Attenuated Total Reflection Fourier-Transform Infrared Spectral Discrimination in Human Tissue of Oesophageal Transformation to Adenocarcinoma
Abstract
1. Introduction
2. Material and Methods
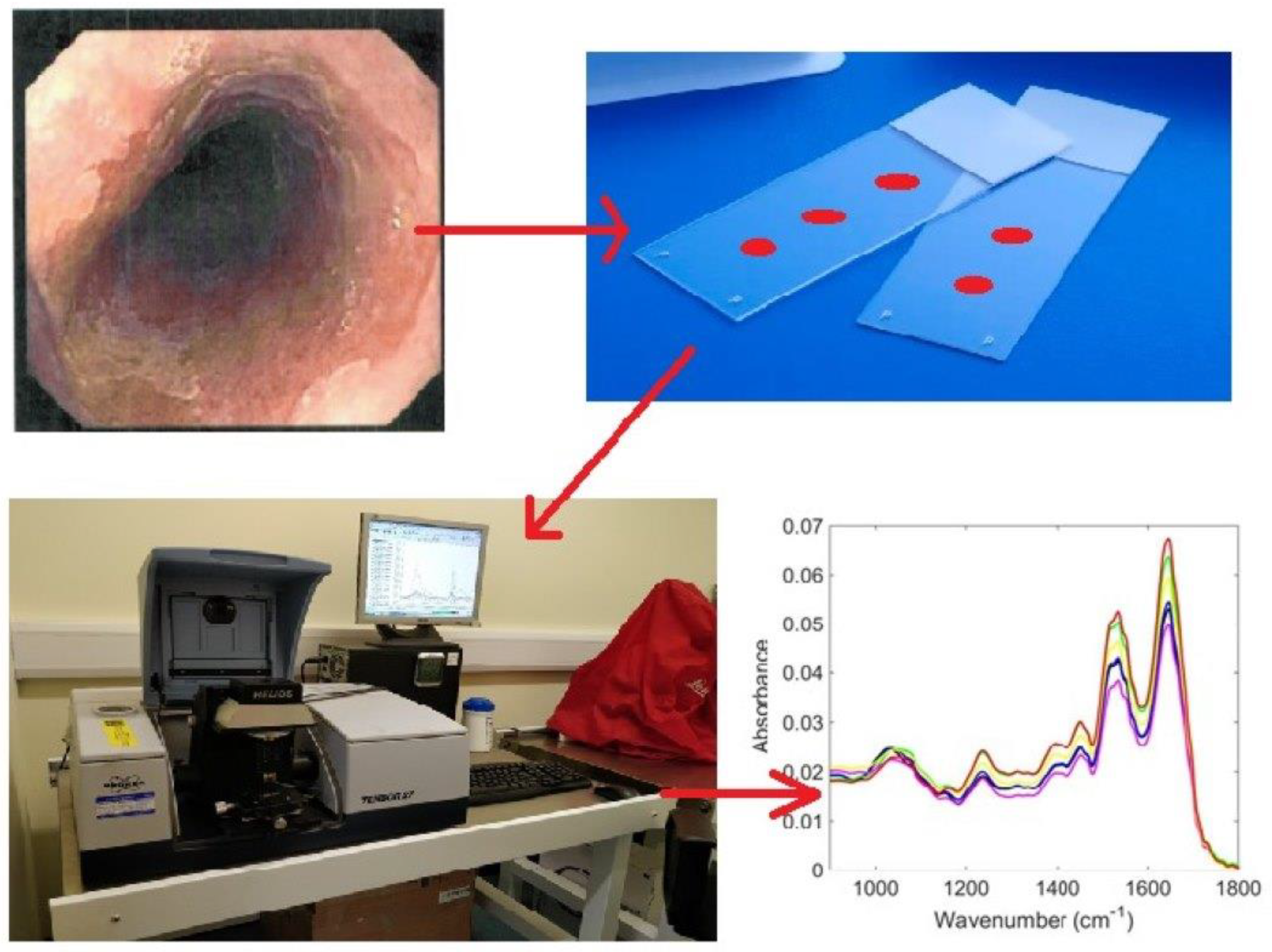
2.1. Sample Collection
2.2. ATR-FTIR Spectroscopy
2.3. Data Analysis and Chemometric Methods
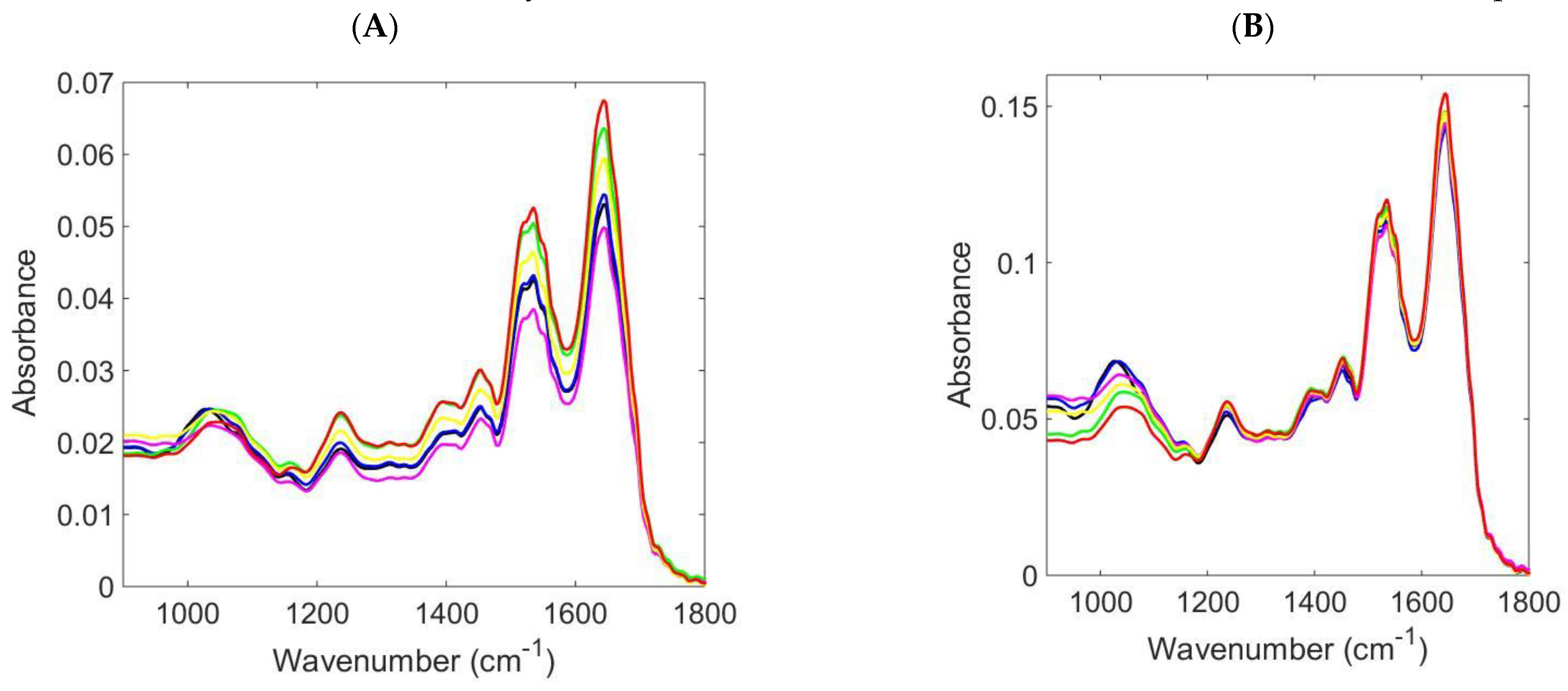
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bunaciu, A.A.; Hoang, V.D.; Aboul-Enein, H.Y. Vibrational Micro-Spectroscopy of Human Tissues Analysis: Review. Crit. Rev. Anal. Chem. 2017, 47, 194–203. [Google Scholar] [CrossRef] [PubMed]
- Hanna, K.; Krzoska, E.; Shaaban, A.M.; Muirhead, D.; Abu-Eid, R.; Spiers, V. Raman spectroscopy: Current applications in breast cancer diagnosis, challenges and future prospects. Br. J. Cancer 2022, 126, 1125–1139. [Google Scholar] [CrossRef]
- Jeng, M.J.; Sharma, M.; Sharma, L.; Chao, T.Y.; Huang, S.F.; Chang, L.B.; Wu, S.L.; Chow, L. Raman Spectroscopy Analysis for Optical Diagnosis of Oral Cancer Detection. J. Clin. Med. 2019, 8, 1313. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.B.; Sun, X.J.; Xu, Y.Z.; Yang, L.M.; Zhang, Y.F.; Weng, S.F.; Shi, J.S.; Wu, J.G. Use of Fourier-transform infrared spectroscopy to rapidly diagnose gastric endoscopic biopsies. World J. Gastroenterol. 2005, 11, 3842–3845. [Google Scholar] [CrossRef][Green Version]
- Song, C.L.; Kazarian, S.G. Micro ATR-FTIR spectroscopic imaging of colon biopsies with a large area Ge crystal. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2020, 228, 117695. [Google Scholar] [CrossRef] [PubMed]
- Noothalapati, H.; Iwasaki, K.; Yamamoto, T. Non-invasive diagnosis of colorectal cancer by Raman spectroscopy: Recent developments in liquid biopsy and endoscopy approaches. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2021, 258, 119819. [Google Scholar] [CrossRef]
- Isabelle, M.; Dorney, J.; Lewis, A.; Lloyd, G.R.; Old, O.; Shepherd, N.; Rodriguez-Justo, M.; Barr, H.; Lau, K.; Bell, I.; et al. Multi-centre Raman spectral mapping of oesophageal cancer tissues: A study to assess system transferability. Faraday Discuss. 2016, 187, 87–103. [Google Scholar] [CrossRef]
- Old, O.J.; Lloyd, G.R.; Nallala, J.; Isabelle, M.; Almond, L.M.; Shepherd, N.A.; Kendall, C.A.; Shore, A.C.; Barr, H.; Stone, N. Rapid infrared mapping for highly accurate automated histology in Barrett’s oesophagus. Analyst 2017, 142, 1227–1234. [Google Scholar] [CrossRef]
- Maziak, D.E.; Do, M.T.; Shamji, F.M.; Sundaresan, S.R.; Perkins, D.G.; Wong, P.T. Fourier-transform infrared spectroscopic study of characteristic molecular structure in cancer cells of esophagus: An exploratory study. Cancer Detect. Prev. 2007, 31, 244–253. [Google Scholar] [CrossRef]
- Wallace, M.B.; Wax, A.; Roberts, D.N.; Graf, R.N. Reflectance spectroscopy. Gastrointest. Endosc. Clin. N. Am. 2009, 19, 233–242. [Google Scholar] [CrossRef]
- Maitra, I.; Date, R.S.; Martin, F.L. Towards screening Barrett’s oesophagus: Current guidelines, imaging modalities and future developments. Clin. J. Gastroenterol. 2020, 13, 635–649. [Google Scholar] [CrossRef] [PubMed]
- Lipman, G.; Haidry, R. Endoscopic management of Barrett’s and early oesophageal neoplasia. Frontline Gastroenterol. 2017, 8, 138–142. [Google Scholar] [CrossRef] [PubMed]
- Tropini, C.; Moss, E.L.; Merrill, B.D.; Ng, K.M.; Higginbottom, S.K.; Casavant, E.P.; Gonzalez, C.G.; Fremin, B.; Bouley, D.M.; Elias, J.E.; et al. Transient Osmotic Perturbation Causes Long-Term Alteration to the Gut Microbiota. Cell 2018, 173, 1742–1754.e17. [Google Scholar] [CrossRef] [PubMed]
- Shaheen, N.J.; Falk, G.W.; Iyer, P.G.; Gerson, L.B. ACG Clinical Guideline: Diagnosis and Management of Barrett’s Esophagus. Am. J. Gastroenterol. 2016, 111, 30–50. [Google Scholar] [CrossRef] [PubMed]
- Pahlow, S.; Weber, K.; Popp, J.; Wood, B.R.; Kochan, K.; Rüther, A.; Perez-Guaita, D.; Heraud, P.; Stone, N.; Dudgeon, A.; et al. Application of Vibrational Spectroscopy and Imaging to Point-of-Care Medicine: A Review. Appl. Spectrosc. 2016, 72 (Suppl. 1), 52–84. [Google Scholar] [CrossRef]
- Baker, M.J.; Trevisan, J.; Bassan, P.; Bhargava, R.; Butler, H.J.; Dorling, K.M.; Fielden, P.R.; Fogarty, S.W.; Fullwood, N.J.; Heys, K.A.; et al. Using Fourier transform IR spectroscopy to analyze biological materials. Nat. Protoc. 2014, 9, 1771–1791. [Google Scholar] [CrossRef]
- Morais, C.L.M.; Paraskevaidi, M.; Cui, L.; Fullwood, N.J.; Isabelle, M.; Lima, K.M.G.; Martin-Hirsch, P.L.; Sreedhar, H.; Trevisan, J.; Walsh, M.J.; et al. Standardization of complex biologically derived spectrochemical datasets. Nat. Protoc. 2019, 14, 1546–1577. [Google Scholar] [CrossRef]
- Wiens, R.; Rak, M.; Cox, N.; Abraham, S.; Juurlink, B.H.J.; Kulyk, W.M.; Gough, K.M. Synchrotron FTIR microspectroscopic analysis of the effects of anti-inflammatory therapeutics on wound healing in laminectomized rats. Anal. Bioanal. Chem. 2007, 387, 1679–1689. [Google Scholar] [CrossRef]
- Meuse, C.W.; Barker, P.E. Quantitative Infrared Spectroscopy of Formalin-fixed, Paraffin-embedded Tissue Specimens, Paraffin Wax Removal with Organic Solvents. Appl. Immunohistochem. Mol. Morphol. 2009, 17, 547–552. [Google Scholar] [CrossRef]
- Nallala, J.; Lloyd, G.R.; Stone, N. Evaluation of different tissue de-paraffinization procedures for infrared spectral imaging. Analyst 2015, 140, 2369–2375. [Google Scholar] [CrossRef]
- Fitzgerald, R.C.; Pietro, M.; Ragunath, K.; Ang, Y.; Kang, J.; Watson, P.; Trudgill, N.; Patel, P.; Kaye, P.V.; Sanders, S.; et al. British Society of Gastroenterology guidelines on the diagnosis and management of Barrett’s oesophagus. Gut 2014, 63, 7–42. [Google Scholar] [CrossRef] [PubMed]
- Maitra, I.; Morais, C.L.M.; Lima, K.M.G.; Ashton, K.M.; Date, R.S.; Martin, F.L. Attenuated total reflection Fourier-transform infrared spectral discrimination in human bodily fluids of oesophageal transformation to adenocarcinoma. Analyst 2019, 144, 7447–7456. [Google Scholar] [CrossRef] [PubMed]
- Wood, B.R.; Quinn, M.A.; Tait, B.; Ashdown, M.; Hislop, T.; Romeo, M.; McNaughton, D. FTIR microspectroscopic study of cell types and potential confounding variables in screening for cervical malignancies. Biospectroscopy 1998, 4, 75–91. [Google Scholar] [CrossRef]
- Xu, Q.-S.; Liang, Y.-Z. Monte Carlo cross validation. Chemom. Intell. Lab. Syst. 2001, 56, 1–11. [Google Scholar] [CrossRef]
- Morais, C.L.M.; Lima, K.M.G.; Martin, F.L. Uncertainty estimation and misclassification probability for classification models based on discriminant analysis and support vector machines. Anal. Chim. Acta 2019, 1063, 40–46. [Google Scholar] [CrossRef] [PubMed]
- Sahu, R.K.; Salman, A.; Mordechai, S. Tracing overlapping biological signals in mid-infrared using colonic tissues as a model system. World J. Gastroenterol. 2017, 23, 286–296. [Google Scholar] [CrossRef]
- Wang, J.S.; Shi, J.S.; Xu, Y.Z.; Duan, X.Y.; Zhang, L.; Wang, J.; Yang, L.M.; Weng, S.F.; Wu, J.G. FT-IR spectroscopic analysis of normal and cancerous tissues of esophagus. World J. Gastroenterol. 2003, 9, 1897–1899. [Google Scholar] [CrossRef]
- Wu, J.-G.; Xu, Y.-Z.; Sun, C.-W.; Soloway, R.D.; Xu, D.-F.; Wu, Q.-G.; Sun, K.-H.; Weng, S.-F.; Xu, G.-X. Distinguishing malignant from normal oral tissues using FTIR fiber-optic techniques. Biopolymer 2001, 62, 185–192. [Google Scholar] [CrossRef]
- Wood, B.R.; Quinn, M.A.; Burden, F.R.; McNaughton, D. An investigation into FT-IR spectroscopy as a bio-diagnostic tool for cervical cancer. Biospectroscopy 1996, 2, 143–153. [Google Scholar] [CrossRef]
- Chiriboga, L.; Xie, P.; Yee, H.; Vigorita, V.; Zarou, D.; Zakim, D.; Diem, M. Infrared spectroscopy of human tissue. I. Differentiation and maturation of epithelial cells in the human cervix. Biospectroscopy 1998, 4, 47–53. [Google Scholar] [CrossRef]
- Sukuta, S.; Bruch, R. Factor analysis of cancer Fourier transform infrared evanescent wave fiberoptical (FTIR-FEW) spectra. Lasers Surg. Med. 1999, 24, 382–388. [Google Scholar] [CrossRef]
- Quaroni, L.; Casson, A.G. Characterization of Barrett esophagus and esophageal adenocarcinoma by Fourier-transform infrared microscopy. Analyst 2009, 134, 1240–1246. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.D.; Triadafilopoulos, G.; Crawford, J.M.; Dixon, L.R.; Bhandari, T.; Sahbaie, P.; Friedland, S.; Soetikno, R.; Contag, C.H. Detection of endogenous biomolecules in Barrett’s esophagus by Fourier transform infrared spectroscopy. Proc. Natl. Acad. Sci. USA 2007, 104, 15864–15869. [Google Scholar] [CrossRef]
- Theophilou, G.; Lima, K.M.G.; Martin-Hirsch, P.L.; Stringfellow, H.F.; Martin, F.L. ATR-FTIR spectroscopy coupled with chemometric analysis discriminates normal, borderline and malignant ovarian tissue: Classifying subtypes of human cancer. Analyst 2016, 141, 585–594. [Google Scholar] [CrossRef] [PubMed]
- Andleed, F.; Janjua, H.U.; Atiq, A.; Atiq, M.; Malik, S. Attenuated total reflection spectroscopy to diagnose skin cancer and to distinguish different metastatic potential of melanoma cell. Cancer Biomark. 2018, 1, 1–8. [Google Scholar]
- Vazquez-Zapien, G.J.; Mata-Miranda, M.M.; Sanchez-Monroy, V.; Delgado-Macuil, R.J.; Perez-Ishiwara, D.G.; Rojas-Lopez, M. FTIR Spectroscopic and Molecular Analysis during Differentiation of Pluripotent Stem Cells to Pancreatic Cells. Stem Cells Int. 2016, 2016, 6709714. [Google Scholar] [CrossRef] [PubMed]
- Paraskevaidi, M.; Morais, C.L.M.; Lima, K.M.G.; Ashton, K.M.; Stringfellow, H.F.; Martin-Hirsch, P.L.; Martin, F.L. Potential of mid-infrared spectroscopy as a non-invasive diagnostic test in urine for endometrial or ovarian cancer. Analyst 2018, 143, 3156–3163. [Google Scholar] [CrossRef]
- Cameron, J.M.; Rinaldi, C.; Butler, H.J.; Hegarty, M.G.; Brennan, P.M.; Jenkinson, M.D.; Syed, K.; Ashton, K.M.; Dawson, T.P.; Palmer, D.S.; et al. Stratifying Brain Tumour Histological Sub-Types: The Application of ATR-FTIR Serum Spectroscopy in Secondary Care. Cancers 2020, 12, 1710. [Google Scholar] [CrossRef]
- Leal, L.B.; Nogueirab, M.S.; Canevaria, R.A.; Carvalho, L.F.C.S. Vibration spectroscopy and body biofluids: Literature review for clinical applications. Photodiagnosis Photodyn. Ther. 2018, 24, 237–244. [Google Scholar] [CrossRef]
- Diem, M.; Ergin, A.; Remiszewski, S.; Mu, X.; Akalin, A.; Raz, D. Infrared micro-spectroscopy of human tissue: Principles and future promises. Faraday Discuss. 2016, 187, 9–42. [Google Scholar] [CrossRef]
- Wang, H.P.; Wang, H.-C.; Huang, Y.J. Microscopic FTIR studies of lung cancer cells in pleural fluid. Sci. Total Environ. 1997, 204, 283–287. [Google Scholar]
- Movasaghi, Z.; Rehman, S.; ur Rehman, I. Fourier Transform Infrared (FTIR) Spectroscopy of Biological Tissues. Appl. Spectrosc. Rev. 2008, 43, 75–91. [Google Scholar]
| FOM | PCA-QDA (Accuracy = 90.9%) | |||||
|---|---|---|---|---|---|---|
| Normal | Inflammatory | Barrett’s Oesophagus | LGD | HGD | OAC | |
| Sensitivity (%) | 71.4 | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 |
| Specificity (%) | 100.0 | 89.5 | 100.0 | 100.0 | 100.0 | 100.0 |
| F-Score (%) | 83.3 | 94.5 | 100.0 | 100.0 | 100.0 | 100.0 |
| FOM | SPA-QDA (Accuracy = 86.4%) | |||||
| Normal | Inflammatory | Barrett’s oesophagus | LGD | HGD | OAC | |
| Sensitivity (%) | 100.0 | 0.00 | 100.0 | 100.0 | 100.0 | 100.0 |
| Specificity (%) | 80.0 | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 |
| F-Score (%) | 88.9 | 0.00 | 100.0 | 100.0 | 100.0 | 100.0 |
| FOM | GA-QDA (Accuracy = 63.6%) | |||||
| Normal | Inflammatory | Barrett’s oesophagus | LGD | HGD | OAC | |
| Sensitivity (%) | 28.6 | 0.00 | 100.0 | 100.0 | 100.0 | 100.0 |
| Specificity (%) | 80.0 | 73.7 | 100.0 | 100.0 | 100.0 | 100.0 |
| F-Score (%) | 42.1 | 0.00 | 100.0 | 100.0 | 100.0 | 100.0 |
| Real/Predicted | PCA-QDA | |||||
|---|---|---|---|---|---|---|
| Normal | Inflammatory | Barrett’s Oesophagus | LGD | HGD | OAC | |
| Normal (n = 35) | 25 | 10 | 0 | 0 | 0 | 0 |
| Inflammatory (n = 13) | 0 | 13 | 0 | 0 | 0 | 0 |
| Barrett’s oesophagus (n = 26) | 0 | 0 | 26 | 0 | 0 | 0 |
| LGD (n = 4) | 0 | 0 | 0 | 4 | 0 | 0 |
| HGD (n = 9) | 0 | 0 | 0 | 0 | 9 | 0 |
| OAC (n = 22) | 0 | 0 | 0 | 0 | 0 | 22 |
| Real/Predicted | SPA-QDA | |||||
| Normal | Inflammatory | Barrett’s oesophagus | LGD | HGD | OAC | |
| Normal (n = 35) | 35 | 0 | 0 | 0 | 0 | 0 |
| Inflammatory (n = 13) | 13 | 0 | 0 | 0 | 0 | 0 |
| Barrett’s oesophagus (n = 26) | 0 | 0 | 26 | 0 | 0 | 0 |
| LGD (n = 4) | 0 | 0 | 0 | 4 | 0 | 0 |
| HGD (n = 9) | 0 | 0 | 0 | 0 | 9 | 0 |
| OAC (n = 22) | 0 | 0 | 0 | 0 | 0 | 22 |
| Real/Predicted | GA-QDA | |||||
| Normal | Inflammatory | Barrett’s oesophagus | LGD | HGD | OAC | |
| Normal (n = 35) | 10 | 25 | 0 | 0 | 0 | 0 |
| Inflammatory (n = 13) | 13 | 0 | 0 | 0 | 0 | 0 |
| Barrett’s oesophagus (n = 26) | 0 | 0 | 26 | 0 | 0 | 0 |
| LGD (n = 4) | 0 | 0 | 0 | 4 | 0 | 0 |
| HGD (n = 9) | 0 | 0 | 0 | 0 | 9 | 0 |
| OAC (n = 22) | 0 | 0 | 0 | 0 | 0 | 22 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maitra, I.; Morais, C.L.M.; Lima, K.M.G.; Ashton, K.M.; Bury, D.; Date, R.S.; Martin, F.L. Attenuated Total Reflection Fourier-Transform Infrared Spectral Discrimination in Human Tissue of Oesophageal Transformation to Adenocarcinoma. J. Pers. Med. 2023, 13, 1277. https://doi.org/10.3390/jpm13081277
Maitra I, Morais CLM, Lima KMG, Ashton KM, Bury D, Date RS, Martin FL. Attenuated Total Reflection Fourier-Transform Infrared Spectral Discrimination in Human Tissue of Oesophageal Transformation to Adenocarcinoma. Journal of Personalized Medicine. 2023; 13(8):1277. https://doi.org/10.3390/jpm13081277
Chicago/Turabian StyleMaitra, Ishaan, Camilo L. M. Morais, Kássio M. G. Lima, Katherine M. Ashton, Danielle Bury, Ravindra S. Date, and Francis L. Martin. 2023. "Attenuated Total Reflection Fourier-Transform Infrared Spectral Discrimination in Human Tissue of Oesophageal Transformation to Adenocarcinoma" Journal of Personalized Medicine 13, no. 8: 1277. https://doi.org/10.3390/jpm13081277
APA StyleMaitra, I., Morais, C. L. M., Lima, K. M. G., Ashton, K. M., Bury, D., Date, R. S., & Martin, F. L. (2023). Attenuated Total Reflection Fourier-Transform Infrared Spectral Discrimination in Human Tissue of Oesophageal Transformation to Adenocarcinoma. Journal of Personalized Medicine, 13(8), 1277. https://doi.org/10.3390/jpm13081277







