The Challenge of Pneumatosis Intestinalis: A Contemporary Systematic Review
Abstract
1. Introduction
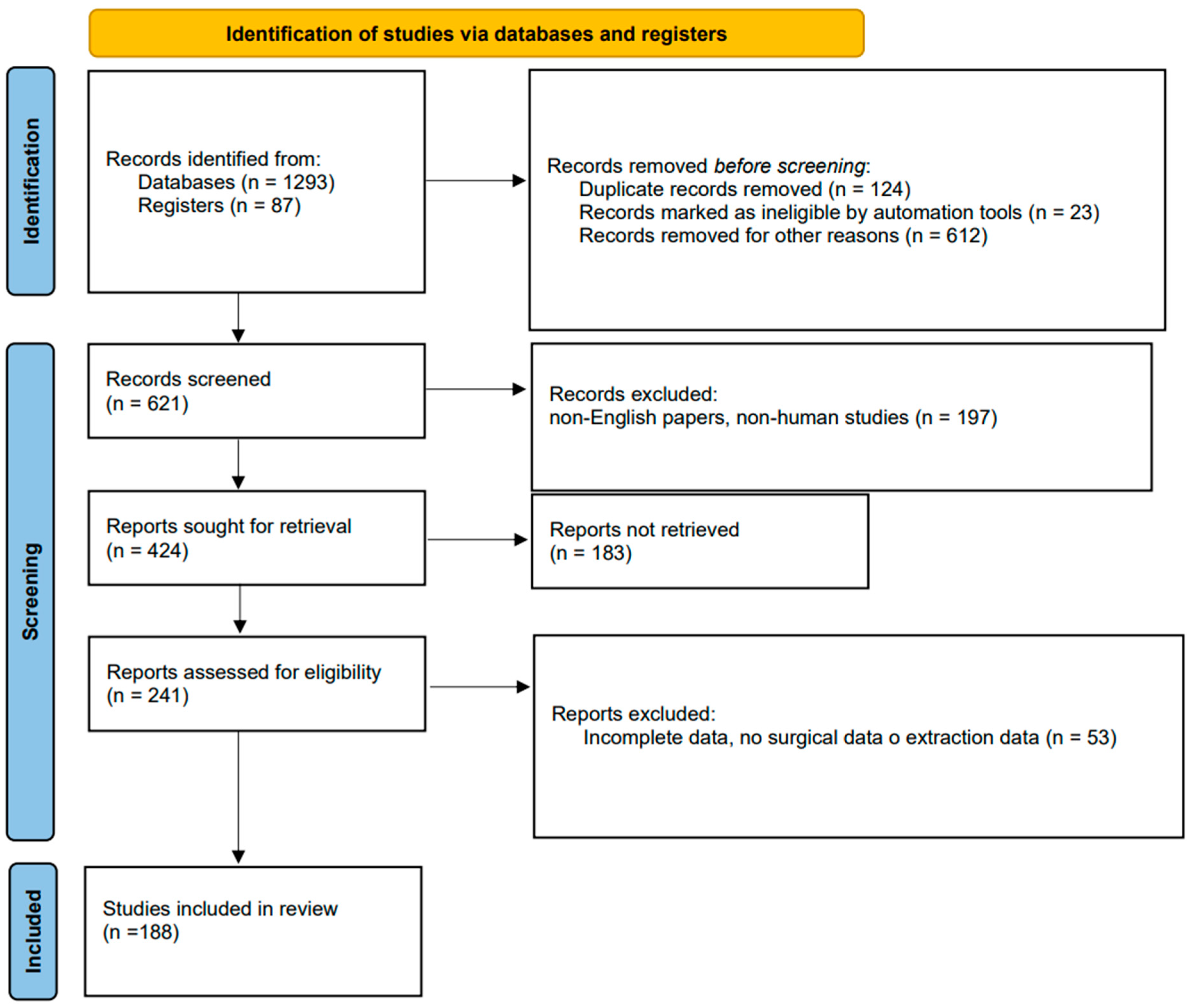
2. Methods
Design
3. Results
3.1. General Characteristics
3.2. Laboratory and Diagnostic Tests
3.3. Therapy
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Du Vernoy, J.G. Aer intestinorum tam sub extima quam intimatunica inclusus: Observationes anatomicae: Comment. Acad. Acient Imp. Petropol. 1730, 5, 213–225. [Google Scholar]
- Wu, L.L.; Yang, Y.S.; Dou, Y.; Liu, Q.S. A systematic analysis of pneumatosis cystoids intestinalis. World J. Gastroenterol. 2013, 19, 4973–4978. [Google Scholar] [CrossRef]
- Barmase, M.; Kang, M.; Wig, J.; Kochhar, R.; Gupta, R.; Khandelwal, N. Role of multidetector CT angiography in the evaluation of suspected mesenteric ischemia. Eur. J. Radiol. 2011, 80, e582–e587. [Google Scholar] [CrossRef] [PubMed]
- Duron, V.P.; Rutigliano, S.; Machan, J.T.; Dupuy, D.E.; Mazzaglia, P.J. Computed tomographic diagnosis of pneumatosis intestinalis: Clinical measures predictive of the need for surgical intervention. Arch. Surg. 2011, 146, 506–510. [Google Scholar] [CrossRef] [PubMed]
- Pickhardt, P.J.; Kim, D.H.; Taylor, A.J. Asymptomatic pneumatosis at CT colonography: A benign self-limited imaging finding distinct from perforation. AJR Am. J. Roentgenol. 2008, 190, W112–W117. [Google Scholar] [CrossRef]
- DuBose, J.J.; Lissauer, M.; Maung, A.A.; Piper, G.L.; O’Callaghan, T.A.; Luo-Owen, X.; Inaba, K.; Okoye, O.; Shestopalov, A.; Fielder, W.D.; et al. Pneumatosis Intestinalis Predictive Evaluation Study (PIPES): A multicenter epidemiologic study of the Eastern Association for the Surgery of Trauma. J. Trauma Acute Care Surg. 2013, 75, 15–23. [Google Scholar] [CrossRef]
- Ho, L.M.; Paulson, E.K.; Thompson, W.M. Pneumatosis intestinalis in the adult: Benign to life-threatening causes. AJR Am. J. Roentgenol. 2007, 188, 1604–1613. [Google Scholar] [CrossRef]
- Knechtle, S.J.; Davidoff, A.M.; Rice, R.P. Pneumatosis intestinalis. Surgical management and clinical outcome. Ann. Surg. 1990, 212, 160–165. [Google Scholar] [CrossRef]
- Ling, F.; Guo, D.; Zhu, L. Pneumatosis cystoides intestinalis: A case report and literature review. BMC Gastroenterol. 2019, 19, 176. [Google Scholar] [CrossRef]
- Gao, Y.; Uffenheimer, M.; Ashamallah, M.; Grimaldi, G.; Swaminath, A.; Sultan, K. Presentation and outcomes among inflammatory bowel disease patients with concurrent pneumatosis intestinalis: A case series and systematic review. Intest. Res. 2020, 18, 289–296. [Google Scholar] [CrossRef]
- Wang, Y.J.; Wang, Y.M.; Zheng, Y.M.; Jiang, H.Q.; Zhang, J. Pneumatosis cystoides intestinalis: Six case reports and a review of the literature. BMC Gastroenterol. 2018, 18, 100. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.S.; Han, J.J.; Kim, S.Y.; Maeng, C.H. Pneumatosis cystoides intestinalis associated with sunitinib and a literature review. BMC Cancer. 2017, 17, 732. [Google Scholar] [CrossRef]
- Amin, S.; Dorer, R.; Irani, S. Full-thickness resection of subepithelial nodules, allowing for the diagnosis of an unusual case of pneumatosis cystoides intestinalis. VideoGIE 2020, 5, 120–122. [Google Scholar] [CrossRef]
- Göbel, T.; Donnez, E. Pneumatosis Cystoides Intestinalis as an Incidental Finding on Endoscopy. Dtsch. Ärzteblatt Int. 2019, 116, 496. [Google Scholar] [CrossRef] [PubMed]
- Smyth, A.I.; Osmanska, J.; Lip, S.; Chong, P.S.; Dalzell, J.R. An unusual gastrointestinal complication following heart transplantation. Ulst. Med. J. 2019, 88, 56. [Google Scholar]
- Lin, W.C.; Wang, K.C. Pneumatosis Cystoides Intestinalis Secondary to Use of an α-Glucosidase Inhibitor. Radiology 2019, 290, 619. [Google Scholar] [CrossRef] [PubMed]
- Cuevas-Castillejos, J.E.; Valdovinos-Díaz, M.A. Pneumatosis cystoides intestinalis: A rare benign cause of chronic pain and bloating with pneumoperitoneum. Neumatosis quística intestinal: Una causa rara y benigna de dolor y distensión abdominal crónicos con neumoperitoneo. Rev. Gastroenterol. Mex. 2019, 84, 402–404. [Google Scholar] [CrossRef]
- Kirmanidis, M.; Boulas, K.; Paraskeva, A.; Kariotis, I.; Barettas, N.; Kariotis, S.; Keskinis, C.; Hatzigeorgiadis, A. Extensive colonic pneumatosis in a patient on adjuvant chemotherapy after right colectomy for primary terminal ileum lymphoma: A decision-making process between surgical and non-surgical management. Int. J. Surg. Case Rep. 2018, 52, 84–88. [Google Scholar] [CrossRef]
- Asahi, Y.; Suzuki, T.; Sawada, A.; Kina, M.; Takada, J.; Gotoda, H.; Masuko, H. Pneumatosis Cystoides Intestinalis Secondary to Sunitinib Treatment for Gastrointestinal Stromal Tumor. Case Rep. Gastroenterol. 2018, 12, 432–438. [Google Scholar] [CrossRef]
- Vecchio, E.; Jamot, S.; Ferreira, J. Pneumatosis Coli Formation via Counterperfusion Supersaturation in a Patient with Severe Diarrhea. Case Rep. Gastrointest. Med. 2018, 2018, 1–4. [Google Scholar] [CrossRef]
- Uruga, H.; Moriguchi, S.; Takahashi, Y.; Ogawa, K.; Murase, K.; Mochizuki, S.; Hanada, S.; Takaya, H.; Miyamoto, A.; Morokawa, N.; et al. Gefitinib successfully administered in a lung cancer patient with leptomeningeal carcinomatosis after erlotinib-induced pneumatosis intestinalis. BMC Cancer 2018, 18, 825. [Google Scholar] [CrossRef] [PubMed]
- Akarsu, M.; Duran, Y.; Htway, Z. A pneumatosis intestinalis case diagnosed with water-immersion technique. Endoscopy 2018, 50, E267–E268. [Google Scholar] [CrossRef] [PubMed]
- Iwamuro, M.; Tanaka, T.; Kawabata, T.; Sugihara, Y.; Harada, K.; Hiraoka, S.; Okada, H. Pseudolipomatosis of the Colon and Cecum Followed by Pneumatosis Intestinalis. Intern. Med. 2018, 57, 2501–2504. [Google Scholar] [CrossRef] [PubMed]
- Cho, Y.M.; Behrenbruch, C.; Smart, P. Colonic lymphoma or pneumatosis coli: Should I biopsy? ANZ J. Surg. 2020, 90, 1198–1200. [Google Scholar] [CrossRef]
- Tharmaradinam, S.; Kanthan, S.; Kanthan, R. Pneumatosis cystoides intestinalis and hyperganglionosis—Cause or effect? A review. Pathol. Res. Pr. 2020, 216, 152879. [Google Scholar] [CrossRef]
- Toyota, S.; Nagata, S.; Yoshino, S.; Kono, S.; Kawanami, S.; Maeda, S.; Kuramitsu, E.; Ichimannda, M.; Nagamatsu, S.; Kai, S.; et al. Mesenteric venous thrombosis as a rare complication of decompression sickness. Surg. Case Rep. 2020, 6, 1–5. [Google Scholar] [CrossRef]
- Tsai, N.Y.; Chou, C.H.; Cheng, Y.C. Pneumatosis cystoides intestinalis in a patient with aseptic meningitis: A case report. Int. J. Color. Dis. 2019, 34, 1805–1808. [Google Scholar] [CrossRef] [PubMed]
- Brighi, M.; Vaccari, S.; Lauro, A.; D’andrea, V.; Pagano, N.; Marino, I.R.; Cervellera, M.; Tonini, V. “Cystamatic” Review: Is Surgery Mandatory for Pneumatosis Cystoides Intestinalis? Dig. Dis. Sci. 2019, 64, 2769–2775. [Google Scholar] [CrossRef]
- Tirumanisetty, P.; Sotelo, J.W.; Disalle, M.; Sharma, M. Pneumatosis intestinalis: Cost paid for rheumatoid arthritis treatment. BMJ Case Rep. 2019, 12, e229329. [Google Scholar] [CrossRef]
- Lee, C.I.; Wu, Y.H. Pneumatosis intestinalis and pneumoretroperitoneum post steroid use in a patient with superior mesenteric artery syndrome. Am. J. Emerg. Med. 2019, 37, 1993.e1–1993.e3. [Google Scholar] [CrossRef]
- Arora, A.; Jouhra, F. Pneumatosis cystoides intestinalis with pneumoperitoneum in a renal transplant patient. Br. J. Hosp. Med. 2014, 75, 407. [Google Scholar] [CrossRef] [PubMed]
- Abidali, H.; Cole, L.; Seetharam, A.B. Rapid reversal of colonic pneumatosis with restoration of mesenteric arterial supply. Clin. J. Gastroenterol. 2018, 11, 461–464. [Google Scholar] [CrossRef] [PubMed]
- Poor, A.; Braman, S.S. Pneumatosis Intestinalis Associated with the Tyrosine Kinase Inhibitor Nintedanib. Lung 2018, 196, 373–375. [Google Scholar] [CrossRef] [PubMed]
- Yamasaki, M.; Teshima, H.; Okanobu, H.; Hattori, N. Pneumatosis cystoides intestinalis in pulmonary mycobacterial disease. Br. J. Hosp. Med. 2019, 80, iii. [Google Scholar] [CrossRef] [PubMed]
- Peng, F.B.; Kim, H.S.; Agarwal, S.K. Abnormal Polypoid Finding During Colonoscopy. Gastroenterology 2019, 157, 940–941. [Google Scholar] [CrossRef] [PubMed]
- Nukii, Y.; Miyamoto, A.; Mochizuki, S.; Moriguchi, S.; Takahashi, Y.; Ogawa, K.; Murase, K.; Hanada, S.; Uruga, H.; Takaya, H.; et al. Pneumatosis intestinalis induced by osimertinib in a patient with lung adenocarcinoma harbouring epidermal growth factor receptor gene mutation with simultaneously detected exon 19 deletion and T790 M point mutation: A case report. BMC Cancer 2019, 19, 186. [Google Scholar] [CrossRef] [PubMed]
- Chaudhry, N.S.; Bi, W.L.; Gupta, S.; Keraliya, A.; Shimizu, N.; Chiocca, E.A. Pneumatosis Intestinalis After Molecular-Targeted Therapy. World Neurosurg. 2019, 125, 312–315. [Google Scholar] [CrossRef]
- González-Olivares, C.; Palomera-Rico, A.; Blanca, N.R.-P.; Sánchez-Aldehuelo, R.; Figueroa-Tubío, A.; de la Filia, I.G.; López-Sanromán, A. Neumatosis intestinal en la enfermedad de Crohn. Gastroenterol. Hepatol. 2019, 42, 181–183. [Google Scholar] [CrossRef]
- Kelly, G.S.; Grandy, B.; Rice, J. Diffuse Pneumatosis Coli. J. Emerg. Med. 2018, 54, e137–e139. [Google Scholar] [CrossRef]
- Shindo, M.; Ito, K.; Tabei, K.; Morishita, Y. Severe pneumatosis intestinalis with portal venous gas. Saudi J. Gastroenterol. 2018, 24, 69. [Google Scholar] [CrossRef]
- Okuda, Y.; Mizuno, S.; Koide, T.; Suzaki, M.; Isaji, S. Surgical treatment of pneumatosis cystoides intestinalis with pneumoperitoneum secondary. Turk. J. Gastroenterol. 2018, 29, 129–131. [Google Scholar] [CrossRef]
- Liu, T.; Zhang, S.; Mao, H. Gastrointestinal malignant neoplasms disguised as pneumatosis cystoids intestinalis: A case report and literature review. Medicine 2017, 96, e9410. [Google Scholar] [CrossRef]
- Zimmer, V.; Schnabel, P.A.; Lammert, F. A Flat Tire in the Colon. Gastroenterology 2018, 154, e8–e9. [Google Scholar] [CrossRef]
- Telegrafo, M.; Stabile Ianora, A.A.; Angelelli, G.; Moschetta, M. Reversible pneumatosis cystoides intestinalis after liver transplantation. G. Chir. 2017, 38, 239–242. [Google Scholar] [CrossRef]
- Liang, J.T.; Chen, T.C. Pneumatosis cystoides intestinalis. Asian J. Surg. 2018, 41, 98. [Google Scholar] [CrossRef]
- Ohkuma, K.; Saraya, T.; Shimoda, M.; Takizawa, H. A case of pneumatosis cystoides intestinalis. J. Gen. Fam. Med. 2017, 18, 481–482. [Google Scholar] [CrossRef] [PubMed]
- Mikami, T.; Kakimoto, K.; Hirata, Y.; Kawakami, K.; Takeuchi, T.; Higuchi, K. Pneumatosis Cystoides Intestinalis in a Patient with Ulcerative Colitis. J. Gastrointest. Liver Dis. 2017, 26, 337. [Google Scholar] [CrossRef] [PubMed]
- Ribaldone, D.G.; Bruno, M.; Gaia, S.; Maria Saracco, G.; De Angelis, C. Endoscopic ultrasound to diagnose pneumatosis cystoides intestinalis (with video). Endosc. Ultrasound 2017, 6, 416–417. [Google Scholar] [CrossRef] [PubMed]
- Sugihara, Y.; Okada, H. Pneumatosis Cystoides Intestinalis. N. Engl. J. Med. 2017, 377, 2266. [Google Scholar] [CrossRef] [PubMed]
- Robinson, A.J.; Quigley, J.-P.; Banks, A.; Farmer, M. Ogilvie’s syndrome treated with an emergency laparotomy, right hemicolectomy and end ileostomy. BMJ Case Rep. 2017, 2017, bcr-2017-220916. [Google Scholar] [CrossRef] [PubMed]
- Rachapalli, V.; Chaluvashetty, S.B. Pneumatosis Cystoides Intestinalis. J. Clin. Diagn. Res. 2017, 11, TJ01–TJ02. [Google Scholar] [CrossRef]
- Beetz, O.; Kleine, M.; Vondran, F.W.R.; Cammann, S.; Klempnauer, J.; Kettler, B. A Case of Recurrent Pneumoperitoneum and Pneumatosis Intestinalis After Bilateral Lung Transplant. Transplantation 2019, 17, 124–127. [Google Scholar] [CrossRef]
- Kanwal, D.; Attia, K.M.E.; Fam, M.N.A.; Khalil, S.M.F.; Alblooshi, A.M. Stercoral perforation of the rectum with faecal peritonitis and pneumatosis coli: A case Report. J. Radiol. Case Rep. 2017, 11, 1–6. [Google Scholar] [CrossRef]
- Suzuki, E.; Kanno, T.; Hazama, M.; Kobayashi, H.; Watanabe, H.; Ohira, H. Four Cases of Pneumatosis Cystoides Intestinalis Complicated by Connective Tissue Diseases. Intern. Med. 2017, 56, 1101–1106. [Google Scholar] [CrossRef]
- Tsuji, T.; Sonobe, S.; Koba, T.; Maekura, T.; Takeuchi, N.; Tachibana, K. Systemic Air Embolism Following Diagnostic Bronchoscopy. Intern. Med. 2017, 56, 819–821. [Google Scholar] [CrossRef][Green Version]
- Nishimura, J.M.; Farzaneh, T.; Pigazzi, A. Pneumatosis coli causing pneumoperitoneum. J. Surg. Case Rep. 2017, 2017, rjw233. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Faria, L.D.; Anjos, C.H.; Fernandes, G.D.; Carvalho, I.F. Pneumatosis intestinalis after etoposide-based chemotherapy in a patient with metastatic small cell lung cancer: Successful conservative management of a rare condition. Einstein 2016, 14, 420–422. [Google Scholar] [CrossRef][Green Version]
- Fujiya, T.; Iwabuchi, M.; Sugimura, M.; Ukai, K.; Tadokoro, K. A Case of Intussusception Associated with Pneumatosis Cystoides Intestinalis. Case Rep. Gastroenterol. 2016, 10, 494–498. [Google Scholar] [CrossRef] [PubMed]
- Furihata, T.; Furihata, M.; Ishikawa, K.; Kosaka, M.; Satoh, N.; Kubota, K. Does massive intraabdominal free gas require surgical intervention? World J. Gastroenterol. 2016, 22, 7383–7388. [Google Scholar] [CrossRef] [PubMed]
- Maeda, A.; Nakata, M.; Shimizu, K.; Yukawa, T.; Saisho, S.; Okita, R. Pneumatosis intestinalis after gefitinib therapy for pulmonary adenocarcinoma: A case report. World J. Surg. Oncol. 2016, 14, 1–4. [Google Scholar] [CrossRef][Green Version]
- Fraga, M.; Silva, M.J.N.D.; Lucas, M. Idiopathic Pneumatosis Intestinalis, Radiological and Endoscopic Images. GE Port. J. Gastroenterol. 2016, 23, 270–272. [Google Scholar] [CrossRef] [PubMed]
- Gassend, J.L.; Dimitrief, M.; Roulet, D.; Cherbanyk, F. Large bowel pneumatosis intestinalis: To operate or not to operate? BMJ Case Rep. 2016, 2016, bcr2016214612. [Google Scholar] [CrossRef]
- Castren, E.E.; Hakeem, A.R.; Mahmood, N.S.; Aryal, K. Case of pneumatosis intestinalis and hepatic portal venous gas following a laparoscopic right hemicolectomy. BMJ Case Rep. 2016, 2016, bcr2016214431. [Google Scholar] [CrossRef] [PubMed]
- Waterland, P.; Jones, A.D.; Peleki, A.; Zilvetti, M. Fulminant pneumatosis coli: A rare presentation of hollow viscus injury after blunt abdominal trauma. J. Emerg. Trauma. Shock. 2016, 9, 40–42. [Google Scholar] [CrossRef] [PubMed]
- Keklik, F.; Cayci, Z.; Arndt, P.; Ustun, C. Spontaneous complete resolution of pneumomediastinum pneumatosis intestinalis caused by acute GVHD. Am. J. Hematol. 2016, 91, 749–750. [Google Scholar] [CrossRef]
- Ksiadzyna, D.; Peña, A.S. Segmental pneumatosis cystoides coli: Computed tomography-facilitated diagnosis. Rev. Esp. Enferm. Dig. 2016, 108, 510–513. [Google Scholar] [CrossRef][Green Version]
- Vargas, A.; Pagés, M.; Buxó, E. Pneumatosis intestinalis due to 5-fluorouracil chemotherapy. Neumatosis intestinal secundaria a quimioterapia con 5-fluorouracilo. Gastroenterol. Hepatol. 2016, 39, 672–673. [Google Scholar] [CrossRef]
- Ling, F.Y.; Zafar, A.M.; Angel, L.F.; Mumbower, A.L. Benign pneumatosis intestinalis after bilateral lung transplantation. BMJ Case Rep. 2015, 2015, bcr2015210701. [Google Scholar] [CrossRef]
- Rottenstreich, A.; Agmon, Y.; Elazary, R. A Rare Case of Benign Pneumatosis Intestinalis with Portal Venous Gas and Pneumoperitoneum Induced by Acarbose. Intern. Med. 2015, 54, 1733–1736. [Google Scholar] [CrossRef]
- Balasuriya, H.D.; Abeysinghe, J.; Cocco, N. Portal venous gas and pneumatosis coli in severe cytomegalovirus colitis. ANZ J. Surg. 2018, 88, 113–114. [Google Scholar] [CrossRef]
- Pulat, H.; Celik, G.; Sabuncuoglu, M.Z.; Benzin, M.F.; Karakose, O.; Cetin, R. Pneumatosis cystoides intestinalis: A rare cause of intraabdominal free air. Turk. J. Surg. 2017, 33, 315–317. [Google Scholar] [CrossRef]
- Helo, N.; Rhee, K.; Dugum, M. An Intriguing Case of Bright Red Blood per Rectum. Pneumatosis Cystoids Intestinalis. Gastroenterology 2015, 149, e1–e2. [Google Scholar] [CrossRef]
- Araújo, T.F.; Pedroto, I. Endoscopic ultrasound of pneumatosis cystoides intestinalis. Endoscopy 2015, 47 (Suppl. 1), E274. [Google Scholar] [CrossRef]
- Ooi, S.M. Fulminant Shigellosis in a HIV Patient. Case Rep. Infect. Dis. 2015, 2015, 128104. [Google Scholar] [CrossRef]
- Blair, H.A.; Baker, R.; Albazaz, R. Pneumatosis intestinalis an increasingly common radiological finding, benign or life-threatening? A case series. BMJ Case Rep. 2015, 2015, bcr2014207234. [Google Scholar] [CrossRef]
- Chandola, R.; Elhenawy, A.; Lien, D.; Laing, B. Massive gas under diaphragm after lung transplantation: Pneumatosis intestinalis simulating bowel perforation. Ann. Thorac. Surg. 2015, 99, 687–689. [Google Scholar] [CrossRef]
- Grimm, J.C.; Berger, J.C.; Lipsett, P.A.; Haut, E.R.; Ferrada, P. A case of profound pneumatosis intestinalis in a patient with recent polytrauma. J. Trauma: Inj. Infect. Crit. Care 2015, 78, 209–210. [Google Scholar] [CrossRef]
- Choi, J.Y.; Cho, S.B.; Kim, H.H.; Lee, I.H.; Lee, H.Y.; Kang, H.S.; Lee, H.Y.; Lee, S.Y. Pneumatosis Intestinalis Complicated by Pneumoperitoneum in a Patient with Asthma. Tuberc. Respir. Dis. 2014, 77, 219–222. [Google Scholar] [CrossRef] [PubMed]
- Aziret, M.; Erdem, H.; Ülgen, Y.; Kahramanca, Ş.; Çetinkünar, S.; Bozkurt, H.; Bali, I.; İrkörücü, O. The appearance of free-air in the abdomen with related pneumatosis cystoides intestinalis: Three case reports and review of the literature. Int. J. Surg. Case Rep. 2014, 5, 909–913. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues-Pinto, E.; Ramalho, R.; Baldaia, H.; Macedo, G. Pneumatosis cystoides intestinalis secondary to increased intraluminal air pressure of previous colonoscopies. Endoscopy 2014, 46 (Suppl. S1), E572–E573. [Google Scholar] [CrossRef] [PubMed]
- Jacob, T.; Paracha, M.; Penna, M.; Arul, D.; Wilson, J. Pneumatosis Coli Mimicking Colorectal Cancer. Case Rep. Surg. 2014, 2014, 428989. [Google Scholar] [CrossRef]
- Neesse, A.; Nimphius, W.; Schoppet, M.; Gress, T.M. Abdominal pain following percutaneous mitral valve repair (MitraClip). Pneumatosis intestinalis (PI) of the ascending colon. Gut 2015, 64, 458–494. [Google Scholar] [CrossRef]
- Santos-Antunes, J.; Ramalho, R.; Lopes, S.; Guimarães, S.; Carneiro, F.; Macedo, G. Asymptomatic pneumatosis cystoides intestinalis diagnosed in the follow-up of a dysplastic polyp. Endoscopy 2013, 46, E425–E426. [Google Scholar] [CrossRef] [PubMed]
- Martis, N.; Baque-Juston, M.; Buscot, M.; Marquette, C.-H.; Leroy, S. An unexpected discovery complicating sicca syndrome. Eur. Respir. Rev. 2014, 23, 397–398. [Google Scholar] [CrossRef] [PubMed]
- Krüger, A.K.; Schmidt, C. LocG. Portal venous gas—Always an ominous sign? Ultraschall Med. 2014, 35, 495–496. [Google Scholar] [CrossRef] [PubMed]
- Tseng, P.Y.; Tarng, D.C.; Yang, W.C.; Yang, C.Y. Benign pneumatosis intestinalis. Intern. Med. 2014, 53, 1589–1590. [Google Scholar] [CrossRef][Green Version]
- Rajpal, S.; Akkus, N.I. Pneumatosis intestinalis, a dreaded complication of intra-aortic balloon pump use. J. Invasive Cardiol. 2014, 26, E104–E105. [Google Scholar] [PubMed]
- Bamakhrama, K.; Abdulhady, L.; Vilmann, P. Endoscopic ultrasound diagnosis of pneumatosis cystoides coli initially misdiagnosed as colonic polyps. Endoscopy 2014, 46 (Suppl. S1), E195–E196. [Google Scholar] [CrossRef] [PubMed]
- Chao, C.T. Prolonged ileus with pneumatosis cystoides intestinalis. Acta Clin. Belg. 2014, 69, 224–225. [Google Scholar] [CrossRef]
- Jurado-Román, M.; Calero-García, P.; Flores-Garnica, L.M.; Sagredo, M.d.P.; Scortechini, M.; Calderón-Gómez, M.; Valiente-Carrillo, J. Pneumatosis cystoides intestinalis. Uncommon cause of pneumoperitoneum. Rev. Esp. Enferm. Dig. 2014, 106, 71–72. [Google Scholar] [CrossRef]
- Pinto Pais, T.; Pinho, R.; Carvalho, J. Hepatic portal venous gas and intestinal pneumatosis as initial presentation of Crohn’s disease: First case report. J. Crohns Colitis 2014, 8, 1329–1330. [Google Scholar] [CrossRef]
- Lemos, A.A.; Cioffi, U.; Ossola, M.W.; Conte, D. Appendicitis-related pneumatosis intestinalis and portal venous gas in a pregnant woman. Dig. Liver Dis. 2014, 46, 476–477. [Google Scholar] [CrossRef]
- Qin, Q.; Ma, T.; Wang, L. Acute dyspnea during diagnostic sigmoidoscopy. Gastroenterology 2014, 146, e1–e3. [Google Scholar] [CrossRef]
- Lim, C.X.; Tan, W.J.; Goh, B.K. Benign pneumatosis intestinalis. Clin. Gastroenterol. Hepatol. 2014, 12, xxv–xxvi. [Google Scholar] [CrossRef] [PubMed]
- Nakajima, N.; Ueda, M.; Yamamoto, K.; Katayama, Y. Pneumatosis intestinalis in a patient with myasthenia gravis. Intern. Med. 2013, 52, 2835–2836. [Google Scholar] [CrossRef][Green Version]
- Bareggi, E.; Tonolini, M.; Ardizzone, S. Pneumatosis intestinalis and perforation in Crohn’s disease: Worrisome or not? J. Crohns Colitis 2014, 8, 338–339. [Google Scholar] [CrossRef] [PubMed]
- Fong, K.Y.; Siao, F.Y.; Yen, H.H. Cecal pneumatosis intestinalis in obstructing sigmoid cancer: Emergency metallic stenting. Am. J. Emerg. Med. 2014, 32, 395.e1–395.e3. [Google Scholar] [CrossRef]
- Zarbalian, Y.; von Rosenvinge, E.C.; Twadell, W.; Mikdashi, J. Recurrent pneumatosis intestinalis in a patient with dermatomyositis. BMJ Case Rep. 2013, 2013, bcr2013200308. [Google Scholar] [CrossRef]
- Lommen, M.J.; Zineldine, O.; I Mehta, T.; E Radtke, L.; Serrano, O. Pneumatosis Cystoides Intestinalis Identified on Screening Colonoscopy With Associated Pneumoperitoneum. Cureus 2020, 12, e9512. [Google Scholar] [CrossRef] [PubMed]
- Ezuka, A.; Kawana, K.; Nagase, H.; Takahashi, H.; Nakajima, A. Improvement of pneumatosis cystoides intestinalis after steroid tapering in a patient with bronchial asthma: A case report. J. Med. Case Rep. 2013, 7, 163. [Google Scholar] [CrossRef]
- Siddiqui, M.; Jain, A.; Rizvi, S.; Ahmad, K.; Ullah, E.; Ahmad, I. Necrotizing colitis complicating necrotized pancreatitis: Look out for intestinal pneumatosis. J. Belg. Soc. Radiol. 2013, 96, 19. [Google Scholar] [CrossRef][Green Version]
- Tanabe, S.; Shirakawa, Y.; Takehara, Y.; Maeda, N.; Katsube, R.; Ohara, T.; Sakurama, K.; Noma, K.; Fujiwara, T. Successfully treated pneumatosis cystoides intestinalis with pneumoperitoneum onset in a patient administered α-glucosidase inhibitor. Acta Medica Okayama 2013, 67, 123–128. [Google Scholar] [CrossRef]
- Adar, T.; Paz, K. Images in clinical medicine. Pneumatosis intestinalis. N. Engl. J. Med. 2013, 368, e19. [Google Scholar] [CrossRef]
- Rahim, H. Gastrointestinal sarcoidosis associated with pneumatosis cystoides intestinalis. World J. Gastroenterol. 2013, 19, 1135–1139. [Google Scholar] [CrossRef]
- Liang, H.-H.; Wang, W.; Hung, C.-S.; Wei, P.-L.; Yen, K.-L.; Kuo, L.-J.; Tu, C.-C. Branch-type Gas in the Liver. J. Emerg. Med. 2013, 44, e363–e364. [Google Scholar] [CrossRef] [PubMed]
- de Leon, M.P.; Bertarelli, C.; Casadei, G.P.; Grilli, A.; Bacchini, P.; Pedroni, M.; Jovine, E. A case of pneumatosis cystoides intestinalis mimicking familial adenomatous polyposis. Fam. Cancer 2012, 12, 573–576. [Google Scholar] [CrossRef] [PubMed]
- Mourra, N.; Fontugne, J. Extensive pneumatosis coli misdiagnosed and mismanaged as polyposis. Scand. J. Gastroenterol. 2013, 48, 119–120. [Google Scholar] [CrossRef]
- Masuda, N.; Nagata, K.; Mitsushima, T.; Fujiwara, M. Computed tomographic colonography in diagnosis of asymptomatic pneumatosis cystoides intestinalis. Dig. Liver Dis. 2013, 45, 79. [Google Scholar] [CrossRef] [PubMed]
- Kashima, T.; Ohno, Y.; Tachibana, M. Pneumatosis intestinalis and hepatic portal venous gas in a patient receiving sorafenib. Int. J. Urol. 2012, 19, 1041–1042. [Google Scholar] [CrossRef] [PubMed]
- Aitken, E.; Lyon, A.; Felstenstein, I. An unusual case of acalculous cholecystitis heralding presentation of acute mesenteric ischaemia with typical radiological findings. Int. J. Surg. Case Rep. 2012, 3, 346–348. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Makni, A.; Daghfous, A.; Rebai, W.; Zghab, S.; Jouini, M.; Kacem, M.; Safta, B. Idiopathic aeroportia and gastric pneumatosis. Tunis. Med. 2012, 90, 336–337. [Google Scholar]
- Balbir-Gurman, A.; Brook, O.R.; Chermesh, I.; Braun-Moscovici, Y. Pneumatosis cystoides intestinalis in scleroderma-related conditions. Intern. Med. J. 2012, 42, 323–329. [Google Scholar] [CrossRef]
- Schieber, J.R.; Sherman, S.C. Pneumatosis intestinalis and ulcerative colitis. J. Emerg. Med. 2013, 44, 472–473. [Google Scholar] [CrossRef]
- Lee, J.Y.; Han, H.-S.; Lim, S.-N.; Shim, Y.K.; Choi, Y.H.; Lee, O.-J.; Lee, K.H.; Kim, S.T. Pneumatosis intestinalis and portal venous gas secondary to Gefitinib therapy for lung adenocarcinoma. BMC Cancer 2012, 12, 87. [Google Scholar] [CrossRef] [PubMed]
- Vijayakanthan, N.; Dhamanaskar, K.; Stewart, L.; Connolly, J.; Leber, B.; Walker, I.; Trus, M. A Review of Pneumatosis Intestinalis in the Setting of Systemic Cancer Treatments, Including Tyrosine Kinase Inhibitors. Can. Assoc. Radiol. J. 2012, 63, 312–317. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.J.; Lin, M.S.; Tsai, K.C.; Sun, J.T. Pneumatosis intestinalis and portomesenteric vein gas in ischemic bowel disease. J. Emerg. Med. 2012, 43, e475–e476. [Google Scholar] [CrossRef] [PubMed]
- Martin-Smith, J.D.; Larkin, J.O.; Hogan, J.G.; Ravi, N.; Reynolds, J.V. Necrotizing pancreatitis presenting with pneumatosis coli and hepatic portal venous gas. ANZ J. Surg. 2011, 81, 467–468. [Google Scholar] [CrossRef]
- Hong, K.D.; Lee, S.I.; Moon, H.Y. Pneumatosis intestinalis and laparoscopic exploration: Beware of gas explosion. J. Laparoendosc. Adv. Surg. Tech. A 2012, 22, 273–275. [Google Scholar] [CrossRef] [PubMed]
- Hoot, N.R.; Pfennig, C.L.; Johnston, M.N.; Jones, I. An incidental finding? Pneumatosis intestinalis after minor trauma. J. Emerg. Med. 2013, 44, e145–e147. [Google Scholar] [CrossRef]
- Shimada, R.; Hayama, T.; Yamazaki, N.; Akahane, T.; Horiuchi, A.; Shibuya, H.; Yamada, H.; Ishihara, S.; Nozawa, K.; Matsuda, K.; et al. Intestinal Pneumatosis in Which CT Colonography Was of Significant Diagnostic Value: Case Report. Int. Surg. 2011, 96, 217–219. [Google Scholar] [CrossRef]
- Iwasaku, M.; Yoshioka, H.; Korogi, Y.; Kunimasa, K.; Nishiyama, A.; Nagai, H.; Ishida, T. Pneumatosis Cystoides Intestinalis After Gefitinib Therapy for Pulmonary Adenocarcinoma. J. Thorac. Oncol. 2012, 7, 257. [Google Scholar] [CrossRef] [PubMed]
- Nancy Fu, Y.T.; Kim, E.; Bressler, B. Pneumatosis intestinalis after colonoscopy in a Crohn’s disease patient with mucosal healing. Inflamm. Bowel Dis. 2013, 19, E7–E8. [Google Scholar] [CrossRef] [PubMed]
- Sagara, A.; Kitagawa, K.; Furuichi, K.; Kitajima, S.; Toyama, T.; Okumura, T.; Hara, A.; Sakai, Y.; Kaneko, S.; Wada, T. Three cases of pneumatosis intestinalis presenting in autoimmune diseases. Mod. Rheumatol. 2011, 22, 610–615. [Google Scholar] [CrossRef] [PubMed]
- Jarkowski, A., 3rd; Hare, R.; Francescutti, V.; Wilkinson, N.; Khushalani, N. Case report of pneumatosis intestinalis secondary to sunitinib treatment for refractory gastrointestinal stromal tumor. Anticancer. Res. 2011, 31, 3429–3432. [Google Scholar]
- Wu, S.S.; Yen, H.H. Images in clinical medicine. Pneumatosis cystoides intestinalis. N. Engl. J. Med. 2011, 365, e16. [Google Scholar] [CrossRef] [PubMed]
- Yoon, S.; Hong, Y.S.; Park, S.H.; Lee, J.L.; Kim, T.W. Pneumatosis Intestinalis After Cetuximab-containing Chemotherapy for Colorectal Cancer. Ultrasound Med. Biol. 2011, 41, 1225–1228. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lioger, B.; Diot, E. Pneumatosis cystoides intestinalis in dermatomyositis. QJM 2012, 105, 801–802. [Google Scholar] [CrossRef][Green Version]
- Arenal, J.J.; Said, A.; Tinoco, C.; Mato, M.J.; Otero, M.; Guerro, J. Colonic pneumatosis intestinalis in a patient with neutropenia secondary to anorexia nervosa. Rev. Esp. Enferm. Dig. 2011, 103, 391–392. [Google Scholar] [CrossRef]
- Amrein, K.; Högenauer, C.; Spreizer, C.; Spuller, E.; Langner, C. Pneumatosis coli—An underrecognized lesion mimicking neoplastic disease. Wien. Klin. Wochenschr. 2011, 123, 515–518. [Google Scholar] [CrossRef]
- García-Castellanos, R.; López, R.; de Vega, V.M.; Ojanguren, I.; Piñol, M.; Boix, J.; Domènech, E.; Cabré, E. Idiopathic myointimal hyperplasia of mesenteric veins and pneumatosis intestinalis: A previously unreported association. J. Crohn’s Colitis 2011, 5, 239–244. [Google Scholar] [CrossRef]
- Strote, S.R.; Caroon, L.V.; Reardon, R.F. Identification of portal venous air with bedside ultrasound in the emergency department. J. Emerg. Med. 2012, 43, 698–699. [Google Scholar] [CrossRef]
- Gil Kim, Y.; Kim, K.-J.; Noh, S.H.; Yang, D.H.; Jung, K.W.; Ye, B.D.; Byeon, J.-S.; Myung, S.-J.; Yang, S.-K. Clear water filling and puncture: Sufficient for endoscopic diagnosis of pneumatosis cystoides intestinalis? (with video). Gastrointest. Endosc. 2011, 74, 1170–1171. [Google Scholar] [CrossRef]
- Shimojima, Y.; Ishii, W.; Matsuda, M.; Tojo, K.; Watanabe, R.; Ikeda, S. Pneumatosis cystoides intestinalis in neuropsychiatric systemic lupus erythematosus with diabetes mellitus: Case report and literature review. Mod. Rheumatol. 2011, 21, 415–419. [Google Scholar] [CrossRef]
- Wright, N.J.; Wiggins, T.; Stubbs, B.M.; Engledow, A. Benign pneumatosis intestinalis with pneumoperitoneum and typhlitis: Side-effects of drug or disease induced immunosuppression. BMJ Case Rep. 2011, 2011, bcr0720114518. [Google Scholar] [CrossRef]
- Marinello, D.K.; Rafael, D.; Paiva Edos, S.; Dominoni, R.L. Systemic lupus erythematosus complicated by intestinal vasculitis and pneumatosis intestinalis. Rev. Bras. Reumatol. 2010, 50, 596–602. [Google Scholar] [CrossRef]
- Pasquier, M.; Waeber, G. Non-surgical pneumoperitoneum. Emerg. Med. J. 2011, 28, 170. [Google Scholar] [CrossRef]
- Bamba, S.; Tsujikawa, T.; Saotome, T.; Okuno, T.; Saito, Y.; Sasaki, M.; Andoh, A.; Fujiyama, Y. Pneumatosis coli with ulcerative colitis as a rare complication of colonoscopy. Clin. J. Gastroenterol. 2010, 3, 233–236. [Google Scholar] [CrossRef]
- Huang, C.-T.; Liu, C.-Y.; Wu, H.-T.; Yu, Y.-B.; Tzeng, C.-H. Pneumatosis intestinalis and pneumoperitoneum after allogeneic haematopoietic stem cell transplantation. Br. J. Haematol. 2010, 151, 118. [Google Scholar] [CrossRef] [PubMed]
- Liao, Y.T.; Lin, T.H.; Ko, W.J. The early presence of pneumatosis in traumatic colonic perforation: A sequential computed tomography demonstration. Am. J. Emerg. Med. 2010, 28, 645.e1–645.e4. [Google Scholar] [CrossRef] [PubMed]
- Chaput, U.; Ducrotté, P.; Denis, P.; Nouveau, J. Pneumatosis cystoides intestinalis: An unusual cause of distal constipation. Gastroenterol. Clin. Biol. 2010, 34, 502–504. [Google Scholar] [CrossRef] [PubMed]
- Ong, K.-P.J.; Ng, K.-H.; Lim, K.-H.; Low, S.-C.A.; Eu, K.-W. Pneumoperitoneum resulting from pneumatosis cystoides intestinalis: A rare complication of massive colonic dilatation. Tech. Coloproctol. 2010, 14, 287–288. [Google Scholar] [CrossRef]
- Newman, J.A.; Candfield, S.; Howlett, D.; McKenzie, P.; Sahu, S. Graft-versus-host disease. BMJ Case Rep. 2010, 2010. [Google Scholar] [CrossRef]
- Syed, M.P.; Thapa, S.; Kate, Y.; Subedi, P.; Chemmanur, A. Pneumatosis Cystoides Intestinalis in the Setting of Clindamycin Use: An Association or a Coincidence? Balk. Med J. 2020, 38, 53–54. [Google Scholar] [CrossRef] [PubMed]
- Meini, S.; Zini, C.; Passaleva, M.T.; Frullini, A.; Fusco, F.; Carpi, R.; Piani, F. Pneumatosis intestinalis in COVID-19. BMJ Open Gastroenterol. 2020, 7, e000434. [Google Scholar] [CrossRef] [PubMed]
- Miwa, W.; Hiratsuka, T.; Sato, K.; Kato, Y. Pneumatosis cystoides intestinalis lesions changing into yellowish plaque-like elastosis lesions during healing. Clin. J. Gastroenterol. 2020, 13, 1165–1172. [Google Scholar] [CrossRef] [PubMed]
- Kielty, J.; Duggan, W.P.; O’Dwyer, M. Extensive pneumatosis intestinalis and portal venous gas mimicking mesenteric ischaemia in a patient with SARS-CoV-2. Ann. R Coll. Surg. Engl. 2020, 102, e145–e147. [Google Scholar] [CrossRef] [PubMed]
- Lakshmanan, S.; Toubia, N. Pneumatosis Intestinalis in COVID-19. Clin. Gastroenterol. Hepatol. 2020, 19, E99. [Google Scholar] [CrossRef] [PubMed]
- Hokama, A.; Haranaga, S.; Tanaka, T.; Kinjo, T.; Hirata, T.; Fujita, J. Pneumatosis intestinalis and hepatic portal venous gas by paralytic ileus from Strongyloides stercoralis infestation. Pol. Arch. Intern. Med. 2020, 130, 681–682. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Song, J.; Gong, S.; Yu, Y.; Hu, W.; Wang, Y. Hepatic portal venous gas with pneumatosis intestinalis secondary to mesenteric ischemia in elderly patients. Medicine 2020, 99, e19810. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Jun, S.L.; Brennan, T.V. Pneumatosis intestinalis: Not always a surgical indication. Case Rep. Surg. 2012, 2012, 719713. [Google Scholar] [CrossRef]
- Ribolla, M.; Conti, L.; Baldini, E.; Palmieri, G.; Grassi, C.; Banchini, F.; Dacco’, M.D.; Capelli, P. Asymptomatic pneumoperitoneum in pneumatosis coli: A misleading operative indication. Int. J. Surg. Case Rep. 2020, 69, 92–95. [Google Scholar] [CrossRef]
- Ferrada, P.; Callcut, R.; Bauza, G.; O’bosky, K.R.; Luo-Owen, X.; Mansfield, N.J.; Inaba, K.; Pasley, J.; Bugaev, N.; Pereira, B.; et al. Pneumatosis Intestinalis Predictive Evaluation Study. J. Trauma Inj. Infect. Crit. Care 2017, 82, 451–460. [Google Scholar] [CrossRef]
- Matsumoto, S.; Sekine, K.; Funaoka, H.; Funabiki, T.; Yamazaki, M.; Orita, T.; Hayashida, K.; Kitano, M. Diagnostic value of intestinal fatty acid-binding protein for pneumatosis intestinalis. Am. J. Surg. 2016, 212, 961–968. [Google Scholar] [CrossRef]
- Hani, M.B.; Kamangar, F.; Goldberg, S.; Greenspon, J.; Shah, P.; Volpe, C.; Turner, D.J.; Horton, K.; Fishman, E.K.; Francis, I.R.; et al. Pneumatosis and portal venous gas: Do CT findings reassure? J. Surg. Res. 2013, 185, 581–586. [Google Scholar] [CrossRef]
- Gupta, A.K.; Vazquez, O.A.; Lopez-Viego, M. Idiopathic Pneumatosis of Small Bowel and Bladder. Cureus 2020, 12, e8313. [Google Scholar] [CrossRef]
- Muhammad Nawawi, K.N.; Abd Samat, A.H.; Nik Fuad, N.F.; Yi, L.L. Pneumatosis intestinalis: An important radiological clue in a case of missed perforated appendicitis. Turk. J. Emerg. Med. 2020, 20, 86–89. [Google Scholar] [CrossRef] [PubMed]
- Gomes, A.F.; Fernandes, S.; Costa Gomes, O.; Coutinho, J. Aeroportia and pneumatosis intestinalis: Discrepancy between radiological and intraoperative findings. BMJ Case Rep. 2020, 13, e233132. [Google Scholar] [CrossRef] [PubMed]
- Takimoto, T.; Minomo, S.; Azuma, K.; Tsuyuguchi, K.; Suzuki, K. A rare cause of pneumatosis intestinalis: Intestinal amyloidosis reactive to pulmonary Mycobacterium avium infection. Int. J. Infect. Dis. 2020, 97, 329–330. [Google Scholar] [CrossRef] [PubMed]
- Lim, J.; Ashmore, D.; Oommen, C. A Rare Case of Chronic Small Bowel Pseudo-Obstruction. Cureus 2020, 12, e8003. [Google Scholar] [CrossRef]
- Fairley, L.J.; Smith, S.L.; Fairley, S.K. A case report of iatrogenic gas gangrene post colonoscopy successfully treated with conservative management- is surgery always necessary? BMC Gastroenterol. 2020, 20, 163. [Google Scholar] [CrossRef] [PubMed]
- A Molina, G.; Fuentes, G.; Orejuela, M.E.; Herrera, J.M.; Jiménez, G.E.; Pinto, J.C.; Cobo, M.M. Pneumatosis cystoides intestinalis in an elderly patient, better to be safe than sorry. J. Surg. Case Rep. 2020, 2020, rjaa053. [Google Scholar] [CrossRef] [PubMed]
- Arai, M.; Kim, S.; Ishii, H.; Takiguchi, T.; Yokota, H. Portal Venous Gas in Adults: Clinical Significance, Management, and Outcomes of 25 Consecutive Patients. J. Nippon. Med Sch. 2021, 88, 88–96. [Google Scholar] [CrossRef] [PubMed]
- Police, A.; Charre, L.; Volpin, E.; Antonopulos, C.; Braham, H.; El Arbi, N. Pneumatosis cystoides intestinalis induced by the alpha-glucosidase inhibitor complicated from sigmoid volvulus in a diabetic patient. Int. J. Color. Dis. 2020, 35, 943–946. [Google Scholar] [CrossRef] [PubMed]
- Wheatley, L.; Williams, M.; Swinson, B.; Crawley-Smith, T. Benign harbinger of portal venous gas and pneumatosis intestinalis. ANZ J. Surg. 2020, 90, 1803–1805. [Google Scholar] [CrossRef]
- Tsang, C.L.N.; Lim, C.S.H.; Chen, M.Z.; Tay, Y.K.; Phan-Thien, K. Pneumatosis intestinalis: Benign or life-threatening? ANZ J. Surg. 2019, 90, 1790–1792. [Google Scholar] [CrossRef]
- Chen, P.A.; Sun, J.T.; Lien, W.C.; Huang, C.Y. Ultrasound Imaging of Pneumatosis Intestinalis. J. Med. Ultrasound 2019, 27, 211–212. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Hwang, H.Y.; Kim, M.J.; Park, K.J.; Kim, K.-B. Early laparoscopic exploration for acute mesenteric ischemia after cardiac surgery. Acute Crit. Care 2020, 35, 213–217. [Google Scholar] [CrossRef]
- Furutani, Y.; Hiranuma, C.; Hattori, M.; Doden, K.; Hashizume, Y. A case of portal venous gas after obstructive transverse colon cancer surgery. Surg. Case Rep. 2019, 5, 1–4. [Google Scholar] [CrossRef]
- Varelas, L.J.; Klinge, M.J.; Malik, S.M.; A Borhani, A.; Neal, M. Idiopathic pneumatosis intestinalis secondary to lactulose use in patients with cirrhosis. J. Gastroenterol. Hepatol. 2019, 35, 1065–1068. [Google Scholar] [CrossRef]
- Arai, M.; Kim, S.; Ishii, H.; Hagiwara, J.; Takiguchi, T.; Ishiki, Y.; Yokota, H. Delayed development of portal vein thrombosis in a patient initially detected with portal venous gas and pneumatosis intestinalis: A case report. Acute Med. Surg. 2019, 6, 419–422. [Google Scholar] [CrossRef]
- Belkhir, A.; Jrad, M.; Sebei, A.; Soudani, M.; Haddad, A.; Boukriba, S.; Frikha, W.; Mizouni, H. Pneumatosis cystoides intestinalis revealed after a hand-to-hand aggression: A case report. Int. J. Surg. Case Rep. 2019, 62, 100–102. [Google Scholar] [CrossRef]
- Khan, T.; Mujtaba, M.; Flores, M.S.; Nahum, K.; Carson, M.P. A Case of Pneumatosis Intestinalis With Pneumoperitoneum as a Potential Delayed Adverse Effect of Capecitabine. World J. Oncol. 2019, 10, 151–152. [Google Scholar] [CrossRef] [PubMed]
- Di Pietropaolo, M.; Trinci, M.; Giangregorio, C.; Galluzzo, M.; Miele, V. Pneumatosis cystoides intestinalis: Case report and review of literature. Clin. J. Gastroenterol. 2019, 13, 31–36. [Google Scholar] [CrossRef]
- Rivera, C.J.P.; Ramirez, N.A.; Gonzalez-Orozco, A.; Caicedo, I.; Cabrera, P. Pneumoperitoneum, pneumatosis intestinalis and portal venous gas: Rare gastrostomy complications case report. Int. J. Surg. Case Rep. 2019, 58, 174–177. [Google Scholar] [CrossRef]
- Ibrahim, A.; Edirimanne, S. Portal venous gas and pneumatosis intestinalis: Ominous findings with an idiopathic aetiology. J. Surg. Case Rep. 2019, 2019, rjy352. [Google Scholar] [CrossRef] [PubMed]
- Harris, E.; Giannotti, G. Is pneumatosis cystoides intestinalis a lymphatic pathology? A case of small bowel lymphangioma and subsequent development of pneumatosis cystoides intestinalis in a 57-year-old female. J. Surg. Case Rep. 2019, 2019, rjy334. [Google Scholar] [CrossRef] [PubMed]
- Bansal, R.; Shankar, U.; Walfish, A. Emphysematous Gut. Am. J. Med. Sci. 2019, 357, e9. [Google Scholar] [CrossRef] [PubMed]
- Dhadlie, S.; Mehanna, D.; McCourtney, J. Pneumatosis intestinalis a trap for the unwary: Case series and literature review. Int. J. Surg. Case Rep. 2018, 53, 214–217. [Google Scholar] [CrossRef] [PubMed]
- Sanford, Z.; Brown, S.; Tran, M.N.; Zahiri, H.R.; Feather, C.; Wormuth, J.; Buckley, B. Updates on the Utility of Diagnostic Laparoscopy in the Management of Pneumatosis Intestinalis: An Improvement to the Current Treatment Algorithm. Surg. Innov. 2018, 25, 648–650. [Google Scholar] [CrossRef]
- Guan, J.; Munaf, A.; Simmonds, A.V.; Inayat, I. When the Benign Pneumatosis Intestinalis Becomes No Longer Benign: A Rare Case of Bowel Perforation in a Patient with Systemic Sclerosis. Case Rep. Gastrointest. Med. 2018, 2018, 5124145. [Google Scholar] [CrossRef]
- Gray, S.; Katzen, M.; Vudatha, V. A case of recurrent pneumatosis intestinalis. J. Surg. Case Rep. 2018, 2018, rjy077. [Google Scholar] [CrossRef]
- Fujimi, A.; Sakamoto, H.; Kanisawa, Y.; Minami, S.; Nagamachi, Y.; Yamauchi, N.; Ibata, S.; Kato, J. Pneumatosis intestinalis during chemotherapy with nilotinib in a patient with chronic myeloid leukemia who tested positive for anti-topoisomerase I antibodies. Clin. J. Gastroenterol. 2016, 9, 358–364. [Google Scholar] [CrossRef]
- Yamamoto, S.; Takahashi, Y.; Ishida, H. Pneumatosis cystoides intestinalis. Ann. Gastroenterol. 2020, 33, 541. [Google Scholar] [CrossRef]
- Dibra, R.; Picciariello, A.; Trigiante, G.; Labellarte, G.; Tota, G.; Papagni, V.; Martines, G.; Altomare, D.F. Pneumatosis Intestinalis and Hepatic Portal Venous Gas: Watch and Wait or Emergency Surgery? A Case Report and Literature Review. Am. J. Case Rep. 2020, 21, e923831. [Google Scholar] [CrossRef]
- Fukunaga, N.; Yoshida, S.; Shimoji, A.; Maeda, T.; Mori, O.; Yoshizawa, K.; Okada, T.; Tamura, N. Pneumatosis intestinalis and hepatic portal venous gas caused by enteral feeding after a heart valve surgery. J. Cardiol. Cases 2022, 26, 412–414. [Google Scholar] [CrossRef] [PubMed]
- Furtado, T.; Domingues, P.; Piedade, A.; Parreira, L.; Natário, A. A Rare and Severe Cause of Abdominal Pain in a Hemodialysis Patient. Cureus 2022, 14, e30800. [Google Scholar] [CrossRef] [PubMed]
- Sharp, J.; Chuang, K. Benign Pneumatosis Intestinalis: A Case Report and Review of the Literature. Fed. Pract. 2022, 39, 281–285. [Google Scholar] [CrossRef] [PubMed]
- Gefen, R.; Helou, B.; Shussman, N.; Elia, A.; Appelbaum, L.; Pikarsky, A.; Demma, J.A. Pneumatosis Cystoides Coli Presenting as Acute Abdomen in a Patient with Complicated Behcet’s Disease: A Case Report. Am. J. Case Rep. 2022, 23, e937677. [Google Scholar] [CrossRef] [PubMed]
- Miratashi Yazdi, S.A.; Chinisaz, F.; Mohammadi, L.; Najjari, K.; Zabihi Mahmoudabadi, H. Intestinal volvulus secondary to pneumatosis intestinalis: A case report. Int. J. Surg. Case Rep. 2021, 88, 106515. [Google Scholar] [CrossRef]
- Yeo, I.H.; Kim, Y.J. Two case reports of pneumatosis intestinalis in patients with cancer: Is surgical management mandatory? Clin. Exp. Emerg. Med. 2021, 8, 237–241. [Google Scholar] [CrossRef] [PubMed]
- Brocchi, S.; Parmeggiani, A.; Gaudiano, C.; Balacchi, C.; Renzulli, M.; Brandi, N.; Dall’Olio, F.G.; Rihawi, K.; Ardizzoni, A.; Golfieri, R. Pneumatosis intestinalis and spontaneous perforation associated with drug toxicity in oncologic patients: A case series. Acta Gastro-Enterol. Belg. 2021, 84, 497–499. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, S.; Mukai, T.; Fujita, S.; Morita, Y. Barium-induced pneumatosis intestinalis in a patient with anti-synthetase syndrome. Clin. Case Rep. 2021, 9, e04775. [Google Scholar] [CrossRef] [PubMed]
- Della Seta, M.; Kloeckner, R.; Pinto Dos Santos, D.; Walter-Rittel, T.C.; Hahn, F.; Henze, J.; Gropp, A.; Pratschke, J.; Hamm, B.; Geisel, D.; et al. Pneumatosis intestinalis and porto-mesenteric venous gas: A multicenter study. BMC Med. Imaging 2021, 21, 129. [Google Scholar] [CrossRef] [PubMed]
- Adachi, W.; Matsushita, T.; Yashiro, Y.; Imura, J.; Shiozawa, H.; Kishimoto, K. Clinical characteristics of pneumoperitoneum with pneumatosis intestinalis detected using computed tomography: A descriptive study. Medicine 2020, 99, e22461. [Google Scholar] [CrossRef] [PubMed]
- Epin, A.; Passot, G.; Christou, N.; Monneuse, O.; Mabrut, J.Y.; Ferrero, P.A.; Caudron, S.; Pezet, D.; Magnin, B.; Grange, R.; et al. Gastric Pneumatosis with Portal Venous Gas can be Treated Non-operatively: A Retrospective Multi-institutional Study. World J. Surg. 2022, 46, 784–790. [Google Scholar] [CrossRef] [PubMed]
- Treyaud, M.O.; Duran, R.; Zins, M.; Knebel, J.F.; Meuli, R.A.; Schmidt, S. Clinical significance of pneumatosis intestinalis—Correlation of MDCT-findings with treatment and outcome. Eur. Radiol. 2017, 27, 70–79. [Google Scholar] [CrossRef] [PubMed]
- Koss, L.G. Abdominal gas cysts (pneumatosis cystoides intestinorum hominis): An analysis with a report of a case and a critical review of the literature. AMA Arch. Pathol. 1952, 53, 523–549. [Google Scholar]
- St Peter, S.D.; Abbas, M.A.; Kelly, K.A. The spectrum of pneumatosis intestinalis. Arch. Surg. 2003, 138, 68–75. [Google Scholar] [CrossRef]
- Borns, P.F.; Johnston, T.A. Indolent pneumatosis of the bowel wall associated with immune suppressive therapy. Ann. Radiol. 1973, 16, 163–166. [Google Scholar]
- Sato, T.; Ohbe, H.; Fujita, M.; Kushimoto, S. Clinical characteristics and prediction of the asymptomatic phenotype of pneumatosis intestinalis in critically ill patients: A retrospective observational study. Acute Med. Surg. 2020, 7, e556. [Google Scholar] [CrossRef]
- Tahiri, M.; Levy, J.; Alzaid, S.; Anderson, D. An approach to pneumatosis intestinalis: Factors affecting your management. Int. J. Surg. Case Rep. 2015, 6, 133–137. [Google Scholar] [CrossRef] [PubMed]
- Kinoshita, H.; Shinozaki, M.; Tanimura, H.; Umemoto, Y.; Sakaguchi, S.; Takifuji, K.; Kawasaki, S.; Hayashi, H.; Yamaue, H. Clinical features and management of hepatic portal venous gas: Four case reports and cumulative review of the literature. Arch. Surg. 2001, 136, 1410–1414. [Google Scholar] [CrossRef] [PubMed]
- Jamart, J. Pneumatosis cystoides intestinalis. A statistical study of 919 cases. Acta Hepato-Gastroenterol. 1979, 26, 419–422. [Google Scholar]
- Perrone, G.; Sartelli, M.; Mario, G.; Chichom-Mefire, A.; Labricciosa, F.M.; Abu-Zidan, F.M.; Ansaloni, L.; Biffl, W.L.; Ceresoli, M.; Coccolini, F.; et al. Management of intra-abdominal-infections: 2017 World Society of Emergency Surgery guidelines summary focused on remote areas and low-income nations. Int. J. Infect. Dis. 2020, 99, 140–148. [Google Scholar] [CrossRef]
| References | No. of Patients | Gender | Age | Diagnostic Test | Etiology | Location | Treatment |
|---|---|---|---|---|---|---|---|
| Ling 2019 [9] | 1 | M | 64 | CT-Colonoscopy-US | Unknown | Sigmoid Colon | Conservative |
| Gao 2019 [10] | 4 | M (3) F (1) | 61.2 (Mean) | CT (4) | IBD | Cecum (2) Ascending colon (2) | Conservative |
| Wang 2018 [11] | 6 | M (2) F (4) | 55.5 (Mean) | CT (4)-Colonoscopy (4)-US (1) | Unknown (2)-glucocorticoid-TCE | Small bowel (1)-sigmoid (2)-descending colon (1)-transverse colon (1)-rectum | Conservative |
| Lee 2017 [12] | 1 | F | 68 | CT-Colonoscopy | Sunitinib | Small bowel-cecum | Conservative (Sunitinib suspension) |
| Wu 2013 [2] | 1 | M | 70 | CT-US | Unknown | All colon | Conservative |
| Amin 2020 [13] | 1 | M | 61 | CT-PET | Unknown | Descending colon | Conservative |
| Göbel 2019 [14] | 1 | M | 46 | Colonoscopy-X-ray | Unknown | Ascending Colon | Conservative |
| Smyth 2019 [15] | 1 | M | 29 | CT | Steroid therapy | All colon | Conservative |
| Lin 2019 [16] | 1 | M | 65 | CT-Colonoscopy | Acarbose | Sigmoid | Conservative (Acarbose suspension) |
| Cuevas 2019 [17] | 1 | F | 65 | CT | Unknown | Small bowel-ascending colon | Conservative |
| Kirmanidis 2018 [18] | 1 | F | 82 | CT-X-ray | Unknown | All colon | Conservative |
| Asahi 2018 [19] | 1 | M | 67 | CT | Sunitinib | Cecum | Conservative (Sunitinib suspension) |
| Vecchio 2018 [20] | 1 | M | 86 | CT | Myeloma Therapy | Transverse—descending colon | Conservative |
| Uruga 2018 [21] | 1 | F | 71 | CT-X-ray | Erlotinib | All colon | Conservative (Erlotinib suspension) |
| Akarsu 2018 [22] | 1 | M | 65 | CT-Colonoscopy | Unknown | Sigmoid | Conservative |
| Iwamuro 2018 [23] | 1 | F | 74 | CT-Colonoscopy | Pseudolipomatosis coli | Cecum-ascending colon | Conservative |
| Cho 2020 [24] | 1 | M | 63 | CT-Colonoscopy | Unknown | Sigmoid | Conservative |
| Tharmaradinam 2020 [25] | 1 | M | 66 | CT | Hyperganglionosis | Cecum-ascending colon | Right colectomy |
| Toyota 2020 [26] | 1 | M | 59 | CT | Decompression Sickness (DCS) | Transverse | Transverse resection-colostomy |
| Tsai 2019 [27] | 1 | M | 46 | CT-Colonoscopy | Meningititis | Ascending colon | Exploratory laparoscopy |
| Brighi 2019 [28] | 1 | M | 70 | CT | Unknown | All colon | Conservative |
| Tirumanisetty 2019 [29] | 1 | F | 75 | CT-X-ray | Unknown | Ascending colon | Right colectomy |
| Lee 2019 [30] | 1 | M | 50 | CT | Steroid therapy | - | Conservative |
| Arora 2014 [31] | 1 | M | 68 | CT-X-ray | Steroid therapy | - | Conservative |
| Abidali 2018 [32] | 1 | F | 60 | CT-Colonoscopy | AAA | Transverse colon | Endovascular AAA repair |
| Poor 2018 [33] | 1 | M | 84 | CT | Nintedanib | Cecum | Conservative (Nintedanib suspension) |
| Yamasaki 2019 [34] | 1 | F | 63 | CT-Colonoscopy | Unknown | All colon | Conservative |
| Peng 2019 [35] | 1 | F | 60 | Colonoscopy | Unknown | Descending colon | Conservative |
| Nukii 2019 [36] | 1 | F | 69 | CT | Osimertinib | Transverse colon | Conservative (Osimertinib suspension) |
| Chaundhry 2019 [37] | 1 | M | 67 | CT | Bevacizumab | Sigmoid | Conservative (Bevacizumab suspension) |
| González-Olivares 2019 [38] | 2 | F (2) | 59 (Mean) | CT (2) | IBD (2) | Ascending (1) Ascending-transverse colon (1) | Conservative (2) |
| Kelly 2018 [39] | 1 | F | 59 | CT-X-ray | Unknown | All colon | Conservative |
| Shindo 2018 [40] | 1 | M | 81 | CT | Unknown | Small bowel-all colon | Conservative |
| Okuda 2018 [41] | 1 | F | 91 | CT | Sigmoid volvulus | Sigmoid | Sigmoid resection-colostomy |
| Liu 2017 [42] | 1 | M | 55 | CT-Colonoscopy | Intussusception terminal ileum | Ascending colon | Ileocecal resection |
| Zimmer 2018 [43] | 1 | M | 45 | CT-Colonoscopy | Unknown | Transverse colon | Conservative |
| Telegrafo 2017 [44] | 1 | M | 54 | CT | Steroid therapy | All colon | Conservative (Steroid suspension) |
| Liang 2018 [45] | 1 | F | 66 | Colonoscopy-Barium Enema | Unknown | Descending colon | Left colectomy |
| Ohkuma 2017 [46] | 1 | F | 76 | CT | Unknown | Descending colon | Conservative |
| Mikami 2017 [47] | 1 | M | 72 | CT-Colonoscopy | Salazosulfapyridine | All colon | Conservative (Salazosulfapyridine suspension) |
| Ribaldone 2017 [48] | 1 | F | 59 | CT-Colonoscopy-US | Unknown | Descending colon | Conservative |
| Sugihara 2017 [49] | 1 | F | 48 | CT | Unknown | Descending colon | Conservative |
| Robinson 2017 [50] | 1 | M | 76 | CT-X-ray | Ogilvie’s syndrome | Ascending colon | Right colectomy-ileostomy |
| Rachapalli 2017 [51] | 1 | M | 50 | CT | GVHD | Transverse colon | Conservative |
| Beetz 2019 [52] | 1 | M | 60 | CT-Colonoscopy | Steroid therapy | Transverse colon | Conservative (Steroid suspension) |
| Kanwal 2017 [53] | 1 | F | 79 | CT-X-ray | Colic perforation | Sigmoid | Left colectomy-colostomy |
| Suzuki 2017 [54] | 3 | M (1) F (2) | 70.3 (Mean) | CT (3) | Voglibose (2) Unknown (1) | Small bowel (1)-all colon | Conservative (2 voglibose suspension) |
| Tsuji 2017 [55] | 1 | M | 51 | CT | Unknown | All colon | Conservative |
| Nishimura 2017 [56] | 1 | M | 54 | CT | Unknown | Ascending colon | Conservative |
| Faria 2016 [57] | 1 | M | 69 | CT | Chemotherapy | - | Conservative |
| Fujiya 2016 [58] | 1 | M | 29 | CT-Colonoscopy | Intussusception | Sigmoid | Intussusception reduction |
| Furihata 2016 [59] | 1 | M | 81 | CT-Colonoscopy | Unknown | Sigmoid | Conservative |
| Maeda 2016 [60] | 1 | F | 80 | CT-X-ray | Gefitinib | Small bowel-transverse colon | Conservative |
| Fraga 2016 [61] | 1 | F | 66 | CT-Colonoscopy | Unknown | Ascending colon | Conservative |
| Gassend 2016 [62] | 1 | M | 72 | CT-X-ray | Unknown | All colon | Subtotal colectomy |
| Castren 2016 [63] | 1 | F | 74 | CT | Unknown | Small bowel-all colon | Ileostomy |
| Waterland 2016 [64] | 1 | M | 76 | CT | GVHD | Ascending colon | Conservative |
| Keklik 2016 [65] | 1 | M | 31 | CT | Trauma | Small bowel-all colon | Conservative |
| Ksiadzyna 2016 [66] | 1 | M | 64 | CT-Colonoscopy | Acarbose | Ascending-transverse colon | Conservative (Acarbose suspension) |
| Vargas 2016 [67] | 1 | M | 65 | CT | 5-FU | All colon | Conservative |
| Ling 2015 [68] | 2 | M (1) F (1) | 60 (Mean) | CT (2) | Steroid therapy | Ascending colon (2) | Conservative (2) |
| Rottenstreich 2015 [69] | 1 | M | 73 | CT | Acarbose | Small bowel-ascending colon | Conservative (Acarbose suspension) |
| Balasuriya 2018 [70] | 1 | M | 32 | CT | Unknown | Ascending colon | Appendicectomy |
| Pülat 2015 [71] | 1 | M | 33 | CT-US-EGDS | Unknown | Descending colon | Conservative |
| Helo 2015 [72] | 1 | M | 36 | CT-X-ray | Unknown | All colon | Conservative |
| Castro-Poças 2015 [73] | 1 | M | 65 | Colonoscopy-US | Unknown | Sigmoid | Conservative |
| Ooi 2015 [74] | 1 | M | 44 | CT | Unknown | Descending colon | Hartmann’s procedure |
| Blair 2015 [75] | 1 | F | 86 | CT-X-ray | Unknown | All colon | Conservative |
| Chandola 2015 [76] | 1 | M | 59 | CT-X-ray | Unknown | Ascending-transverse colon | Conservative |
| Grimm 2015 [77] | 1 | M | 21 | CT | Unknown | Ascending colon | Conservative |
| Choi 2014 [78] | 1 | F | 74 | CT-X-ray | Unknown | Ascending-transverse colon | Conservative |
| Aziret 2014 [79] | 1 | M | 62 | CT-X-ray | Unknown | Small bowel-cecum | Ileocecal resection |
| Rodrigues-Pinto 2014 [80] | 1 | M | 67 | Colonoscopy | Unknown | - | Conservative |
| Jacob 2014 [81] | 1 | M | 40 | Colonoscopy | Unknown | - | LAR-ileostomy |
| Neesse 2015 [82] | 1 | M | 81 | CT-US | Unknown | Ascending colon | Right colectomy |
| Santos-Antunes 2014 [83] | 1 | M | 73 | Colonoscopy | Unknown | Ascending colon | Conservative |
| Martis 2014 [84] | 1 | F | 77 | CT | Unknown | Descending colon | Conservative |
| Krüger 2014 [85] | 1 | M | 54 | CT-US | Unknown | - | Conservative |
| Tseng 2014 [86] | 1 | F | 50 | CT-X-ray | Unknown | Ascending colon | Conservative |
| Rajpal 2014 [87] | 1 | F | 56 | CT-X-ray | Unknown | Ascending colon | Colic resection |
| Bamakhrama 2014 [88] | 1 | F | 85 | CT-colonoscopy-US | Unknown | Descending colon | Conservative |
| Chao 2014 [89] | 1 | F | 40 | CT | Unknown | Small bowel | Conservative |
| Jurado-Romàn 2014 [90] | 1 | M | 87 | CT | Unknown | Small bowel | Conservative |
| Pinto Pais 2014 [91] | 1 | F | 43 | CT-US | Unknown | Small bowel-cecum | Conservative |
| Lemos 2014 [92] | 1 | F | 39 | CT-colonoscopy-X-ray | Appendicitis | Cecum-ascending colon | Right colectomy |
| Qin 2014 [93] | 1 | M | 29 | CT-colonoscopy | Colonoscopy complication | Ascending-transverse colon | Conservative |
| Lim 2014 [94] | 1 | F | 28 | CT-X-ray | Unknown | - | Conservative |
| Nakajima 2013 [95] | 1 | M | 52 | CT-X-ray | Steroid therapy | - | Conservative |
| Bareggi 2014 [96] | 1 | F | 32 | CT | Unknown | - | Conservative |
| Fong 2014 [97] | 1 | M | 85 | CT-colonoscopy-X-ray | Sigmoid cancer | Ascending colon | Endoscopic stent |
| Zarbalian 2013 [98] | 1 | F | 51 | CT | Steroid therapy | - | Right colectomy |
| Lommen 2020 [99] | 1 | F | 65 | Colonoscopy-barium enema | Unknown | All colon | Conservative |
| Ezuka 2013 [100] | 1 | F | 62 | CT | Steroid therapy | Ascending colon | Conservative |
| Siddiqui 2013 [101] | 1 | M | 35 | CT-X-ray | Pancreatitis | Ascending colon | Conservative |
| Tanabe 2013 [102] | 1 | F | 80 | CT-X-ray | Alpha-Glucosidasy Inhibitor | - | Conservative (Alpha-Glucosidasy Inhibitor suspension) |
| Adar 2013 [103] | 1 | M | 63 | CT-colonoscopy- X-ray | Unknown | Descending colon | Alpha-Glucosidasy Inhibitor |
| Rahim 2013 [104] | 1 | M | 39 | CT | Steroid therapy | Cecum | Right colectomy |
| Liang 2013 [105] | 1 | M | 88 | CT | Unknown | Cecum-ascending colon | Conservative |
| Ponz de Leon 2013 [106] | 1 | M | 54 | Colonoscopy | Unknown | All colon | Total colectomy (FAP) |
| Mourra 2013 [107] | 1 | M | 50 | Colonoscopy | Unknown | All colon | Total colectomy (FAP) |
| Masuda 2013 [108] | 1 | F | 68 | Colonoscopy | Unknown | Ascending colon | Conservative |
| Kashima 2012 [109] | 1 | F | 77 | CT | Sorafenib | Unknown | None (death) |
| Aitken 2012 [110] | 1 | F | 69 | CT | Unknown | All colon | None (death) |
| Makni 2012 [111] | 1 | M | 56 | CT-X-ray | Unknown | Unknown | Conservative |
| Balbir-Gurman 2012 [112] | 1 | F | 76 | CT-X-ray | Unknown | Sigmoid | Conservative |
| Schieber 2012 [113] | 1 | F | 19 | CT-colonoscopy | IBD | Cecum-ascending colon | Conservative |
| Lee 2012 [114] | 1 | F | 66 | CT-X-ray | Gefitinib | All colon | Conservative (Gefitinib suspension) |
| Vijayakanthan 2012 [115] | 2 | M (2) | 27.5 (Mean) | CT-X-ray | Imatinib | Cecum (1)-Transverse colon | Conservative (Imatinib suspension) |
| Chang 2012 [116] | 1 | M | 85 | CT-X-ray | Bowel Ischemia | Ascending colon | None (death) |
| Martin-Smith 2011 [117] | 1 | M | 34 | CT | Necrotizing pancreatitis | Cecum-ascending colon | Conservative |
| Hong 2012 [118] | 1 | F | 75 | CT | Unknown | Ascending colon | Laparoscopic exploration (ileostomy) |
| Hoot 2013 [119] | 1 | F | 57 | CT | Trauma | Ascending-transverse-sigmoid colon | Conservative |
| Shimada 2011 [120] | 1 | M | 43 | CT | Unknown | Cecum-ascending colon | Conservative |
| Iwasaku 2012 [121] | 1 | F | 82 | CT | Gefitinib | Ascending colon | Conservative (Gefitinib suspension) |
| Nancy 2013 [122] | 1 | M | 22 | CT | Colonoscopy complication | Ascending-transverse colon | Conservative |
| Sagara 2012 [123] | 2 | F (2) | 48.5 (Mean) | CT (2) | Steroid therapy (1) | Sigmoid (1) | Colostomy (1)- Conservative (1) |
| Jarkowski 2011 [124] | 1 | M | 73 | CT | Sunitinib | Ascending-transverse colon | Conservative (Sunitinib suspension) |
| Wu 2011 [125] | 1 | F | 67 | CT-colonoscopy | Alpha-Glucosidasy Inhibitor | Ascending colon | Conservative (Alpha-Glucosidasy Inhibitor suspension) |
| Yoon 2011 [126] | 3 | M (1) F (2) | 59.6 (Mean) | CT (3) | Cetuximab | Cecum (2)-ascending (2)-transverse colon (2) | Conservative (Cetuximab suspension) |
| Lioger 2012 [127] | 1 | M | 67 | CT | Collagen Disorders | Unknown | Unknown |
| Arenal 2011 [128] | 1 | F | 18 | CT | Unknown | Cecum | Conservative |
| Amrein 2011 [129] | 2 | M (1) F (1) | 61.5 (Mean) | CT-colonoscopy | Unknown | Ascending colon (2) | Right colectomy (1) Conservative (1) |
| García-Castellanos 2011 [130] | 1 | F | 32 | CT-colonoscopy | Unknown | Descending-sigmoid colon-rectum | Left colectomy |
| Strote 2012 [131] | 1 | M | 57 | CT-US | Unknown | Ascending colon | Small Bowel Resection-Superior Mesentery Artery Thrombectomy |
| Kim 2011 [132] | 1 | F | 40 | CT-colonoscopy | Unknown | Sigmoid | Conservative |
| Shimojima 2011 [133] | 1 | M | 48 | CT-X-ray | Glimepiride Voglibose | Ascending colon | Conservative (Glimepiride Voglibose suspension) |
| Wright 2011 [134] | 1 | F | 42 | CT-X-ray | Unknown | Cecum | Conservative |
| Marinello 2010 [135] | 1 | M | 20 | CT | LES | Unknown | Conservative |
| Pasquier 2011 [136] | 1 | F | 96 | CT | Unknown | Unknown | Conservative |
| Bamba 2010 [137] | 2 | M (1) F (1) | 43.5 (Mean) | CT-colonoscopy-X-ray (1) | Colonoscopy complication | Cecum (1)-ascending (1)-transverse colon (1) | Conservative |
| Huang 2010 [138] | 1 | M | 30 | CT-X-ray | Transplantation complication | Ascending colon | Conservative |
| Liao 2010 [139] | 1 | M | 48 | CT-US | Colonic trauma | Ascending colon | Right colectomy |
| Chaput 2010 [140] | 1 | M | 57 | Colonoscopy-X-ray-Manometry | Unknown | Rectum | Conservative |
| Ong 2010 [141] | 1 | M | 69 | CT-X-ray | Volvulus | All colon | Total colectomy |
| Newman 2010 [142] | 1 | M | 26 | CT-X-ray | GVHD | Unknown | Conservative |
| Syed 2020 [143] | 1 | M | 60 | CT-colonoscopy | Clindamycin | Sigmoid | Conservative |
| Meini 2020 [144] | 1 | M | 44 | CT | COVID-19 | Ascending colon | Conservative |
| Miwa 2020 [145] | 1 | M | 58 | CT-colonoscopy | Unknown | Ascending-transverse colon | Conservative |
| Kielty 2020 [146] | 1 | M | 47 | CT | COVID-19 | Small bowel-cecum | Conservative |
| Lakshmanan 2020 [147] | 1 | M | 72 | CT | COVID-19 | Ascending-sigmoid colon | Conservative |
| Hokama 2020 [148] | 1 | M | 91 | CT | Strongyloides Stercoralis | Small bowel-all colon | Conservative |
| Wang 2020 [149] | 2 | M (2) | 90 (Mean) | CT | Unknown | Small bowel-all colon | Conservative |
| Ribolla 2020 [150] | 1 | F | 65 | CT | Unknown | Ascending colon | Laparotomy exploration |
| Zhang 2012 [151] | 1 | M | 60 | CT | IBD | Transverse-descending-sigmoid colon | Conservative |
| Ferrada 2017 [152] | 127 | - | 57 (Mean) | CT (117)-X-ray (8) | Unknown | Small bowel (61)-cecum (40)-ascending (60)-transverse (17)-descending colon (12)-sigmoid (11)-rectum (3) | Surgery (70) Conservative (57) |
| Matsumoto 2016 [153] | 70 | M (38) F (32) | 72 (Mean) | CT (70) | Unknown | Small bowel (42)-ascending (20)-descending colon (8) | Surgery (39) Conservative |
| Bani 2013 [154] | 209 | - | 56.8 (Mean) | CT (209) | Obstruction (53)- ischemia (53) | Unknown | Surgery |
| DuBose 2013 [6] | 500 | M (283) F (217) | 56.6 (Mean) | CT (500) | IBD (18)- Colonoscopy complication (57) | Small bowel (305)-colon (285)-rectum (3) | Surgery (199) Conservative (301) |
| Gupta 2020 [155] | 1 | F | 81 | CT | Unknown | Small bowel | Conservative |
| Muhammad Nawawi 2020 [156] | 1 | M | 38 | CT | Unknown | Small bowel | Conservative |
| Gomes 2020 [157] | 1 | F | 90 | CT | Sigmoid cancer | Small bowel | Surgery |
| Takimoto 2020 [158] | 1 | F | 75 | CT | M. avium-amyloidosis | Colon | Conservative |
| Lim 2020 [159] | 1 | M | 68 | CT | Unknown | Small bowel | Surgery |
| Fairley 2020 [160] | 1 | M | 71 | CT | Colonoscopy complication | Colon | Conservative |
| Molina 2020 [161] | 1 | F | 72 | CT-X-ray | Unknown | Small bowel | Surgery |
| Arai 2020 [162] | 25 | M (17) F (8) | 75 (Mean) | CT | Unknown | Colon-Small bowel | Surgery (17) Conservative (8) |
| Police 2020 [163] | 1 | M | 72 | CT | Sigmoid volvulus | Sigmoid colon | Surgery (Sigmoidectomy) |
| Wheatley 2020 [164] | 1 | F | 52 | CT | Unknown | Small bowel | Surgery |
| Tsang 2019 [165] | 2 | M (1) F (1) | 67.5 (Mean) | CT (2) | Unknown | Small bowel-ascending colon | Surgery (1) Conservative (1) |
| Chen 2019 [166] | 1 | M | 63 | CT-US | Unknown | Small bowel | Surgery |
| Kim 2019 [167] | 2 | M (2) | 75.5 (Mean) | CT | Cardiac surgery | Small bowel | Suergery (2) |
| Furutani 2019 [168] | 1 | M | 69 | CT | Colic resection | Ascending colon | Conservative |
| Varelas 2019 [169] | 11 | M (9) F (2) | 61 (Mean) | CT | Lactulose (9) Unknown (2) | Small bowel (1)-colon (11) | Surgery (2) Conservative (9) |
| Arai 2019 [170] | 1 | M | 51 | CT | Unknown | Small bowel | Surgery |
| Belkhir 2019 [171] | 1 | M | 28 | CT-US | Unknown | Small bowel | Surgery |
| Khan 2019 [172] | 1 | F | 70 | CT | Capecitabine | Small bowel | Surgery |
| Di Pietropaolo 2019 [173] | 1 | F | 78 | CT | Chemotherapy | Small bowel-ascending colon | Surgery |
| Perez Rivera 2019 [174] | 1 | M | 19 | CT | Previous gastrostomy | Small bowel-colon | Conservative |
| Ibrahim 2019 [175] | 1 | M | 69 | CT | Unknown | Small bowel | Surgery |
| Harris 2019 [176] | 1 | F | 57 | CT-X-ray | Jejunal lymphangioma | Small bower | Surgery |
| Bansal 2019 [177] | 1 | M | 52 | CT | Unknown | Small bowel | Surgery |
| Dhadlie 2018 [178] | 2 | M (2) | 80.5 (Mean) | CT (1)-X-ray (1) | Unknown | Small bowel | Surgery (1) Conservative (1) |
| Sanford 2018 [179] | 4 | M (2) F (2) | 77 (Mean) | CT (4) | Unknown | Small bowel (2)-cecum (2)-ascending-sigmoid colon | Surgery (3) Conservative (1) |
| Guan 2018 [180] | 1 | F | 78 | CT | Systemic sclerosis | Small bowel-colon | Surgery |
| Gray 2018 [181] | 1 | F | 64 | CT | Unknown | Small bowel | Surgery |
| Fujimi 2016 [182] | 1 | M | 55 | CT | Nilotinib | Small bowel | Conservative |
| Yamamamoto 2020 [183] | 1 | F | 70 | CT-colonoscopy | Unknown | Ascending colon | Conservative |
| Dibra 2020 [184] | 1 | F | 60 | CT-X-ray | Unknown | Small bowel | Surgery |
| Fukunaga 2022 [185] | 1 | F | 81 | CT | Cardiac surgery | Small bowel | Surgery |
| Furtado 2022 [186] | 1 | F | 81 | CT | Unknown | Small Bowel | Conservative |
| Sharp 2022 [187] | 1 | M | 61 | CT-X-ray | Pseudomonas aeruginosa | Small bowel | Conservative |
| Gefen 2022 [188] | 1 | M | 40 | CT-X-ray | Steroid therapy | Ascending colon | Surgery |
| Yadzi 2021 [189] | 1 | M | 30 | CT | Ileal volvulus | Small bowel | Surgery |
| Yeo 2021 [190] | 2 | M | 71.5 (Mean) | CT-X-ray | Steroid therapy Chemotherapy | Small bowel | Surgery (1) Conservative (1) |
| Brocchi 2021 [191] | 8 | M (5) F (3) | 65.5 (Mean) | CT | Chemotherapy | Small bowel (2) Colon (6) | Surgery Conservative |
| Yamamoto 2021 [192] | 1 | M | 43 | CT | Steroid therapy | Colon | Conservative |
| Della seta 2021 [193] | 290 | M (171) F (119) | 66.7 (Mean) | CT | Obstruction (110) Ischemia (94) Volvulus-Intussusception (43) Sepsis (78) | Unknown | Surgery (155) Conservative (135) |
| Adachi 2020 [194] | 21 | M (12) F (9) | 80.1 (Mean) | CT | Steroid therapy (3) Chemotherapy (1) Alpha-Glucosidasy Inhibitor (1) Unknown (16) | Small bowel (12) Colon (6) | Conservative |
| Epin 2022 [195] | 58 | M (37) F (21) | 72.0 (Mean) | CT | Unknown | Small Bowel | Surgery (25) Conservative (33) |
| Treyaud 2017 [196] | 149 | M (96) F (53) | 64.0 (Mean) | CT | Obstruction (10) Ischemia (80) | Small Bowel (72) Colon (96) | Surgery (51) Conservative (98) |
| Parameters | Analyzed Variable | No, % | Mean ± SD |
|---|---|---|---|
| Sex | Female | 581, 34.7% | |
| Male | 773, 46.2% | ||
| Not reported | 319, 19.0% | ||
| Mean age (years) | All considered patients | 67.1 ± 17.6 | |
| Etiology | Known | 871, 52.0% | |
| Unknown | 802, 47.9% | ||
| Diagnostic findings | Signs of bowel ischemia | 564, 33.7% | |
| Portal vein gas | 556, 33.2% | ||
| Pneumoperitoneum | 301, 17,9% |
| Etiology | (No. of Patients, % *) |
|---|---|
| Bowel Obstruction | 278, 16.6% |
| Steroid Therapy | 120, 7.1% |
| Colonoscopy Complications | 64, 3.8% |
| Large Bowel Ischemia | 228, 13.6% |
| IBD complications | 54, 3.2% |
| Monoclonal Antibody Drugs | 16, 0.9% |
| Other | 68, 4.0% |
| Unknown | 802, 47.9% |
| Total PI (n.) | 1673, 100% |
| Therapeutic Approach | No (1673), 100% * |
|---|---|
| NOM | 824, 49.2% |
| Drugs discontinuation | 96, 11.7% |
| Antibiotics-TPN | 166, 20.1% |
| IBD therapy | 54, 6.5% |
| NR NOM | 508, 61.6% |
| Surgery | 619, 36.9% |
| Bowel resection | 237, 38.2% |
| Laparoscopic/Laparotomy exploration (no resections) | 155, 25.0% |
| NR Surgical treatment | 227, 36.6% |
| NR | 230, 13.7% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Perrone, G.; Giuffrida, M.; Donato, V.; Petracca, G.L.; Rossi, G.; Franzini, G.; Cecconi, S.; Annicchiarico, A.; Bonati, E.; Catena, F. The Challenge of Pneumatosis Intestinalis: A Contemporary Systematic Review. J. Pers. Med. 2024, 14, 167. https://doi.org/10.3390/jpm14020167
Perrone G, Giuffrida M, Donato V, Petracca GL, Rossi G, Franzini G, Cecconi S, Annicchiarico A, Bonati E, Catena F. The Challenge of Pneumatosis Intestinalis: A Contemporary Systematic Review. Journal of Personalized Medicine. 2024; 14(2):167. https://doi.org/10.3390/jpm14020167
Chicago/Turabian StylePerrone, Gennaro, Mario Giuffrida, Valentina Donato, Gabriele Luciano Petracca, Giorgio Rossi, Giacomo Franzini, Sara Cecconi, Alfredo Annicchiarico, Elena Bonati, and Fausto Catena. 2024. "The Challenge of Pneumatosis Intestinalis: A Contemporary Systematic Review" Journal of Personalized Medicine 14, no. 2: 167. https://doi.org/10.3390/jpm14020167
APA StylePerrone, G., Giuffrida, M., Donato, V., Petracca, G. L., Rossi, G., Franzini, G., Cecconi, S., Annicchiarico, A., Bonati, E., & Catena, F. (2024). The Challenge of Pneumatosis Intestinalis: A Contemporary Systematic Review. Journal of Personalized Medicine, 14(2), 167. https://doi.org/10.3390/jpm14020167







