Laboratory Diagnostics Accuracy for COVID-19 versus Post-COVID-19 Syndrome in Lung Disease Patients with Multimorbidity
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Participants
2.2. Quantification of Biomarkers and Analytical Quality Control
2.3. Statistical Analysis
3. Results
Profile of the Biochemical and Hematological Results in COVID-19/PCI
4. Discussion
5. Study Limitations
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- COVID19.who.int. WHO Coronavirus (COVID-19) Dashboard. 2022. Available online: https://covid19.who.int/ (accessed on 10 November 2023).
- Long, B.; Carius, B.M.; Chavez, S.; Liang, S.Y.; Brady, W.J.; Koyfman, A.; Gottlieb, M. Clinical update on COVID-19 for the emergency clinician: Presentation and evaluation. Am. J. Emerg. Med. 2022, 54, 46–57. [Google Scholar] [CrossRef]
- Klok, F.A.; Boon, G.J.A.M.; Barco, S.; Endres, M.; Geelhoed, J.J.M.; Knauss, S.; Rezek, S.A.; Spruit, M.A.; Vehreschild, J.; Siegerink, B. The Post-COVID-19 Functional Status scale: A tool to measure functional status over time after COVID-19. Eur. Respir. J. 2020, 56, 2001494. [Google Scholar] [CrossRef]
- George, P.M.; Barratt, S.L.; Condliffe, R.; Desai, S.R.; Devaraj, A.; Forrest, I.; Gibbons, M.A.; Hart, N.; Jenkins, R.G.; McAuley, D.F.; et al. Respiratory follow-up of patients with COVID-19 pneumonia. Thorax 2020, 75, 1009–1016. [Google Scholar] [CrossRef]
- Al-Saadi, E.A.K.D.; Abdulnabi, M.A. Hematological changes associated with COVID-19 infection. J. Clin. Lab. Anal. 2021, 36, e24064. [Google Scholar] [CrossRef]
- Salehi, S.; Reddy, S.; Gholamrezanezhad, A. Long-term Pulmonary Consequences of Coronavirus Disease 2019 (COVID-19): What We Know and What to Expect. J. Thorac. Imaging 2020, 35, W87–W89. [Google Scholar] [CrossRef]
- Rahi, M.S.; Jindal, V.; Reyes, S.-P.; Gunasekaran, K.; Gupta, R.; Jaiyesimi, I. Hematologic disorders associated with COVID-19: A review. Ann. Hematol. 2021, 100, 309–320. [Google Scholar] [CrossRef]
- Lippi, G.; Sanchis-Gomar, F.; Henry, B.M. COVID-19 and its long-term sequelae: What do we know in 2023? Pol. Arch. Intern. Med. 2023, 133, 16402. [Google Scholar] [CrossRef]
- Ma, Y.; Deng, J.; Liu, Q.; Du, M.; Liu, M.; Liu, J. Long-Term Consequences of COVID-19 at 6 Months and above: A Systematic Review and Meta-Analysis. Int. J. Environ. Res. Public Health 2022, 19, 6865. [Google Scholar] [CrossRef]
- Halle, M.; Bloch, W.; Niess, A.M.; Predel, H.; Reinsberger, C.; Scharhag, J.; Steinacker, J.; Wolfarth, B.; Scherr, J.; Niebauer, J. Exercise and sports after COVID-19—Guidance from a clinical perspective. Transl. Sports Med. 2021, 4, 310–318. [Google Scholar] [CrossRef]
- Terpos, E.; Ntanasis-Stathopoulos, I.; Elalamy, I.; Kastritis, E.; Sergentanis, T.N.; Politou, M.; Psaltopoulou, T.; Gerotziafas, G.; Dimopoulos, M.A. Hematological findings and complications of COVID-19. Am. J. Hematol. 2020, 95, 834–847. [Google Scholar] [CrossRef]
- Wu, C.; Chen, X.; Cai, Y.; Xia, J.; Zhou, X.; Xu, S.; Huang, H.; Zhang, L.; Zhou, X.; Du, C.; et al. Risk Factors Associated with Acute Respiratory Distress Syndrome and Death in Patients with Coronavirus Disease 2019 Pneumonia in Wuhan, China. JAMA Intern. Med. 2020, 180, 934–943, Erratum in JAMA Intern. Med. 2020, 180, 1031. [Google Scholar] [CrossRef]
- Li, X.; Liu, C.; Mao, Z.; Xiao, M.; Wang, L.; Qi, S.; Zhou, F. Predictive values of neutrophil-to-lymphocyte ratio on disease severity and mortality in COVID-19 patients: A systematic review and meta-analysis. Crit. Care 2020, 24, 647. [Google Scholar] [CrossRef]
- Liu, Y.; Du, X.; Chen, J.; Jin, Y.; Peng, L.; Wang, H.H.; Luo, M.; Chen, L.; Zhao, Y. Neutrophil-to-lymphocyte ratio as an independent risk factor for mortality in hospitalized patients with COVID-19. J. Infect. 2020, 81, e6–e12. [Google Scholar] [CrossRef]
- Kouhpayeh, H. Clinical features predicting COVID-19 mortality risk. Eur. J. Transl. Myol. 2022, 32, 10268. [Google Scholar] [CrossRef] [PubMed]
- Wang, L. C-reactive protein levels in the early stage of COVID-19. Med. Mal. Infect. 2020, 50, 332–334. [Google Scholar] [CrossRef] [PubMed]
- Frater, J.L.; Zini, G.; D’onofrio, G.; Rogers, H.J. COVID-19 and the clinical hematology laboratory. Int. J. Lab. Hematol. 2020, 42, 11–18. [Google Scholar] [CrossRef]
- Peach, B.C.; Valenti, M.; Sole, M.L. A Call for the World Health Organization to Create International Classification of Disease Diagnostic Codes for Post-Intensive Care Syndrome in the Age of COVID-19. World Med. Health Policy 2021, 13, 373–382. [Google Scholar] [CrossRef]
- Parasher, A. COVID-19: Current understanding of its Pathophysiology, Clinical presentation and Treatment. Postgrad. Med. J. 2021, 97, 312–320. [Google Scholar] [CrossRef] [PubMed]
- Guan, W.J.; Ni, Z.Y.; Hu, Y.; Liang, W.H.; Ou, C.Q.; He, J.X.; Liu, L.; Shan, H.; Lei, C.L.; Hui, D.S.C.; et al. Clinical characteristics of coronavirus disease 2019 in China. N. Engl. J. Med. 2020, 382, 1708–1720. [Google Scholar] [CrossRef]
- Delshad, M.; Tavakolinia, N.; Pourbagheri-Sigaroodi, A.; Safaroghli-Azar, A.; Bagheri, N.; Bashash, D. The contributory role of lymphocyte subsets, pathophysiology of lymphopenia and its implication as prognostic and therapeutic opportunity in COVID-19. Int. Immunopharmacol. 2021, 95, 107586. [Google Scholar] [CrossRef]
- Ketfi, A.; Chabati, O.; Chemali, S.; Mahjoub, M.; Gharnaout, M.; Touahri, R.; Djenouhat, K.; Selatni, F.; Saad, H.B. Profil clinique, biologique et radiologique des patients Algériens hospitalisés pour COVID-19: Données préliminaires. Pan. Afr. Med. J. 2020, 35 (Suppl. S2), 77. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Fang, Y.-Y.; Deng, Y.; Liu, W.; Wang, M.-F.; Ma, J.-P.; Xiao, W.; Wang, Y.-N.; Zhong, M.-H.; Li, C.-H.; et al. Clinical characteristics of novel coronavirus cases in tertiary hospitals in Hubei Province. Chin. Med. J. 2020, 133, 1025–1031. [Google Scholar] [CrossRef] [PubMed]
- Chalmers, S.; Khawaja, A.; Wieruszewski, P.M.; Gajic, O.; Odeyemi, Y. Diagnosis and treatment of acute pulmonary inflammation in critically ill patients: The role of inflammatory biomarkers. World J. Crit. Care Med. 2019, 8, 59–71. [Google Scholar] [CrossRef] [PubMed]
- Kahn, F.; Bonander, C.; Moghaddassi, M.; Rasmussen, M.; Malmqvist, U.; Inghammar, M.; Björk, J. Risk of severe COVID-19 from the Delta and Omicron variants in relation to vaccination status, sex, age and comorbidities—Surveillance results from southern Sweden, July 2021 to January 2022. Eurosurveillance 2022, 27, 2200121. [Google Scholar] [CrossRef] [PubMed]
- Notarte, K.I.; de Oliveira, M.H.S.; Peligro, P.J.; Velasco, J.V.; Macaranas, I.; Ver, A.T.; Pangilinan, F.C.; Pastrana, A.; Goldrich, N.; Kavteladze, D.; et al. Age, Sex and Previous Comorbidities as Risk Factors Not Associated with SARS-CoV-2 Infection for Long COVID-19: A Systematic Review and Meta-Analysis. J. Clin. Med. 2022, 11, 7314. [Google Scholar] [CrossRef]
- Fadini, G.P.; Morieri, M.L.; Longato, E.; Avogaro, A. Prevalence and impact of diabetes among people infected with SARS-CoV-2. J. Endocrinol. Investig. 2020, 43, 867–869. [Google Scholar] [CrossRef]
- Akter, F.; Mannan, A.; Mehedi, H.H.; Rob, A.; Ahmed, S.; Salauddin, A.; Hossain, S.; Hasan, M. Clinical characteristics and short term outcomes after recovery from COVID-19 in patients with and without diabetes in Bangladesh. Diabetes Metab. Syndr. 2020, 14, 2031–2038. [Google Scholar] [CrossRef]
- Chen, Z.; Peng, Y.; Wu, X.; Pang, B.; Yang, F.; Zheng, W.; Liu, C.; Zhang, J. Comorbidities and complications of COVID-19 associated with disease severity, progression, and mortality in China with centralized isolation and hospitalization: A systematic review and meta-analysis. Front. Public Health 2022, 10, 923485. [Google Scholar] [CrossRef]
- Loosen, S.H.; Jensen, B.-E.O.; Tanislav, C.; Luedde, T.; Roderburg, C.; Kostev, K. Obesity and lipid metabolism disorders determine the risk for development of long COVID syndrome: A cross-sectional study from 50,402 COVID-19 patients. Infection 2022, 50, 1165–1170. [Google Scholar] [CrossRef]
- Li, B.; Yang, J.; Zhao, F.; Zhi, L.; Wang, X.; Liu, L. Prevalence and impact of cardiovascular metabolic diseases on COVID-19 in China. Clin. Res. Cardiol. 2020, 109, 531–538. [Google Scholar] [CrossRef]
- Lee, S.C.; Son, K.J.; Han, C.H.; Jung, J.Y.; Park, S.C. Impact of comorbid asthma on severity of coronavirus disease (COVID-19). Sci. Rep. 2020, 10, 21805. [Google Scholar] [CrossRef] [PubMed]
- Philip, K.E.J.; Buttery, S.; Williams, P.; Vijayakumar, B.; Tonkin, J.; Cumella, A.; Renwick, L.; Ogden, L.; Quint, J.K.; Johnston, S.L.; et al. Impact of COVID-19 on people with asthma: A mixed methods analysis from a UK wide survey. BMJ Open Respir. Res. 2022, 9, e001056. [Google Scholar] [CrossRef] [PubMed]
- Jakubec, P.; Fišerová, K.; Genzor, S.; Kolář, M. Pulmonary Complications after COVID-19. Life 2022, 12, 357. [Google Scholar] [CrossRef] [PubMed]

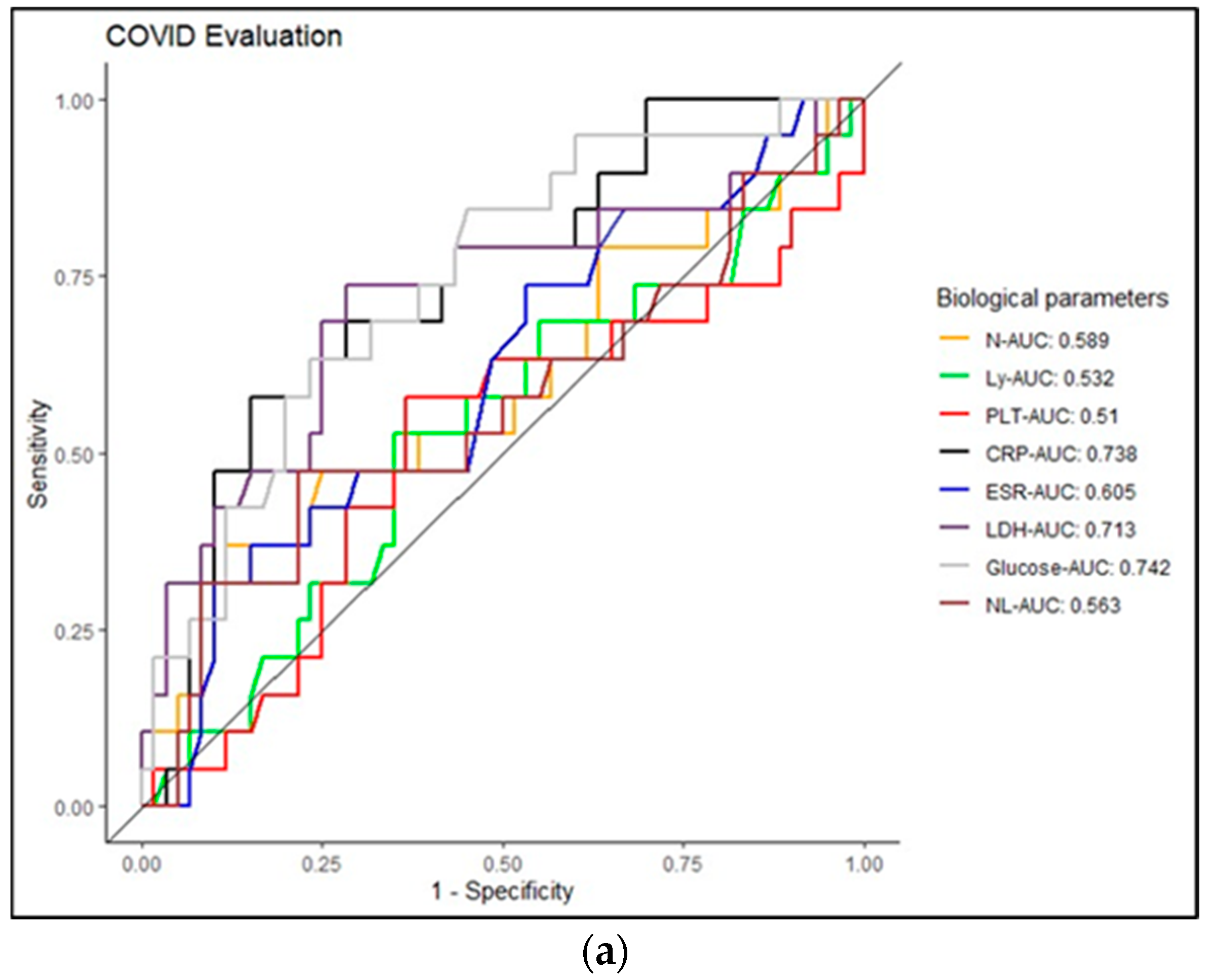


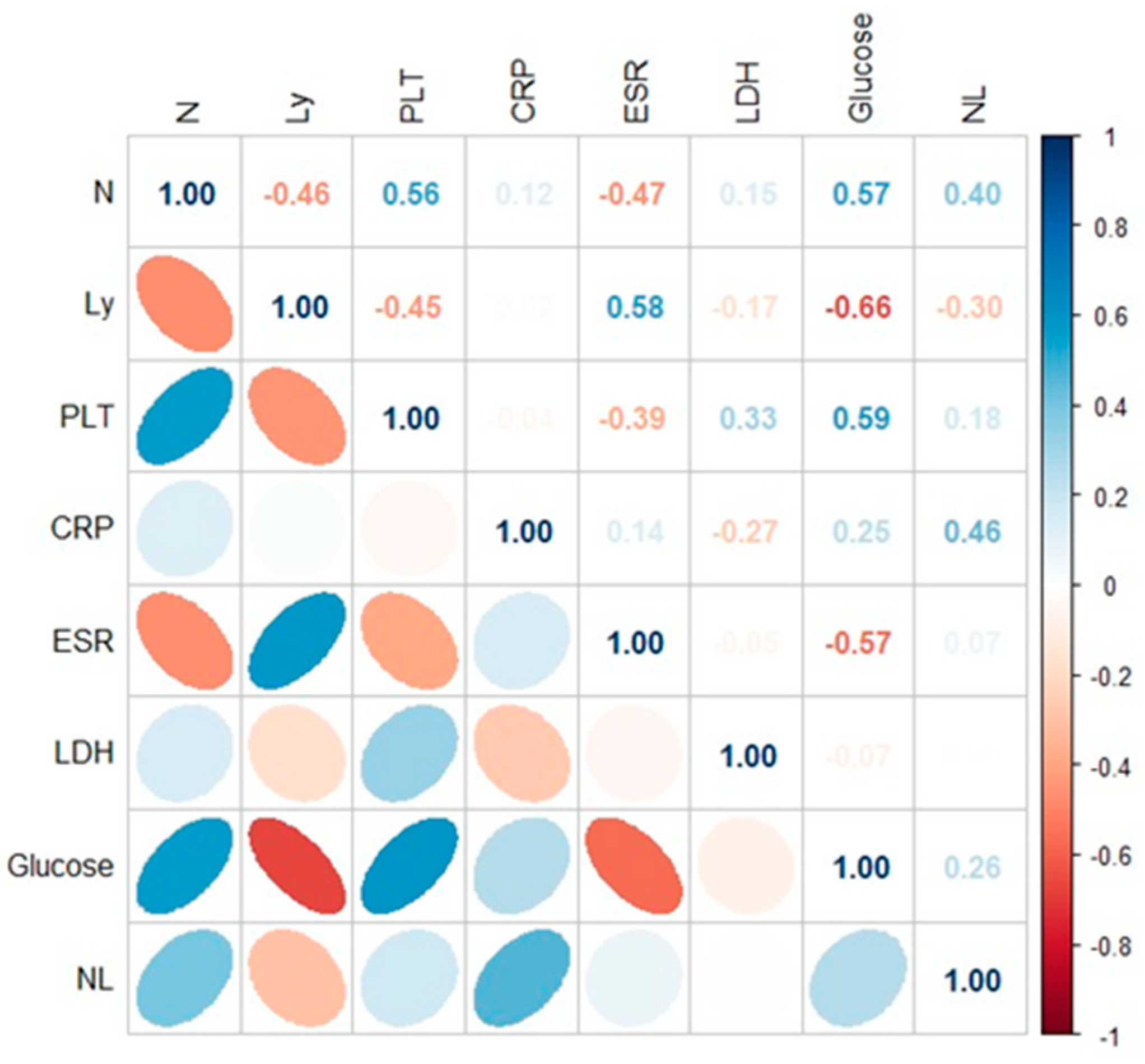
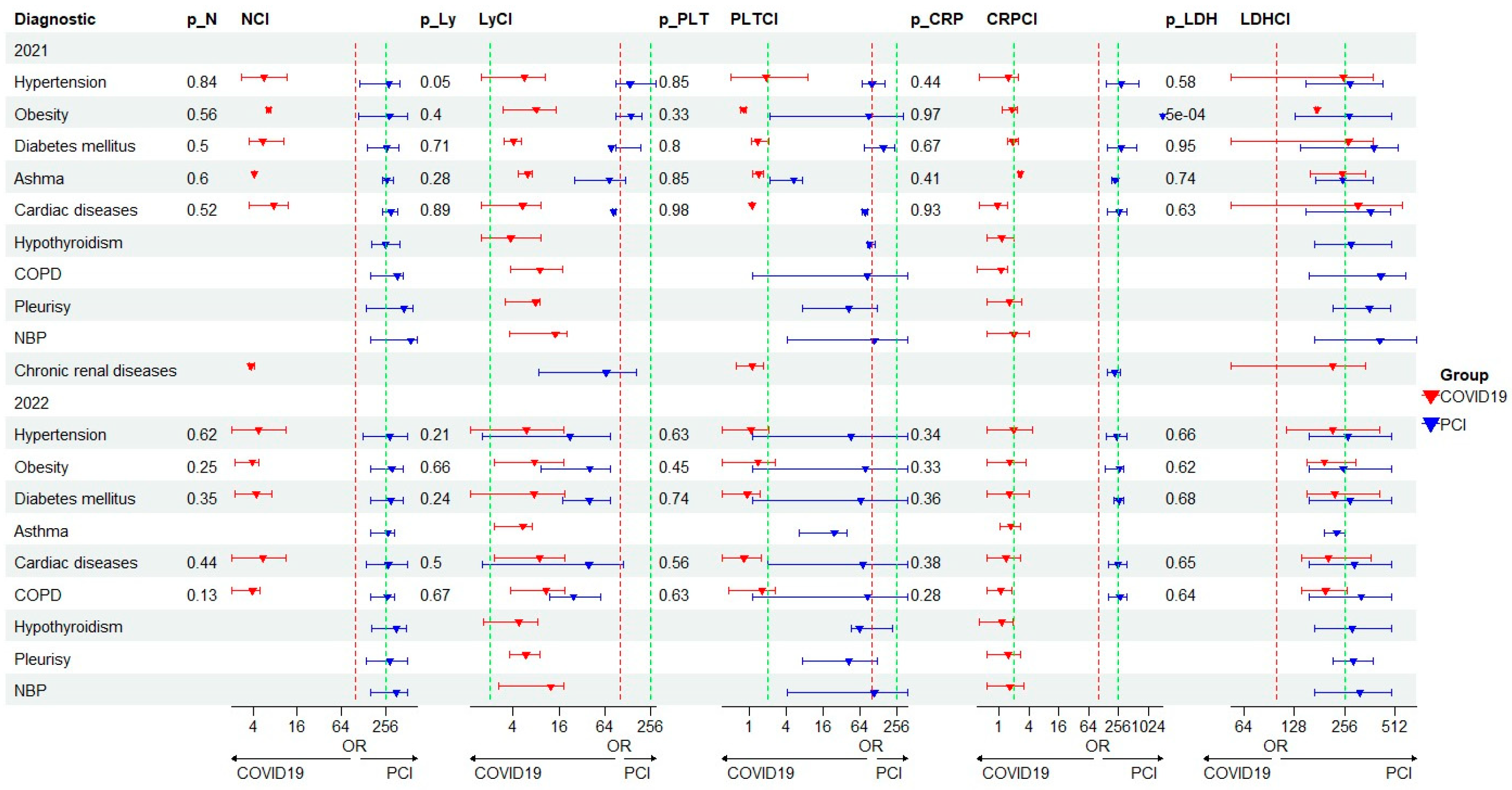
| Characteristics of the Patients | COVID-19/PCI | ||
|---|---|---|---|
| 2020 | 2021 | 2022 | |
| Age (mean ± stdev) | 57.3 ± 13.37 | 61.37 ± 14.80/60.76 ± 17.93) | 68.10 ± 16.06/63.85 ± 15.12 |
| Man | 22 (61%) | 16 (66%)/39 (75%) | 10 (53%)/29 (57%) |
| Woman | 16 (39%) | 8 (34%)/13 (25%) | 9 (47%)/22 (43%) |
| Time for hospitalization | 19.5 ± 6.03 | 13.33 ± 5.46/12.17 ± 7.19 | 9.52 ± 2.98/9.10 ± 8.82 |
| Demographic area | |||
| Area (urban/rural) | 20 (56%)/16 (44%) | (10 (42%)/14 (58%)/(24 (46%)/28 (54%) | (10/9)/(29/22) |
| COVID-19 forms | (n = 36) | (n = 24)/(n = 52) | n = 19/n = 51 |
| Mild | 10 (28%) | 2 (8%)/6 (11%) | 6 (32%)/9 (18%) |
| Moderate | 21 (58%) | 13 (54%)/25 (48%) | 10 (52%)/31 (60%) |
| Severe | 5 (14%) | 9 (38%)/21 (41%) | 3 (16%)/11 (22%) |
| Comorbidities | |||
| Hypertension | 12 (33%) | 13 (54%)/20 (38%) | 11 (58%)/16 (31%) |
| Obesity | 2 (5%) | 2 (8%)/6 (12%) | 2 (10%)/7 (14%) |
| Diabetes mellitus | 8 (22%) | 8 (33%)/4 (8%) | 4 (21%)/7 (14%) |
| Asthma | 2 (5%) | 2 (8%)/6 (12%) | 1/ (5%)/3 (6%) |
| Chronic Kidney diseases | NA | 3 (12%)/1 (2%) | 0/2/(4%) |
| Cardiac disease | 5 (14%) | 7 (29%)/3 (6%) | 7 (37%)/4 (8%) |
| COPD | NA | 2 (8%)/3 (6%) | 6 (32%)/8 (16%) |
| Tuberculosis | NA | NA | 2 (10%)/2 (4%) |
| Pulmonary hypertension | NA | NA | 0/2 (4%) |
| Pleurisy | NA | 0/5 (10%) | 0/14 (27%) |
| Bronchopulmonary neoplasm | NA | 0/2 (4%) | 0/4 (8%) |
| Hypothyroidism | 1 (2%) | 1 (4%)/6 (12%) | 1 (5%)/0 |
| Year 2020 | Mean | Stdev | Range | Mean | Stdev | Range | Mean | Stdev | Range | p Value |
|---|---|---|---|---|---|---|---|---|---|---|
| (n = 36) | Mild Form | Moderate Form | Severe Form | |||||||
| Neutrophils (×103/µL) | 3.10 | 1.09 | 1.21–4.75 | 4.12 | 2.34 | 1.21–4.75 | 3.20 | 1.24 | 2.03–5.11 | 1.00 |
| Ly(×103/µL) | 3.52 | 7.45 | 0.49–24.7 | 1.61 | 0.79 | 0.49–24.70 | 1.17 | 0.87 | 0.35–2.17 | 0.59 |
| PLT (×103/µL) | 257.30 | 62.29 | 162–345 | 217.38 | 87.78 | 162–345 | 175.40 | 31.63 | 138–214 | 0.01 |
| CRP (mg/L) | 26.5 | 33.8 | 0.2–94.9 | 110.6 | 36.61 | 0.2–94.9 | 82.5 | 60.1 | 13–160.3 | 0.03 |
| ESR (mm/1 h) | 37.10 | 34.88 | 6–120 | 48.67 | 39.41 | 6–120 | 42.80 | 32.13 | 34.8–81.00 | 0.67 |
| LDH (U/L) | 261.78 | 156.90 | 172.1–684.3 | 242.97 | 72.12 | 172.1–684.3 | 481.44 | 246.99 | 152.6–744.10 | 0.20 |
| Glucose (mg/dL) | 104.39 | 19.15 | 77.7–132 | 111.06 | 27.50 | 77.7–132.0 | 133.28 | 19.85 | 109.8–158 | 0.07 |
| NLR | 2.87 | 2.20 | 0.14–4.48 | 3.58 | 3.16 | 0.90–11.46 | 4.75 | 5 | 1.16–14.6 | 0.00 |
| Year 2021 | Mean | Stdev | Range | Mean | Stdev | Range | Mean | Stdev | Range | p Value | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Biological Variables * | Mild Form | Moderate Form | Severe Form | ||||||||
| Neutrophils (×103/µL) | COVID-19 | 2.80 | 0.97 | 2.11–3.48 | 5.29 | 2.20 | 2.82–11.44 | 7.75 | 3.29 | 3.50–12.52 | 0.02 |
| PCI ** | 6.66 | 1.55 | 4.72–8.40 | 7.07 | 3.24 | 3.24–14.65 | 7.07 | 3.24 | 3.24–14.65 | 0.14 | |
| Ly (×103/µL) | COVID-19 | 1.12 | 0.07 | 1.07–1.17 | 1.34 | 0.64 | 0.48–2.46 | 2.04 | 2.66 | 0.60–9.04 | 0.69 |
| PCI | 2.24 | 0.57 | 1.35–2.84 | 2.01 | 0.98 | 0.6–5.28 | 1.63 | 0.72 | 0.40–3.65 | 0.06 | |
| PLT(×103/µL) | COVID-19 | 106.50 | 74.25 | 54.00–159 | 234.15 | 86.38 | 123.00–355.00 | 331.33 | 123.601 | 72.00–564.00 | 0.03 |
| PCI | 260.00 | 80.50 | 136.00–365 | 319.20 | 127.32 | 127.32–660.00 | 319.20 | 127.32 | 127.32–660.00 | 0.43 | |
| CRP (mg/L) | COVID-19 | 28.90 | 28.43 | 8.80–49 | 82.22 | 81.50 | 14.20–296.00 | 131.30 | 89.70 | 8.00–27.05 | 0.01 |
| PCI | 8.72 | 9.63 | 2.10–28 | 58.48 | 93.63 | 0.6–323.90 | 50.36 | 65.65 | 1.60–232.400 | 0.00 | |
| ESR (mm/1 h) *** | COVID-19 | 26.00 | 2.83 | 24.00–28 | 37.00 | 17.81 | 5.00–70.00 | 68.11 | 33.38 | 15.00–115.00 | 0.03 |
| PCI | 31.33 | 18.39 | 8.00–60 | 67.96 | 43.88 | 8–140.00 | 68.35 | 35.84 | 16–140.00 | ||
| LDH (U/L) | COVID-19 | 164.00 | 18.38 | 151.00–177 | 287.81 | 133.09 | 147.00–649.00 | 554.43 | 557.56 | 202.90–1998.40 | 0.04 |
| PCI | 247.48 | 105.92 | 145.00–381 | 284.12 | 122.64 | 129.90–659.00 | 305.44 | 130.98 | 114.20–687.80 | 0.00 | |
| Glucose (mg/dL) | COVID-19 | 138.00 | 50.91 | 102.00–174 | 129.68 | 65.48 | 82.30–318.00 | 173.80 | 75.35 | 83.80–324.00 | 0.09 |
| PCI | 119.45 | 31.63 | 90.20–178.5 | 125.72 | 62.87 | 62.87–349.10 | 124.21 | 50.23 | 66.60–240.30 | 0.00 | |
| NLR | COVID-19 | 2.50 | 1.02 | 1.80–3.25 | 5.18 | 5.83 | 1.29–6.65 | 6.41 | 3.48 | 0.81–10.84 | 0.23 |
| PCI | 3.11 | 0.96 | 1.90–4.57 | 4.57 | 3.68 | 1.18–15.42 | 7.46 | 6.78 | 1.33–10.60 | 0.04 | |
| Year 2022 | Mean | Stdev | Range | Mean | Stdev | Range | Mean | Stdev | Range | p Value | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Biological Variables | Mild Form | Moderate Form | Severe Form | ||||||||
| Neutrophils(×103/µL) | COVID-19 | 4.55 | 1.16 | 2.88–5.90 | 5.67 | 3.37 | 2.07–11.28 | 5.10 | 1.92 | 1.92–7.22 | 0.83 |
| PCI | 4.54 | 2.22 | 2.58–9.83 | 6.83 | 4.31 | 1.09–18.91 | 9.00 | 4.99 | 2.25–18.36 | 0.02 | |
| Ly (×103/µL) | COVID-19 | 1.32 | 0.54 | 0.51–2.05 | 0.91 | 0.39 | 0.45–1.49 | 1.60 | 1.00 | 0.62–2.61 | 0.54 |
| PCI | 1.63 | 0.76 | 0.68–3.03 | 1.69 | 0.77 | 0.57–3.16 | 1.95 | 1.48 | 0.56–4.62 | 0.82 | |
| PLT(×103/µL) | COVID-19 | 234.33 | 36.97 | 189–300 | 226.00 | 117.02 | 115–413 | 182.67 | 15.82 | 15.8–200 | 0.36 |
| PCI | 275.33 | 77.96 | 166–368 | 305.06 | 90.37 | 125–499 | 294.18 | 98.93 | 162–438 | 0.71 | |
| CRP (mg/L) | COVID-19 | 32.20 | 43.60 | 1.60–111 | 22.90 | 16.20 | 1.7–44.90 | 56.90 | 42.40 | 14.3–99.10 | 0.26 |
| PCI | 15.80 | 29.49 | 0.6–90.4 | 33.75 | 32.21 | 1.1–120 | 75.02 | 111.19 | 5–381.6 | 0.01 | |
| ESR (mm/1 h) | COVID-19 | 22.00 | 15.89 | 4.00–40 | 31.71 | 34.02 | 7–105.0 | 23.00 | 11.53 | 11.53–35 | 0.73 |
| PCI | 33.00 | 34.06 | 6.00–110 | 49.26 | 32.64 | 1–110 | 70.20 | 43.03 | 15–130 | 0.04 | |
| LDH (U/L) | COVID-19 | 185.43 | 35.93 | 137.7–222.7 | 252.14 | 71.31 | 156–381 | 282.83 | 45.18 | 45.1–324 | 0.02 |
| PCI | 210.04 | 45.59 | 156–312 | 255.18 | 89.65 | 123–478 | 289.78 | 107.38 | 145.7–489 | 0.05 | |
| Glucose (mg/dL) | COVID-19 | 119.33 | 24.76 | 96–151 | 136.44 | 43.17 | 82.1–194 | 150.50 | 58.65 | 58.6–218 | 0.38 |
| PCI | 120.08 | 70.61 | 89.4–307.7 | 106.35 | 33.51 | 63.1–245 | 110.74 | 43.59 | 76.2–226.8 | 0.82 | |
| NLR | COVID-19 | 3.99 | 1.74 | 1.97–6.28 | 7.17 | 6.65 | 3.42–7.57 | 5.20 | 5.58 | 1.77–11.65 | 0.23 |
| PCI | 3.52 | 2.67 | 0.86–4.73 | 5.06 | 5.20 | 1.03–10.26 | 9.35 | 10.11 | 0.9–12.79 | 0.20 | |
| Mann–Whitney U Test | |||||||
|---|---|---|---|---|---|---|---|
| Rank Sum—COVID-19/2021 | Rank Sum—COVID-19/2020 | U | Z | p-value | Valid N—COVID-19/2021 | Valid N—COVID-19/2020 | |
| N | 973 | 857 | 191 | 3.629 | p < 0.005 | 24 | 36 |
| CRP | 1130 | 700 | 34 | 5.998 | p < 0.005 | 24 | 36 |
| Age | 903.5 | 926.5 | 260.5 | 2.580 | p < 0.005 | 24 | 36 |
| Rank Sum—COVID-19/2020 | Rank Sum—COVID-19/2022 | U | Z | p-value | Valid N—COVID-19/2020 | Valid N—COVID-19/2022 | |
| N | 831 | 547 | 165 | −2.429 | p < 0.005 | 36 | 16 |
| CRP | 745 | 633 | 79 | −4.134 | p < 0.005 | 36 | 16 |
| ESR | 1054.5 | 323.5 | 187.5 | 1.983 | p < 0.05 | 36 | 16 |
| Hospitalization time | 1115 | 263 | 127 | 3.182 | p < 0.005 | 36 | 16 |
| Age | 761.5 | 616.5 | 95.5 | −3.807 | p < 0.005 | 36 | 16 |
| Rank Sum—COVID-19/2021 | Rank Sum—PCI/2022 | U | Z | p-value | Valid N—COVID-19/2021 | Valid N—PCI/2022 | |
| CRP | 1230.5 | 1619.5 | 293.5 | 3.612 | p < 0.005 | 24 | 51 |
| LDH | 1086.5 | 1763.5 | 437.5 | 1.976 | p < 0.05 | 24 | 51 |
| Glucose | 1136.5 | 1713.5 | 387.5 | 2.544 | p < 0.05 | 24 | 51 |
| Hospitalization time | 1299.5 | 1550.5 | 224.5 | 4.395 | p < 0.0001 | 24 | 51 |
| Rank Sum—COVID-19/2021 | Rank Sum—PCI/2021 | U | Z | p-value | Valid N—COVID-19/2021 | Valid N—PCI/2021 | |
| N | 732.5 | 2042.5 | 432.5 | −1.928 | p < 0.05 | 24 | 50 |
| Ly | 642 | 2133 | 342 | −2.973 | p < 0.005 | 24 | 50 |
| LDH | 1205.5 | 1569.5 | 294.5 | 3.522 | p < 0.0005 | 24 | 50 |
| Rank Sum—COVID-19/2022 | Rank Sum—PCI/2022 | U | Z | p-value | Valid N—COVID-19/2022 | Valid N—PCI/2022 | |
| Ly | 396.5 | 1881.5 | 260.5 | −2.162 | p < 0.05 | 16 | 51 |
| PLT | 341 | 1937 | 205 | −2.978 | p < 0.005 | 16 | 51 |
| ESR | 380 | 1831 | 244 | −2.327 | p < 0.05 | 16 | 50 |
| Glucose | 731 | 1547 | 221 | 2.743 | p < 0.005 | 16 | 51 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Robu Popa, D.; Melinte, O.E.; Dobrin, M.-E.; Cernomaz, A.T.; Grigorescu, C.; Nemes, A.F.; Todea, D.A.; Vulturar, D.M.; Grosu-Creangă, I.A.; Lunguleac, T.; et al. Laboratory Diagnostics Accuracy for COVID-19 versus Post-COVID-19 Syndrome in Lung Disease Patients with Multimorbidity. J. Pers. Med. 2024, 14, 171. https://doi.org/10.3390/jpm14020171
Robu Popa D, Melinte OE, Dobrin M-E, Cernomaz AT, Grigorescu C, Nemes AF, Todea DA, Vulturar DM, Grosu-Creangă IA, Lunguleac T, et al. Laboratory Diagnostics Accuracy for COVID-19 versus Post-COVID-19 Syndrome in Lung Disease Patients with Multimorbidity. Journal of Personalized Medicine. 2024; 14(2):171. https://doi.org/10.3390/jpm14020171
Chicago/Turabian StyleRobu Popa, Daniela, Oana Elena Melinte, Mona-Elisabeta Dobrin, Andrei Tudor Cernomaz, Cristina Grigorescu, Alexandra Floriana Nemes, Doina Adina Todea, Damiana Maria Vulturar, Ionela Alina Grosu-Creangă, Tiberiu Lunguleac, and et al. 2024. "Laboratory Diagnostics Accuracy for COVID-19 versus Post-COVID-19 Syndrome in Lung Disease Patients with Multimorbidity" Journal of Personalized Medicine 14, no. 2: 171. https://doi.org/10.3390/jpm14020171
APA StyleRobu Popa, D., Melinte, O. E., Dobrin, M.-E., Cernomaz, A. T., Grigorescu, C., Nemes, A. F., Todea, D. A., Vulturar, D. M., Grosu-Creangă, I. A., Lunguleac, T., & Trofor, A. C. (2024). Laboratory Diagnostics Accuracy for COVID-19 versus Post-COVID-19 Syndrome in Lung Disease Patients with Multimorbidity. Journal of Personalized Medicine, 14(2), 171. https://doi.org/10.3390/jpm14020171






