaum, D.; Malik, D.; Radcliffe, N.M.; Cutino, A.; Small, K.W.; Abdelsalam, A.; Shakoor, A.; Moshiri, A.; Barkmeier, A.; et al. The 0.19-mg Fluocinolone Acetonide Intravitreal Implant for Diabetic Macular Edema. Ophthalmol. Retin. 2023, 8, 49–54. [Google Scholar] [CrossRef] [PubMed]
- Baillif, S.; Staccini, P.; Weber, M.; Delyfer, M.-N.; Le Mer, Y.; Gualino, V.; Collot, L.; Merite, P.-Y.; Creuzot-Garcher, C.; Kodjikian, L.; et al. Management of Patients with Diabetic Macular Edema Switched from Dexamethasone Intravitreal Implant to Fluocinolone Acetonide Intravitreal Implant. Pharmaceutics 2022, 14, 2391. [Google Scholar] [CrossRef]
- Kodjikian, L.; Bandello, F.; de Smet, M.; Dot, C.; Zarranz-Ventura, J.; Loewenstein, A.; Sudhalkar, A.; Bilgic, A.; Cunha-Vaz, J.; Dirven, W.; et al. Fluocinolone acetonide implant in diabetic macular edema: International experts’ panel consensus guidelines and treatment algorithm. Eur. J. Ophthalmol. 2022, 32, 1890–1899. [Google Scholar] [CrossRef] [PubMed]
- Moll-Udina, A.; Hernanz, I.; Sainz-De-La-Maza, M.; Pelegrín, L.; Coelho-Borges, A.I.; Pazos, M.; Adán, A.; Llorenç, V. Intravitreal fluocinolone acetonide 0.19 mg (ILUVIEN®) in patients with non-infectious uveitis: Real-world effectiveness and safety outcomes at 12 months. Int. Ophthalmol. 2023, 43, 4181–4195. [Google Scholar] [CrossRef] [PubMed]
- Acharya, N.R.; Vitale, A.T.; Sugar, E.A.; Holbrook, J.T.; Burke, A.E.; Thorne, J.E.; Altaweel, M.M.; Kempen, J.H.; Jabs, D.A. Intravitreal Therapy for Uveitic Macular Edema—Ranibizumab versus Methotrexate versus the Dexamethasone Implant. Ophthalmology 2023, 130, 914–923. [Google Scholar] [CrossRef]
- Yeh, S.; Nussenblatt, R.B. Fluocinolone Acetonide for the Treatment of Uveitis: Weighing the Balance Between Local and Systemic Immuno-suppression. Arch. Ophthalmol. 2008, 126, 1287–1289. [Google Scholar] [CrossRef] [PubMed]
- Kessler, L.J.; Łabuz, G.; Auffarth, G.U.; Khoramnia, R. Biomarkers to Predict the Success of Treatment with the Intravitreal 0.19 mg Fluocinolone Acetonide Implant in Uveitic Macular Edema. Pharmaceutics 2022, 14, 688. [Google Scholar] [CrossRef] [PubMed]
- Cicinelli, M.V.; Rosenblatt, A.; Grosso, D.; Zollet, P.; Capone, L.; Rabiolo, A.; Lattanzio, R.; Loewenstein, A.; Bandello, F.; Nassisi, M.; et al. The outcome of fluocinolone acetonide intravitreal implant is predicted by the response to dexamethasone implant in diabetic macular oedema. Eye 2021, 35, 3232–3242. [Google Scholar] [CrossRef]
- Grewal, D.S.; O’Sullivan, M.L.; Kron, M.; Jaffe, G.J. Association of Disorganization of Retinal Inner Layers With Visual Acuity In Eyes With Uveitic Cystoid Macular Edema. Am. J. Ophthalmol. 2017, 177, 116–125. [Google Scholar] [CrossRef]
- Zur, D.; Iglicki, M.; Sala-Puigdollers, A.; Chhablani, J.; Lupidi, M.; Fraser-Bell, S.; Mendes, T.S.; Chaikitmongkol, V.; Cebeci, Z.; Dollberg, D.; et al. Disorganization of retinal inner layers as a biomarker in patients with diabetic macular oedema treated with dexamethasone implant. Acta Ophthalmol. 2020, 98, E217–E223. [Google Scholar] [CrossRef] [PubMed]

| n = 26 Eyes (22 Patients) | n (%) or Mean ± SD (Range) |
|---|---|
| Gender (females), n (%) | 16 (73.1%) |
| Mean age (years) | 60.4 ± 15.8 (31–87) |
| Mean follow-up duration (months) | 11.4 ± 2.0 (3–12) |
| Uveitis type, n (%) | |
| Anterior Intermediate Posterior Panuveitis | 0 (0%) 3 (11.5%) 9 (34.6%) 14 (53.8%) |
| Etiology of uveitis, n (%) | |
| Idiopathic Sarcoidosis Vogt–Koyanagi–Harada Autoimmune HLA-B27+ Immune restauration syndrome | 15 (57.7%) 5 (19.2%) 2 (7.7%) 2 (7.7%) 1 (3.8%) 1 (3.8%) |
| Baseline signs of inflammation, n (%) | |
| Anterior chamber cells Vitreous haze Vasculitis Optic nerve swelling Macular edema Serous retinal detachment (SRD) Cystoid spaces Hyper-reflective foci (HRF) Disorganization of the inner retinal layers (DRIL) | 11 (42.3%) 17 (65.4%) 10 (38.5%) 10 (38.5%) 26 (100%) 10 (38.5%) 19 (73.1%) 14 (53.8%) 8 (30.8%) |
| Lens status before FAi, n (%) | |
| Pseudophakic | 25 (96.2%) |
| Number of antiglaucoma drops before FAi, n (%) | |
| 0 1 2 3 4 | 20 (76.9%) 3 (11.5%) 3 (11.5%) 0 (0%) 0 (0%) |
| Number of DEXi per eye before FAi, n (%) | |
| Not mentioned 2–5 5–10 10–20 >20 Mean number (per eye) | 2 (7.7%) 7 (26.9%) 11 (42.3%) 5 (19.2%) 1 (3.8%) 8.5 ± 6.1 (2–33) |
| Use of systemic treatments before FAi, n (%) Corticosteroids only Immunosuppressive treatment only Corticosteroids + immunosuppressive treatment | 13 (59.1%) 3 (13.6%) 5 (22.7%) 5 (22.7%) |
| Baseline | After Latest DEXi | After FAi | ||||
|---|---|---|---|---|---|---|
| M2 | M1 | M3 | M6 | M12 | ||
| n (%) or Mean ± SD (Range) | ||||||
| Eyes | 26 | 26 | 24 | 24 | 23 | 24 |
| BCVA (LogMAR), Mean ± SD (range) | 0.43 ± 0.36 (1.40–0) | 0.37 ± 0.44 (1.40–0) | 0.32 ± 0.45 (1.70–0.08) | 0.21 ± 0.27 (1.10–0.08) | 0.18 ± 0.23 (1–0.08) | 0.27 ± 0.35 (1.30–0.08) |
| Central macular thickness (µm), Mean ± SD (range) | 429 ± 112 (275–617) | 293 ± 56 (216–439) | 307 ± 65 (226–483) | 317 ± 91 (218–544) | 320 ± 86 (223–591) | 321 ± 85 (217–626) |
| Intraocular pressure (mmHg), Mean ± SD (range) | 12.9 ± 3.5 (8–21) | 13.9 ± 6.3 (5–25) | 12.9 ± 6.4 (5–33) | 11.0 ± 3.4 (5–21) | 13.8 ± 4.1 (7–26) | 11.1 ± 3.1 (7–19) |
| Glaucoma treatment, n (%) | ||||||
| None Monotherapy Dual therapy Triple therapy Quadruple therapy Filtering surgery | 20 (71.4%) 3 (11.5%) 3 (11.5%) 0 (0%) 0 (0%) 0 (0%) | 15 (57.7%) 4 (15.4%) 5 (19.2%) 0 (0%) 0 (0%) 2 (7.7%) | 15 (62.5%) 3 (12.5%) 6 (25.0%) 0 (0%) 0 (0%) 0 (0%) | 13 (54.2%) 2 (8.3%) 7 (29.2%) 0 (0%) 2 (8.3%) 0 (0%) | 12 (52.2%) 2 (8.7%) 7 (30.4%) 0 (0%) 2 (8.7%) 0 (0%) | 13 (54.2%) 2 (8.3%) 7 (29.2%) 0 (0%) 2 (8.3%) 0 (0%) |
| Anterior chamber cells (+) a, Mean ± SD (range) | 0.8 ± 1.2 (0–3) | 0.0 ± 0.0 (0–0) | 0.0 ± 0.0 (0–0) | 0.08 ± 0.3 (0–1) | 0.0 ± 0.0 (0–0) | 0.2 ± 0.6 (0–2) |
| Anterior chamber cells, N (%) | 11 (42.3%) | 1 (3.8%) | 0 (0%) | 1 (4.2%) | 0 (0%) | 0 (0%) |
| Vitreous Haze (+) a, Mean ± SD (range) | 1.11 ± 0.80 (0–3) | 0.14 ± 0.40 (0–1) | 0.11 ± 0.20 (0–0.5) | 0.04 ± 0.10 (0–0.5) | 0.0 ± 0.0 (0–0) | 0.0 ± 0.0 (0–0) |
| Vitritis, n (%) | 17 (65.4%) | 4 (15.4%) | 2 (8.3%) | 1 (4.2%) | 0 (0%) | 0 (0%) |
| Optic nerve swelling, n (%) | 10 (38.5%) | 4 (15.4%) | 1 (4.2%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Retinal vasculitis, n (%) | 10 (38.5%) | 1 (3.8%) | 1 (4.2%) | 1 (4.2%) | 0 (0%) | 0 (0%) |
| Dexamethasone rescue injection, n (%) | / | / | 0 (0%) | 2 (8.3%) | 4 (17.4%) | 3 (12.5%) |
| FAi reinjection, n (%) | / | / | 0 (0%) | 0 (0%) | 0 (0%) | 1 (4.2%) |
| Baseline | After Latest DEXi | After FAi | ||||
|---|---|---|---|---|---|---|
| M2 | M1 | M3 | M6 | M12 | ||
| n (%) or Mean ± SD (Range) | ||||||
| Eyes | 26 | 26 | 24 | 24 | 23 | 24 |
| Presence of macular edema, n (%) HRF SRD Cystoid spaces DRIL | 26 (100%) 14 (53.8%) 10 (38.5%) 19 (73.1%) 8 (30.8%) | 9 (34.6%) 6 (23.1%) 0 (0%) 9 (34.6%) 3 (11.5%) | 4 (16.7%) 1 (4.1%) 1 (4.1%) 3 (12.5%) 0 (0%) | 4 (16.7%) 2 (8.3%) 1 (4.1%) 3 (12.5%) 2 (8.3%) | 4 (17.4%) 2 (8.7%) 1 (4.3%) 4 (17.4%) 2 (8.7%) | 7 (29.2%) 5 (20.8%) 1 (4.1%) 3 (12.5%) 4 (16.6%) |
| Complete resolution of UME, n (%) | / | 17 (65.4%) | 20 (83.3%) | 20 (83.3%) | 19 (82.6%) | 17 (70.8%) |
| Incomplete resolution of UME, n (%) | / | 2 (7.6%) | 2 (8.3%) | 1 (4.1%) | 1 (4.3%) | 2 (8.3%) |
| Absence of CMT decrease, n (%) | 3 (11.5%) | 0 (0%) | 2 (8.3%) | 2 (8.7%) | 4 (16.6%) | |
| Baseline | After Latest DEXi | After FAi | ||||
|---|---|---|---|---|---|---|
| M1 | M3 | M6 | M12 | |||
| n (%) or Mean ± SD (Range) | ||||||
| Patients | 22 | 22 | 20 | 20 | 19 | 20 |
| Associated systemic treatment, n (%) Corticosteroids (CS) only Immunosuppressive (IS) therapy only CS + IS | 13 (59.1%) 3 (13.6%) 5 (22.7%) 5 (22.7%) | 11 (50.0%) 3 (13.6%) 6 (27.2%) 2 (9.1%) | 10 (50.0%) 5 (25.0%) 4 (20.0%) 1 (5.0%) | 10 (50.0%) 5 (25.0%) 4 (20.0%) 1 (5.0%) | 10 (52.6%) 5 (26.3%) 4 (21.1%) 1 (5.3%) | 9 (45.0%) 2 (10.0%) 4 (20.0%) 3 (15.0%) |
| CS dose (milligrams) among treated, Mean ± SD (range) | 12.4 ± 5.6 (5–20) | 10.6 ± 5.3 (5–20) | 9.5 ± 5.4 (4–20) | 8.9 ± 5.0 (4–20) | 8.8 ± 5.1 (3–20) | 8.3 ± 6.2 (3–20) |
| CS dose, p-value vs. baseline | Ref | 0.50 | 0.19 | 0.19 | 0.19 | 0.37 |
| Anti TNF, n (%) | 4 (18.2%) | 5 (22.7%) | 3 (15.0%) | 3 (15.0%) | 3 (15.8%) | 5 (25.0%) |
| Interferon, n (%) | 1 (4.5%) | 1 (4.5%) | 1 (5.0%) | 1 (5.0%) | 1 (5.3%) | 1 (5.0%) |
| Methotrexate, n (%) | 3 (13.6%) | 3 (13.6%) | 3 (15.0%) | 3 (15.0%) | 3 (15.8%) | 3 (15.0%) |
| Hydroxychloroquine, n (%) | 1 (4.5%) | 1 (4.5%) | 1 (5.0%) | 1 (5.0%) | 1 (5.3%) | 1 (5.0%) |
| Tocilizumab, n (%) | 2 (9.1%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Best Result during Follow-up | 12 Month Follow-up | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean BCVA Gain (LogMAR) | p | Mean CMT Decrease (µm) | p | Complete Anatomical Response n (%) | p | Mean BCVA Gain (LogMAR) | p | Mean CMT Decrease (µm) | p | Complete Anatomical Response n (%) | p | |
| Age | ||||||||||||
| -<60 years ->60 years | 0.27 0.16 | Ref 0.47 | 110 178 | Ref 0.08 | 12 (100%) 12 (86%) | Ref 0.48 | 0.17 0.20 | Ref 0.25 | 61 154 | Ref 0.20 | 10 (83.3%) 7 (58.3%) | Ref 0.37 |
| Gender | ||||||||||||
| -Female -Male | 0.27 0.16 | Ref 0.48 | 110 178 | Ref 0.25 | 18 (94.7%) 6 (85.7%) | Ref 0.47 | 0.23 0.07 | Ref 0.23 | 77 191 | Ref 0.14 | 12 (66.7%) 5 (83.3%) | Ref 0.63 |
| Etiology of uveitis | ||||||||||||
| -Idiopathic -Sarcoidosis -Others | 0.20 0.17 0.35 | Ref 0.66 0.65 | 143 140 96 | Ref 0.88 0.40 | 15 (100%) 4 (80.0%) 5 (83.3%) | Ref 0.25 0.29 | 0.13 0.17 0.33 | Ref 0.83 0.04 | 128 92 81 | Ref 0.86 0.34 | 12 (80.0%) 3 (75.0%) 2 (40%) | Ref 1 0.13 |
| Type of uveitis | ||||||||||||
| -Posterior -Intermediate -Panuveitis | 0.36 0.15 0.17 | Ref 0.71 0.24 | 11 115 129 | Ref 0.48 0.90 | 8 (88.9%) 3 (100%) 13 (92.9%) | Ref 1 1 | 0.31 0.07 0.09 | Ref 0.60 0.26 | 100 223 96 | Ref 0.33 0.74 | 6 (66.7%) 2 (100%) 9 (69.2%) | Ref 1 1 |
| Number of previous DEXi | ||||||||||||
| -1–5 -6–10 ->10 | 0.22 0.24 0.32 | Ref 0.81 0.55 | 53 193 58 | Ref 0.07 1 | 5 (83.3%) 10 (90.9%) 6 (100%) | Ref 1 1 | 0.22 0.16 0.24 | Ref 1 0.97 | −13 188 46 | Ref 0.03 0.69 | 3 (60%) 6 (60%) 5 (83.3) | Ref 1 0.42 |
| Complete anatomic response to DEXi | ||||||||||||
| -No -Yes | 0.34 0.19 | Ref 0.51 | 90 89 | Ref 0.82 | 4 (66.7%) 15 (100%) | Ref 0.07 | 0.36 0.11 | Ref 0.45 | 24 71 | Ref 0.55 | 1 (25.0%) 11 (73.3%) | Ref 0.12 |
| Partial anatomic response to DEXi | ||||||||||||
| -No -Yes | 0.44 0.16 | Ref 0.53 | 102 65 | Ref 1 | 2 (50.0%) 2 (100%) | Ref 0.47 | 0.45 0.08 | Ref 0.80 | 16 49 | Ref 1 | 0 (0%) 1 (100%) | Ref 0.25 |
| Vitritis grade a: | ||||||||||||
| -<1+ -≥1+ | 0.28 0.22 | Ref 0.38 | 145 119 | Ref 0.53 | 13 (100.0%) 11 (84.6%) | Ref 0.48 | 0.21 0.17 | Ref 0.61 | 122 98 | Ref 0.54 | 9 (69.2%) 8 (72.7%) | Ref 1 |
| Posterior inflammation other than UME | ||||||||||||
| -No -Yes | 0.28 0.23 | Ref 0.51 | 172 115 | Ref 0.36 | 9 (100.0%) 15 (88.2%) | Ref 0.53 | 0.23 0.17 | Ref 0.41 | 147 95 | Ref 0.48 | 6 (66.7%) 11 (73.3%) | Ref 1 |
| Associated systemic treatment at the initial visit: | ||||||||||||
| -None -CS only -IS rx only -CS + IS rx | 0.40 0.10 0.18 0.16 | Ref 0.14 0.71 0.35 | 145 172 107 121 | Ref 1 0.67 0.67 | 5 (71.4%) 3 (100%) 5 (100%) 7 (100%) | Ref 1 0.47 1 | 0.38 0.09 0.14 0.06 | Ref 0.31 0.86 0.52 | 83 171 101 101 | Ref 0.55 0.91 0.91 | 4 (57.1%) 3 (100%) 3 (75.0%) 5 (71.4%) | Ref 0.29 1 1 |
| Presence of biomarkers HRF | ||||||||||||
| -None -Presence SRD -None -Presence DRIL -None -Presence | 0.11 0.33 0.35 0.07 0.16 0.32 | Ref 0.17 Ref 0.03 Ref 0.15 | 79 163 121 143 152 160 | Ref 0.09 Ref 0.75 Ref 0.95 | 11 (91.7%) 13 (92.3%) 15 (93.7%) 9 (90.0%) 13 (92.9%) 7 (87.5%) | Ref 1 Ref 1 Ref 1 | 0.04 0.28 0.28 0.01 0.12 0.25 | Ref 0.26 Ref 0.05 Ref 0.16 | 43 151 100 126 139 138 | Ref 0.07 Ref 0.87 Ref 0.92 | 8 (72.7%) 9 (69.2%) 10 (62.5%) 7 (87.5%) 10 (83.3%) 5 (62.5%) | Ref 1 Ref 0.35 Ref 0.35 |
| Eyes without Additional DEXi n = 20 | Eyes with at Least One Rescue DEXi n = 6 | p-Value | |
|---|---|---|---|
| Age | |||
| -<60 years ->60 years | 10 (83.3%) 10 (71.4%) | 2 (16.7%) 4 (28.6%) | Ref 0.65 |
| Gender | |||
| -Female -Male | 16 (84.2%) 4 (57.1%) | 3 (15.8%) 3 (42.9%) | Ref 0.29 |
| Etiology of uveitis | |||
| -Idiopathic -Sarcoidosis -Others | 12 (80.0%) 5 (100%) 3 (50.0%) | 3 (20.0%) 0 (0%) 3 (50.0%) | Ref 0.54 0.29 |
| Type of uveitis | |||
| -Posterior -Intermediate -Panuveitis | 5 (55.6%) 3 (100%) 12 (85.7%) | 4 (44.4%) 0 (0%) 2 (14.3%) | Ref 0.49 0.16 |
| Number of previous DEXi | |||
| -1–5 -6–10 ->10 -No data | 6 (85.7%) 7 (63.4%) 5 (83.3%) 2 (100%) | 1 (16.7%) 4 (36.4%) 1 (16.7%) 0 (0%) | Ref 0.60 1 - |
| Complete anatomic response to DEXi | |||
| -No -Yes -No data | 5 (83.3%) 10 (66.7%) 5 (100%) | 1 (16.7%) 5 (33.3%) 0 (0%) | Ref 0.62 - |
| Partial anatomic response to DEXi | |||
| -No * -Yes ** | 3 (75.0%) 2 (100%) | 1 (25.0%) 0 (0.0%) | Ref 1 |
| Associated systemic treatment at Baseline | |||
| -None -Corticosteroids only -Immunosuppressive rx only -Corticosteroids + immunosuppressive rx -No data | 5 (55.6%) 3 (100%) 6 (100%) 4 (66.7%) 2 (100%) | 4 (44.4%) 0 (0%) 0 (0%) 2 (33.3%) 0 (0%) | Ref 0.49 0.10 0.49 - |
| Presence of biomarkers at baseline | |||
| HRF -None -Presence SDR -None -Presence DRIL -None -Presence -No data | 11 (91.7%) 9 (64.3%) 11 (68.7%) 9 (90.0%) 14 (100%) 2 (25.0%) 4 (100%) | 1 (8.33%) 5 (35.7%) 5 (31.3%) 1 (10.0%) 0 (0%) 6 (75.0%) 0 (0%) | Ref 0.17 Ref 0.35 Ref <0.001 - |
| Baseline | After Latest DEXi | After FAi | ||||
|---|---|---|---|---|---|---|
| M2 | M1 | M3 | M6 | M12 | ||
| n (%) or Mean ± SD (Range) | ||||||
| Eyes | 26 | 26 | 24 | 24 | 23 | 24 |
| Intraocular pressure (mmHg), Mean ± SD (range) | 12.9 ± 3.5 (8–21) | 13.9 ± 6.3 (5–25) | 12.9 ± 6.4 (5–33) | 11.0 ± 3.4 (5–21) | 13.8 ± 4.1 (7–26) | 11.1 ± 3.1 (7–19) |
| p-value vs. baseline | Ref | 0.42 | 0.81 | 0.08 | 0.06 | 0.08 |
| Eyes with IOP > 21 mmHg, n (%) | 0 (0%) | 3 (11.5%) | 2 (8.3%) | 0 (0%) | 1 (4.3%) | 0 (0%) |
| p-value vs. baseline | Ref | 0.62 | 0.62 | 1 | 1 | 1 |
| Eyes with IOP > 30 mmHg, n (%) | 0 (0%) | 1 (3.8%) | 1 (4.2%) | 0 (0%) | 0 (0%) | 0 (0%) |
| p-value vs. baseline | Ref | 1 | 0.50 | 1 | 1 | 1 |
| Glaucoma treatment, n (%) | ||||||
| No treatment Monotherapy Dual therapy Triple therapy Quadruple therapy Filtering surgery | 20 (71.4%) 3 (11.5%) 3 (11.5%) 0 (0%) 0 (0%) 0 (0%) | 15 (57.7%) 4 (15.4%) 5 (19.2%) 0 (0%) 0 (0%) 2 (7.7%) | 15 (62.5%) 3 (12.5%) 6 (25.0%) 0 (0%) 0 (0%) 0 (0%) | 13 (54.2%) 2 (8.3%) 7 (29.2%) 0 (0%) 2 (8.3%) 0 (0%) | 12 (52.2%) 2 (8.7%) 7 (30.4%) 0 (0%) 2 (8.7%) 0 (0%) | 13 (54.2%) 2 (8.3%) 7 (29.2%) 0 (0%) 2 (8.3%) 0 (0%) |
| n of anti-glaucoma drops, Mean ± SD (range) | 0.35 ± 0.70 | 0.50 ± 0.81 | 0.35 ± 0.75 | 0.92 ± 1.30 | 0.88 ± 1.30 | 0.88 ± 1.30 |
| p-value vs. baseline | Ref | 0.73 | 0.98 | 0.05 | 0.08 | 0.08 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jabbour, M.; Kodjikian, L.; Bourdin, A.; Rougier, M.-B.; Serrar, Y.; Weber, M.; Massé, H.; Mazhar, D.; Perez-Roustit, S.; Chiquet, C.; et al. Efficacity and Safety of the Fluocinolone Acetonide Implant in Uveitic Macular Edema: A Real-Life Study from the French Uveitis Network. J. Pers. Med. 2024, 14, 245. https://doi.org/10.3390/jpm14030245
Jabbour M, Kodjikian L, Bourdin A, Rougier M-B, Serrar Y, Weber M, Massé H, Mazhar D, Perez-Roustit S, Chiquet C, et al. Efficacity and Safety of the Fluocinolone Acetonide Implant in Uveitic Macular Edema: A Real-Life Study from the French Uveitis Network. Journal of Personalized Medicine. 2024; 14(3):245. https://doi.org/10.3390/jpm14030245
Chicago/Turabian StyleJabbour, Matthieu, Laurent Kodjikian, Alexandre Bourdin, Marie-Bénédicte Rougier, Yasmine Serrar, Michel Weber, Hélène Massé, Driss Mazhar, Sara Perez-Roustit, Christophe Chiquet, and et al. 2024. "Efficacity and Safety of the Fluocinolone Acetonide Implant in Uveitic Macular Edema: A Real-Life Study from the French Uveitis Network" Journal of Personalized Medicine 14, no. 3: 245. https://doi.org/10.3390/jpm14030245
APA StyleJabbour, M., Kodjikian, L., Bourdin, A., Rougier, M.-B., Serrar, Y., Weber, M., Massé, H., Mazhar, D., Perez-Roustit, S., Chiquet, C., Delyfer, M. N., Bodaghi, B., & Touhami, S. (2024). Efficacity and Safety of the Fluocinolone Acetonide Implant in Uveitic Macular Edema: A Real-Life Study from the French Uveitis Network. Journal of Personalized Medicine, 14(3), 245. https://doi.org/10.3390/jpm14030245








