Stroke after Cardiac Surgery: A Risk Factor Analysis of 580,117 Patients from UK National Adult Cardiac Surgical Audit Cohort
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Statistical Analysis
3. Results
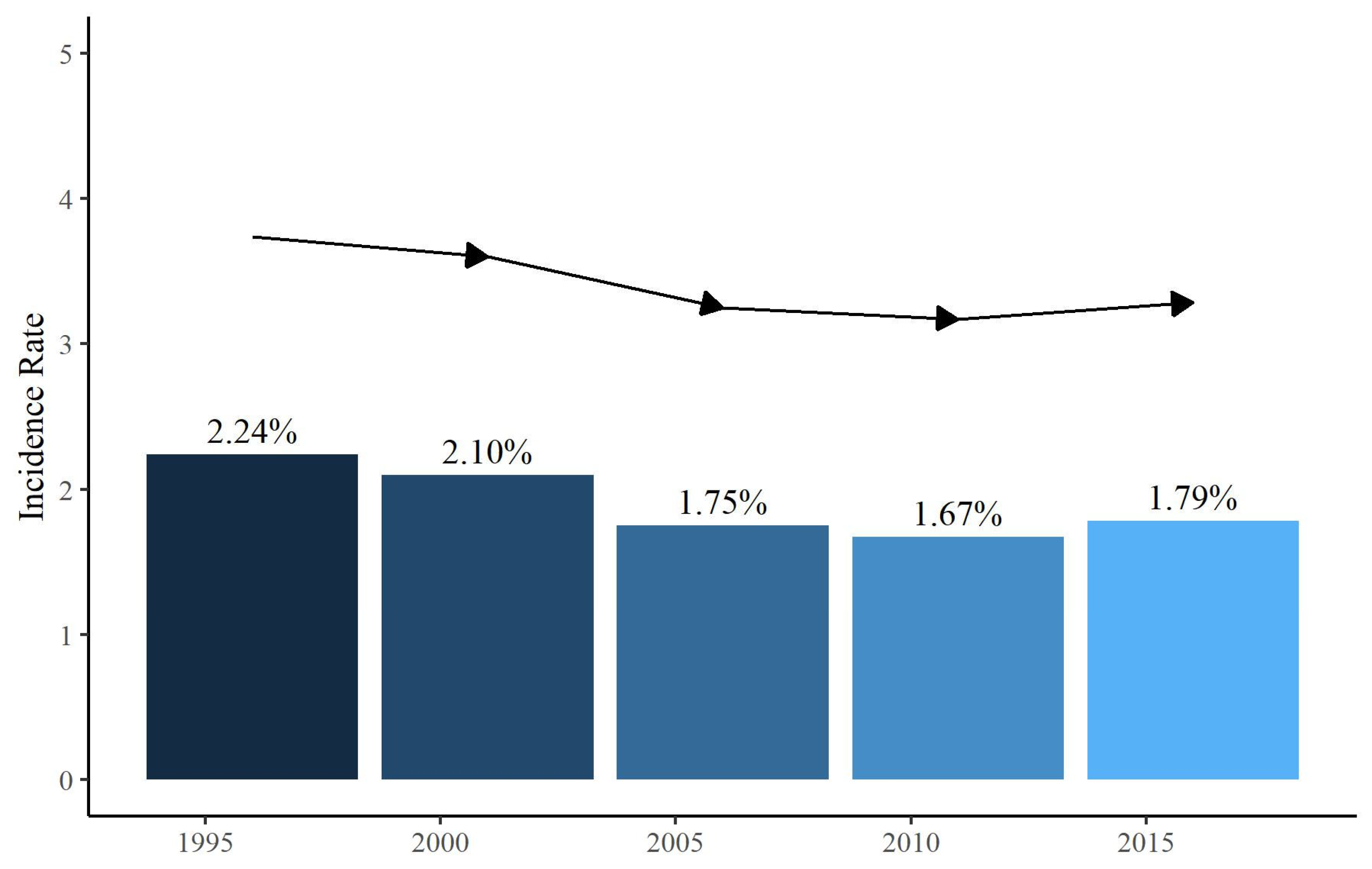
3.1. Patient Characteristics
3.2. Risk Factors
3.3. Type of Procedure
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mashour, G.A.; Moore, L.E.; Lele, A.V.; Robicsek, S.A.; Gelb, A.W. Perioperative care of patients at high risk for stroke during or after non-cardiac, non-neurologic surgery: Consensus statement from the Society for Neuroscience in Anesthesiology and Critical Care. J. Neurosurg. Anesthesiol. 2014, 26, 273–285. [Google Scholar] [CrossRef]
- Vlisides, P.; Mashour, G.A. Perioperative stroke. Can. J. Anaesth. 2016, 63, 193–204. [Google Scholar] [CrossRef] [PubMed]
- Redžek, A.; Mironicki, M.; Gvozdenović, A.; Petrović, M.; Čemerlić-Ađić, N.; Ilić, A.; Velicki, L. Predictors for hospital readmission after cardiac surgery. J. Card. Surg. 2015, 30, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Alrifai, N.; Alhuneafat, L.; AlRobaidi, K.; Al Ghazawi, S.S.; Thirumala, P.D. Perioperative Stroke and Thirty-Day Hospital Readmission After Cardiac Surgeries: State Inpatient Database Study. J. Clin. Med. Res. 2022, 14, 34–44. [Google Scholar] [CrossRef] [PubMed]
- Bucerius, J.; Gummert, J.F.; Borger, M.A.; Walther, T.; Doll, N.; Onnasch, J.F.; Metz, S.; Falk, V.; Mohr, F.W. Stroke after cardiac surgery: A risk factor analysis of 16,184 consecutive adult patients. Ann. Thorac. Surg. 2003, 75, 472–478. [Google Scholar] [CrossRef] [PubMed]
- Benesch, C.; Glance, L.G.; Derdeyn, C.P.; Fleisher, L.A.; Holloway, R.G.; Messé, S.R.; Mijalski, C.; Nelson, M.T.; Power, M.; Welch, B.G.; et al. Perioperative Neurological Evaluation and Management to Lower the Risk of Acute Stroke in Patients Undergoing Noncardiac, Nonneurological Surgery: A Scientific Statement From the American Heart Association/American Stroke Association. Circulation 2021, 143, e923–e946. [Google Scholar] [CrossRef] [PubMed]
- Carrascal, Y.; Guerrero, A.L.; Blanco, M.; Valenzuela, H.; Pareja, P.; Laguna, G. Postoperative stroke related to cardiac surgery in octogenarians. Interact. Cardiovasc. Thorac. Surg. 2014, 18, 596–601. [Google Scholar] [CrossRef] [PubMed]
- Adult Cardiac Surgery (Surgery Audit). Available online: https://www.nicor.org.uk/adult-cardiac-surgery-surgery-audit/ (accessed on 15 January 2023).
- DeLong, E.R.; DeLong, D.M.; Clarke-Pearson, D.L. Comparing the areas under two or more correlated receiver operating characteristic curves: A nonparametric approach. Biometrics 1988, 44, 837–845. [Google Scholar] [CrossRef]
- McKhann, G.M.; Goldsborough, M.A.; Borowicz, L.M., Jr.; Mellits, E.D.; Brookmeyer, R.; Quaskey, S.A.; Baumgartner, W.A.; Cameron, D.E.; Stuart, R.S.; Gardner, T.J. Predictors of stroke risk in coronary artery bypass patients. Ann. Thorac. Surg. 1997, 63, 516–521. [Google Scholar] [CrossRef]
- Floyd, T.F.; Shah, P.N.; Price, C.C.; Harris, F.; Ratcliffe, S.J.; Acker, M.A.; Bavaria, J.E.; Rahmouni, H.; Kuersten, B.; Wiegers, S.; et al. Clinically silent cerebral ischemic events after cardiac surgery: Their incidence, regional vascular occurrence, and procedural dependence. Ann. Thorac. Surg. 2006, 81, 2160–2166. [Google Scholar] [CrossRef]
- Bhatti, F.; Bridgewater, B.; Dalrymple-Hay, M.; Jenkins, D.; Keogh, B.; Rathinam, S.; Venn, G.E. Sixth National Adult Cardiac Surgical Database Report; Dendrite Clinical Systems Ltd.: Henley-on-Thames, UK, 2008. [Google Scholar]
- Jonsson, K.; Barbu, M.; Nielsen, S.J.; Hafsteinsdottir, B.; Gudbjartsson, T.; Jensen, E.M.; Silverborn, M.; Jeppsson, A. Perioperative stroke and survival in coronary artery bypass grafting patients: A SWEDEHEART study. Eur. J. Cardiothorac. Surg. 2022, 62, ezac025. [Google Scholar] [CrossRef]
- Dabrowski, W.; Rzecki, Z.; Pilat, J.; Czajkowski, M. Brain damage in cardiac surgery patients. Curr. Opin. Pharmacol. 2012, 12, 189–194. [Google Scholar] [CrossRef] [PubMed]
- Messé, S.R.; Acker, M.A.; Kasner, S.E.; Fanning, M.; Giovannetti, T.; Ratcliffe, S.J.; Bilello, M.; Szeto, W.Y.; Bavaria, J.E.; Hargrove, W.C., 3rd; et al. Stroke after aortic valve surgery: Results from a prospective cohort. Circulation 2014, 129, 2253–2261. [Google Scholar] [CrossRef] [PubMed]
- Waksman, R.; Minha, S. Stroke after aortic valve replacement: The known and unknown. Circulation 2014, 129, 2245–2247. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.F.; Edelman, J.J.; Seco, M.; Bannon, P.G.; Wilson, M.K.; Byrom, M.J.; Thourani, V.; Lamy, A.; Taggart, D.P.; Puskas, J.D.; et al. Coronary Artery Bypass Grafting With and Without Manipulation of the Ascending Aorta: A Network Meta-Analysis. J. Am. Coll. Cardiol. 2017, 69, 924–936. [Google Scholar] [CrossRef] [PubMed]
- Rodés-Cabau, J.; Kahlert, P.; Neumann, F.J.; Schymik, G.; Webb, J.G.; Amarenco, P.; Brott, T.; Garami, Z.; Gerosa, G.; Lefèvre, T.; et al. Feasibility and exploratory efficacy evaluation of the Embrella Embolic Deflector system for the prevention of cerebral emboli in patients undergoing transcatheter aortic valve replacement: The PROTAVI-C pilot study. JACC Cardiovasc. Interv. 2014, 7, 1146–1155. [Google Scholar] [CrossRef] [PubMed]
- Bozhinovska, M.; Jenko, M.; Stupica, G.T.; Klokočovnik, T.; Kšela, J.; Jelenc, M.; Podbregar, M.; Fabjan, A.; Šoštarič, M. Cerebral microemboli in mini-sternotomy compared to mini- thoracotomy for aortic valve replacement: A cross sectional cohort study. J. Cardiothorac. Surg. 2021, 16, 142. [Google Scholar] [CrossRef]
- Letsou, G.V.; Musfee, F.I.; Zhang, Q.; Loor, G.; Lee, A.D. Stroke and mortality rates after off-pump vs. pump-assisted/no-clamp coronary artery bypass grafting. J. Cardiovasc. Surg. 2022, 63, 742–748. [Google Scholar]
- Abu-Omar, Y.; Balacumaraswami, L.; Pigott, D.W.; Matthews, P.M.; Taggart, D.P. Solid and gaseous cerebral microembolization during off-pump, on-pump, and open cardiac surgery procedures. J. Thorac. Cardiovasc. Surg. 2004, 127, 1759–1765. [Google Scholar] [CrossRef]
- Pires, S.L.; Gagliardi, R.J.; Gorzoni, M.L. Study of the main risk factors frequencies for ischemic cerebrovascular disease in elderly patients. Arq. Neuropsiquiatr. 2004, 62, 844–851. [Google Scholar] [CrossRef]
- Thomas, Q.; Crespy, V.; Duloquin, G.; Ndiaye, M.; Sauvant, M.; Béjot, Y.; Giroud, M. Stroke in women: When gender matters. Rev. Neurol. 2021, 177, 881–889. [Google Scholar] [CrossRef] [PubMed]
- Alloubani, A.; Saleh, A.; Abdelhafiz, I. Hypertension and diabetes mellitus as a predictive risk factors for stroke. Diabetes Metab. Syndr. 2018, 12, 577–584. [Google Scholar] [CrossRef] [PubMed]
- Costa, M.A.; Gauer, M.F.; Gomes, R.Z.; Schafranski, M.D. Risk factors for perioperative ischemic stroke in cardiac surgery. Rev. Bras. Cir. Cardiovasc. 2015, 30, 365–372. [Google Scholar] [CrossRef] [PubMed]
- Freudenberger, R.S.; Hellkamp, A.S.; Halperin, J.L.; Poole, J.; Anderson, J.; Johnson, G.; Mark, D.B.; Lee, K.L.; Bardy, G.H.; SCD-HeFT Investigators. Risk of thromboembolism in heart failure: An analysis from the Sudden Cardiac Death in Heart Failure Trail (SCD-HeFT). Circulation 2007, 115, 2637–2641. [Google Scholar] [CrossRef] [PubMed]
- Aljaber, N.N.; Mattash, Z.A.; Alshoabi, S.A.; Alhazmi, F.H. The prevalence of left ventricular thrombus among patients with low ejection fraction by trans-thoracic echocardiography. Pak. J. Med. Sci. 2020, 36, 673–677. [Google Scholar] [CrossRef]
- Hirotani, T.; Kameda, T.; Kumamoto, T.; Shirota, S.; Yamano, M. Stroke after coronary artery bypass grafting in patients with cerebrovascular disease. Ann. Thorac. Surg. 2000, 70, 1571–1576. [Google Scholar] [CrossRef]
- Raffa, G.M.; Agnello, F.; Occhipinti, G.; Miraglia, R.; Lo Re, V.; Marrone, G.; Tuzzolino, F.; Arcadipane, A.; Pilato, M.; Luca, A. Neurological complications after cardiac surgery: A retrospective case-control study of risk factors and outcome. J. Cardiothorac. Surg. 2019, 14, 23. [Google Scholar] [CrossRef]
- Yang, E.; Spragg, D.; Schulman, S.; Gilotra, N.A.; Kilic, A.; Salenger, R.; Whitman, G.; Metkus, T.S. Rate Versus Rhythm Control in Heart Failure Patients with Post-Operative Atrial Fibrillation After Cardiac Surgery. J. Card. Fail. 2021, 27, 915–919. [Google Scholar] [CrossRef]
- Hindricks, G.; Potpara, T.; Dagres, N.; Arbelo, E.; Bax, J.J.; Blomström-Lundqvist, C.; Boriani, G.; Castella, M.; Dan, G.A.; Dilaveris, P.E.; et al. 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS): The Task Force for the diagnosis and management of atrial fibrillation of the Europea. Eur. Heart J. 2020, 42, 373–498. [Google Scholar] [CrossRef]
- Mathew, J.P.; Fontes, M.L.; Tudor, I.C.; Ramsay, J.; Duke, P.; Mazer, C.D.; Barash, P.G.; Hsu, P.H.; Mangano, D.T.; Investigators of the Ischemia Research and Education Foundation; et al. A multicenter risk index for atrial fibrillation after cardiac surgery. JAMA 2004, 291, 1720–1729. [Google Scholar] [CrossRef]
- Magedanz, E.H.; Guaragna, J.C.V.D.; Albuquerque, L.C.; Wagner, M.B.; Chieza, F.L.; Bueno, N.L.; Bodanese, L.C. Risk Score Elaboration for Stroke in Cardiac Surgery. Braz. J. Cardiovasc. Surg. 2021, 36, 788–795. [Google Scholar] [CrossRef] [PubMed]
- D’Ancona, G.; Saez de Ibarra, J.I.; Baillot, R.; Mathieu, P.; Doyle, D.; Metras, J.; Desaulniers, D.; Dagenais, F. Determinants of stroke after coronary artery bypass grafting. Eur. J. Cardiothorac. Surg. 2003, 24, 552–556. [Google Scholar] [CrossRef] [PubMed]
- Santos, H.N.; Magedanz, E.H.; Guaragna, J.C.; Santos, N.N.; Albuquerque, L.C.; Goldani, M.A.; Petracco, J.B.; Bodanese, L.C. Predictors of stroke in patients undergoing cardiac surgery. Rev. Bras. Cir. Cardiovasc. 2014, 29, 140–147. [Google Scholar] [CrossRef] [PubMed]
- Forlivesi, S.; Cappellari, M.; Bonetti, B. Obesity paradox and stroke: A narrative review. Eat Weight Disord. 2021, 26, 417–423. [Google Scholar] [CrossRef] [PubMed]
- Marchesan, L.Q.; Saffi, M.A.L.; Silveira, L.F.D.; Lovato, M.C.M.; Araujo, P.C.; Chemello, D. Risk Factors Associated with Ischemic Stroke in the Immediate Postoperative Period of Cardiac Surgery. Braz. J. Cardiovasc. Surg. 2023, 8, e20220072. [Google Scholar] [CrossRef] [PubMed]
- Sepehripour, A.H.; Lo, T.T.; McCormack, D.J.; Shipolini, A.R. Is there benefit in smoking cessation prior to cardiac surgery? Interact. Cardiovasc. Thorac. Surg. 2012, 15, 726–732. [Google Scholar] [CrossRef] [PubMed]
- Wagner, B.D.; Grunwald, G.K.; Hossein Almassi, G.; Li, X.; Grover, F.L.; Shroyer, A.L.W. Factors associated with long-term survival in patients with stroke after coronary artery bypass grafting. J. Int. Med. Res. 2020, 48, 300060520920428. [Google Scholar] [CrossRef] [PubMed]
- Jin, X.; Li, P.; Michalski, D.; Li, S.; Zhang, Y.; Jolkkonen, J.; Cui, L.; Didwischus, N.; Xuan, W.; Boltze, J. Perioperative stroke: A perspective on challenges and opportunities for experimental treatment and diagnostic strategies. CNS Neurosci. Ther. 2022, 28, 497–509. [Google Scholar] [CrossRef]

| Variables | All Patients | No SACS | SACS | OR [95%CI] | p-Value |
|---|---|---|---|---|---|
| n = 580,117 | n = 569,360 | n = 10,757 | |||
| Age, years | 67.8 [59.7;74.5] | 67.7 [59.6;74.4] | 71.8 [64.4;77.1] | 1.03 [1.03;1.03] | <0.001 |
| BMI, kg/m2 | 27.6 [24.8;30.8] | 27.6 [24.8;30.8] | 26.8 [24.0;30.1] | 0.97 [0.96;0.97] | <0.001 |
| Gender: | |||||
| F | 157,673 (27.2%) | 154,169 (27.1%) | 3504 (32.6%) | Ref. | Ref. |
| M | 422,204 (72.8%) | 414,961 (72.9%) | 7243 (67.4%) | 0.77 [0.74;0.80] | <0.001 |
| Treated or BP > 140/90 mmHg on >1 occasion before admission | 372,353 (64.9%) | 365,084 (64.9%) | 7269 (68.6%) | 1.19 [1.14;1.24] | <0.001 |
| Diabetes Mellitus: | |||||
| Not Diabetic | 461,350 (79.5%) | 452,928 (79.6%) | 8422 (78.3%) | Ref. | Ref. |
| Diet | 21,721 (3.74%) | 21,283 (3.74%) | 438 (4.07%) | 1.11 [1.00;1.22] | 0.043 |
| Oral therapy | 65,151 (11.2%) | 63,902 (11.2%) | 1249 (11.6%) | 1.05 [0.99;1.12] | 0.105 |
| Insulin | 31,895 (5.50%) | 31,247 (5.49%) | 648 (6.02%) | 1.12 [1.03;1.21] | 0.009 |
| Smoking Status: | |||||
| Never smoked | 211,251 (37.1%) | 207,284 (37.1%) | 3967 (37.6%) | Ref. | Ref. |
| Ex smoker | 298,431 (52.4%) | 292,966 (52.4%) | 5465 (51.8%) | 0.97 [0.94;1.02] | 0.224 |
| Current smoker | 59,878 (10.5%) | 58,756 (10.5%) | 1122 (10.6%) | 1.00 [0.93;1.07] | 0.951 |
| Extracardiac Arteriopathy: | |||||
| No | 515,052 (88.8%) | 506,386 (88.9%) | 8666 (80.6%) | Ref. | Ref. |
| Yes | 65,065 (11.2%) | 62,974 (11.1%) | 2091 (19.4%) | 1.94 [1.85;2.04] | <0.001 |
| Previous Cerebral Vascular Accident: | |||||
| No | 560,619 (96.6%) | 550,649 (96.7%) | 9970 (92.7%) | Ref. | Ref. |
| Yes | 19,498 (3.36%) | 18,711 (3.29%) | 787 (7.32%) | 2.32 [2.16;2.50] | <0.001 |
| Left Ventricular Ejection Fraction: | |||||
| Good ≥ 50% | 400,752 (70.5%) | 393,982 (70.7%) | 6770 (64.2%) | Ref. | Ref. |
| Fair (LVEF 30–50%) | 135,394 (23.8%) | 132,418 (23.8%) | 2976 (28.2%) | 1.31 [1.25;1.37] | <0.001 |
| Poor (LVEF < 30%) | 31,937 (5.62%) | 31,144 (5.59%) | 793 (7.52%) | 1.48 [1.37;1.60] | <0.001 |
| Pre-Operative Atrial Fibrillation: | |||||
| No Atrial Fibrillation | 492,076 (89.7%) | 483,829 (89.7%) | 8247 (84.5%) | Ref. | Ref. |
| Atrial Fibrillation | 56,773 (10.3%) | 55,257 (10.3%) | 1516 (15.5%) | 1.61 [1.52;1.70] | <0.001 |
| CABG operation: | |||||
| No | 171,947 (29.6%) | 167,502 (29.4%) | 4445 (41.3%) | Ref. | Ref. |
| Yes | 408,170 (70.4%) | 401,858 (70.6%) | 6312 (58.7%) | 0.59 [0.57;0.62] | <0.001 |
| Cardiopulmonary bypass: | |||||
| No | 55,332 (9.77%) | 54,869 (9.87%) | 463 (4.39%) | Ref. | Ref. |
| Yes | 510,892 (90.2%) | 500,812 (90.1%) | 10,080 (95.6%) | 2.38 [2.17;2.62] | <0.001 |
| Aortic Valve Surgery: | |||||
| No | 421,908 (72.7%) | 415,069 (72.9%) | 6839 (63.6%) | Ref. | Ref. |
| Yes | 158,209 (27.3%) | 154,291 (27.1%) | 3918 (36.4%) | 1.54 [1.48;1.60] | <0.001 |
| Mitral Valve Surgery: | |||||
| No | 512,843 (88.4%) | 503,835 (88.5%) | 9008 (83.7%) | Ref. | Ref. |
| Yes | 67,274 (11.6%) | 65,525 (11.5%) | 1749 (16.3%) | 1.49 [1.42;1.57] | <0.001 |
| Tricuspid Valve Surgery: | |||||
| No | 566,993 (97.7%) | 556,568 (97.8%) | 10,425 (96.9%) | Ref. | Ref. |
| Yes | 13,124 (2.26%) | 12,792 (2.25%) | 332 (3.09%) | 1.39 [1.24;1.55] | <0.001 |
| Surgery on thoracic aorta: | |||||
| No | 551,169 (95.0%) | 542,108 (95.2%) | 9061 (84.2%) | Ref. | Ref. |
| Yes | 28,948 (4.99%) | 27,252 (4.79%) | 1696 (15.8%) | 3.72 [3.53;3.93] | <0.001 |
| Variable | OR [95%CI] | p-Value |
|---|---|---|
| Age, years | 1.03 [1.03–1.04] | <0.001 |
| BMI, kg/m2 | 0.98 [0.97–0.98] | <0.001 |
| Gender (Ref. F) | ||
| M | 0.92 [0.87–0.98] | 0.008 |
| Systemic Arterial Hypertension (Ref. No hypertension) | ||
| Treated or BP > 140/90 mmHg on > 1 occasion before admission | 1.10 [1.03–1.16] | 0.003 |
| Diabetes Mellitus (Ref. Non-Diabetic) | ||
| Diet | 1.15 [1.01–1.31] | 0.033 |
| Oral therapy | 1.37 [1.23–1.53] | <0.001 |
| Smoking Status (Ref. Never smoke) | ||
| Ex-smoker | 1.20 [0.94–1.06] | 0.987 |
| Current smoker | 1.32 [1.08–1.32] | <0.001 |
| ExtraCardiac Arteriopathy (Ref. No) | ||
| Yes | 1.70 [1.58–1.82] | <0.001 |
| Previous Cerebral Vascular Accident (Ref. No) | ||
| Yes | 1.71 [1.54–1.89] | <0.001 |
| Left Ventricular Ejection Fraction (Ref. Good (≥50%)) | ||
| Fair (LVEF 30–50%) | 1.35 [1.22–1.49] | <0.001 |
| Poor (LVEF < 30%) | 1.24 [1.17–1.31] | <0.001 |
| Pre-Operative Atrial Fibrillation (Ref. No) | ||
| Yes | 1.12 [1.03–1.21] | 0.005 |
| CABG operation (Ref. No) | ||
| Yes | 0.89 [0.83–0.96] | 0.002 |
| Cardiopulmonary bypass (Ref. No) | ||
| Yes | 1.78 [1.57–2.03] | <0.001 |
| Aortic Valve Surgery (Ref. No) | ||
| Yes | 1.16 [1.08–1.23] | <0.001 |
| Mitral Valve Surgery (Ref. No) | ||
| Yes | 1.43 [1.31–1.56] | <0.001 |
| Tricuspid Valve Surgery (Ref. No) | ||
| Yes | 0.88 [0.75–1.04] | 0.133 |
| Surgery on thoracic aorta (Ref. No) | ||
| Yes | 3.30 [3.03–3.60] | <0.001 |
| Ref: Reference category |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Asta, L.; Falco, D.; Benedetto, U.; Porreca, A.; Majri, F.; Angelini, G.D.; Sensi, S.; Di Giammarco, G. Stroke after Cardiac Surgery: A Risk Factor Analysis of 580,117 Patients from UK National Adult Cardiac Surgical Audit Cohort. J. Pers. Med. 2024, 14, 169. https://doi.org/10.3390/jpm14020169
Asta L, Falco D, Benedetto U, Porreca A, Majri F, Angelini GD, Sensi S, Di Giammarco G. Stroke after Cardiac Surgery: A Risk Factor Analysis of 580,117 Patients from UK National Adult Cardiac Surgical Audit Cohort. Journal of Personalized Medicine. 2024; 14(2):169. https://doi.org/10.3390/jpm14020169
Chicago/Turabian StyleAsta, Laura, Daniele Falco, Umberto Benedetto, Annamaria Porreca, Fatma Majri, Gianni D. Angelini, Stefano Sensi, and Gabriele Di Giammarco. 2024. "Stroke after Cardiac Surgery: A Risk Factor Analysis of 580,117 Patients from UK National Adult Cardiac Surgical Audit Cohort" Journal of Personalized Medicine 14, no. 2: 169. https://doi.org/10.3390/jpm14020169
APA StyleAsta, L., Falco, D., Benedetto, U., Porreca, A., Majri, F., Angelini, G. D., Sensi, S., & Di Giammarco, G. (2024). Stroke after Cardiac Surgery: A Risk Factor Analysis of 580,117 Patients from UK National Adult Cardiac Surgical Audit Cohort. Journal of Personalized Medicine, 14(2), 169. https://doi.org/10.3390/jpm14020169







