L-Carnitine and Chronic Kidney Disease: A Comprehensive Review on Nutrition and Health Perspectives
Abstract
1. Introduction
2. Methods
2.1. Search Strategy for the Original Study
2.2. Criteria for the Original Study
2.2.1. Inclusion Criteria
2.2.2. Exclusion Criteria
2.2.3. Study Selection
3. Review Findings
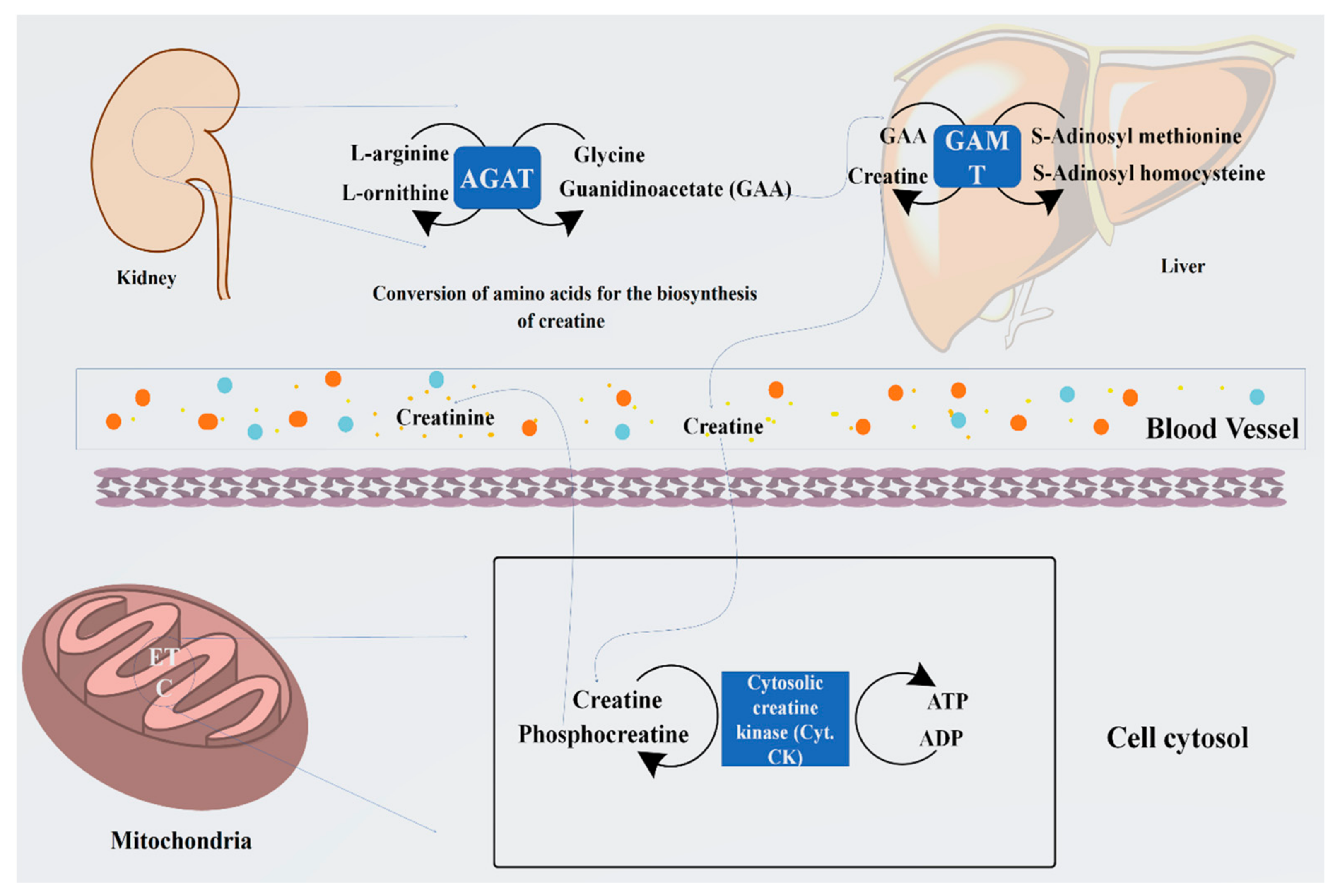
3.1. Sources and Biosynthesis of Creatine
3.2. Perception of LC Supplementation for Kidney Dysfunction
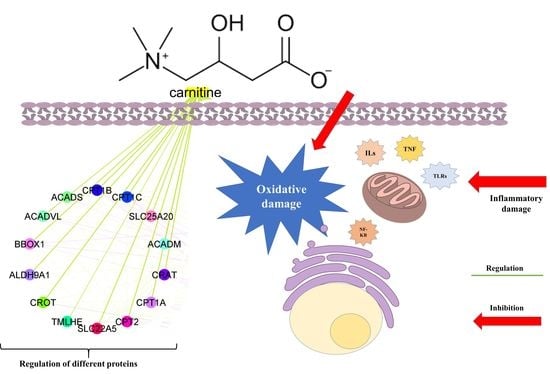
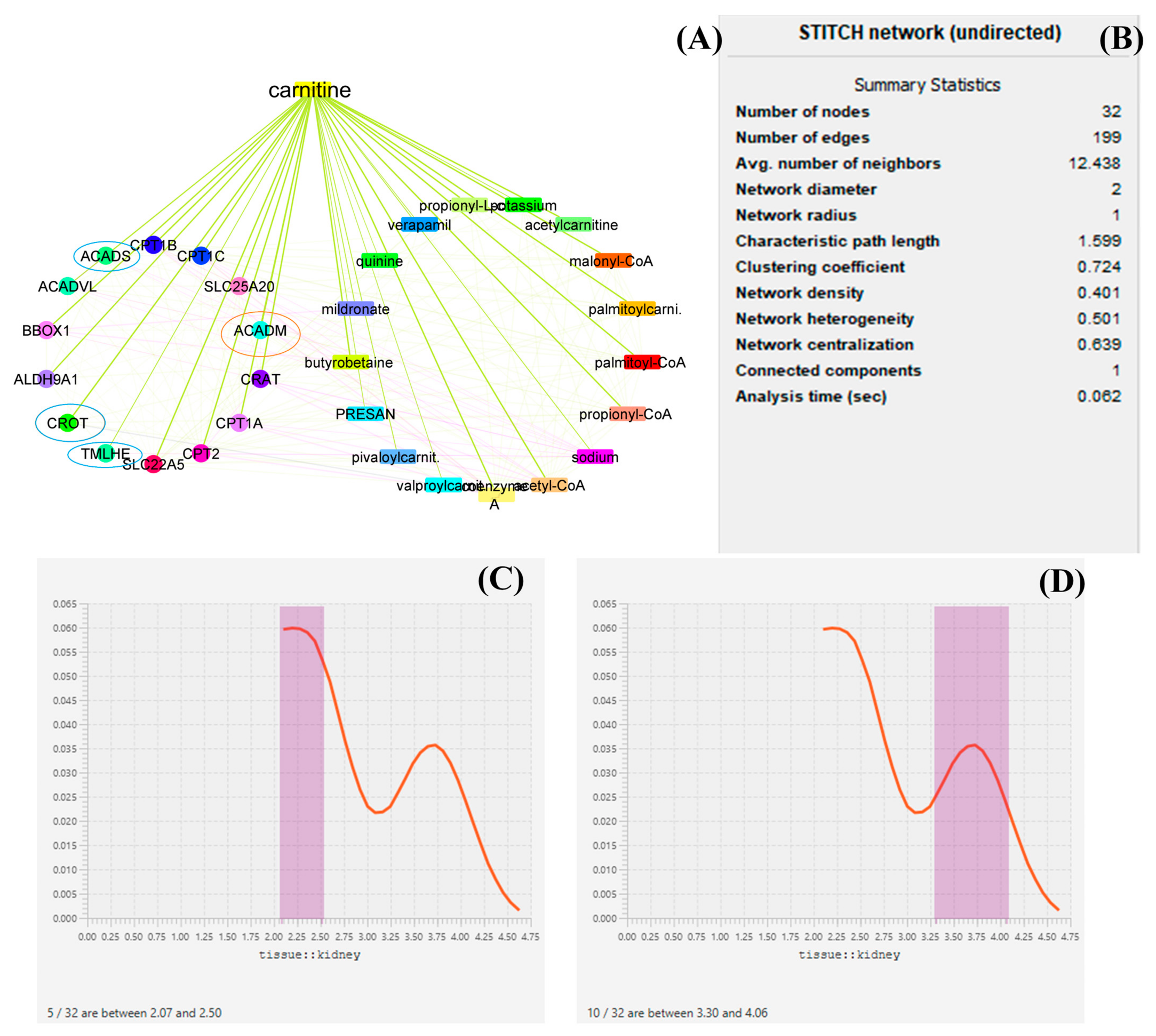
3.3. Network Biology and Polypharmacology of LC
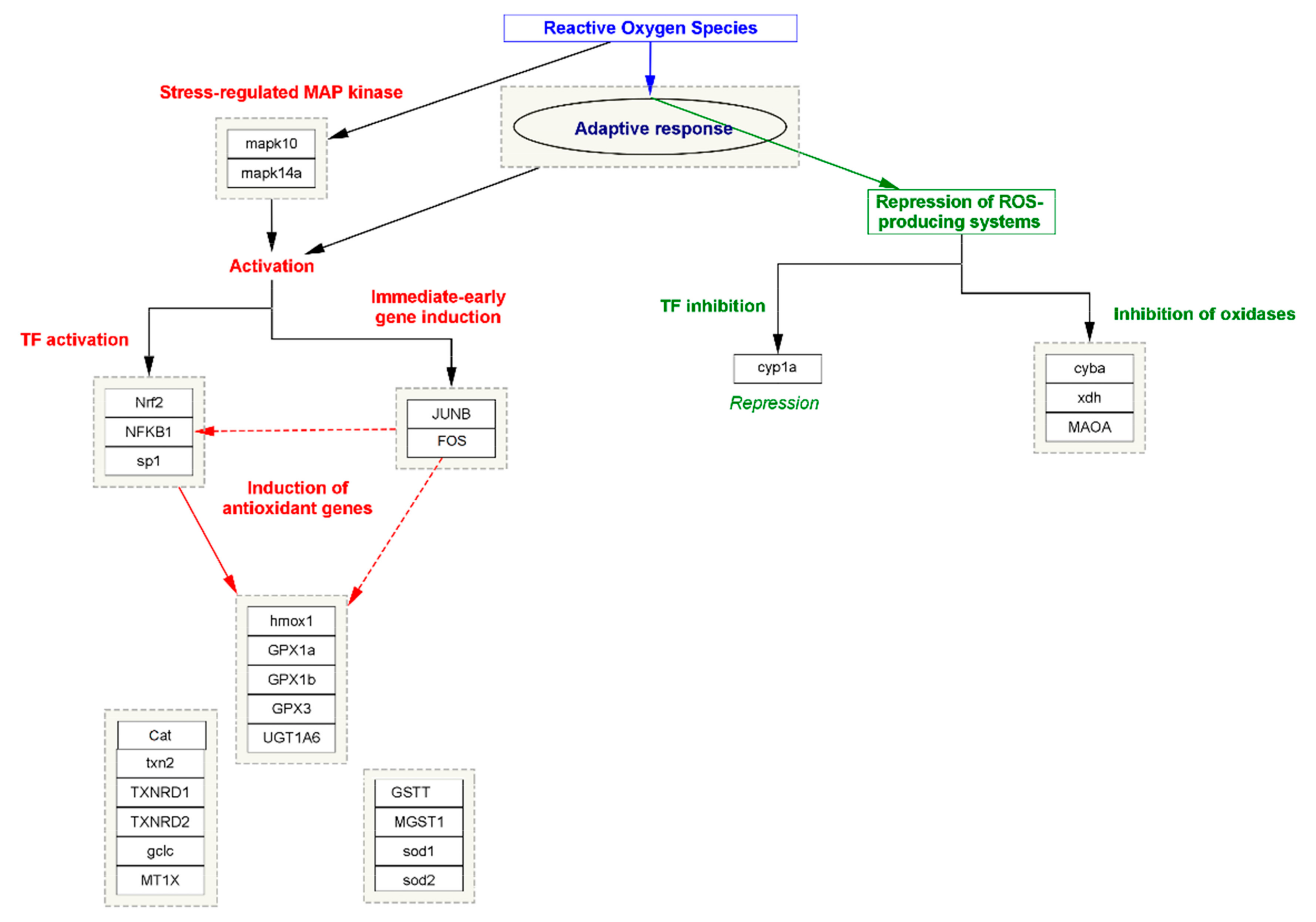
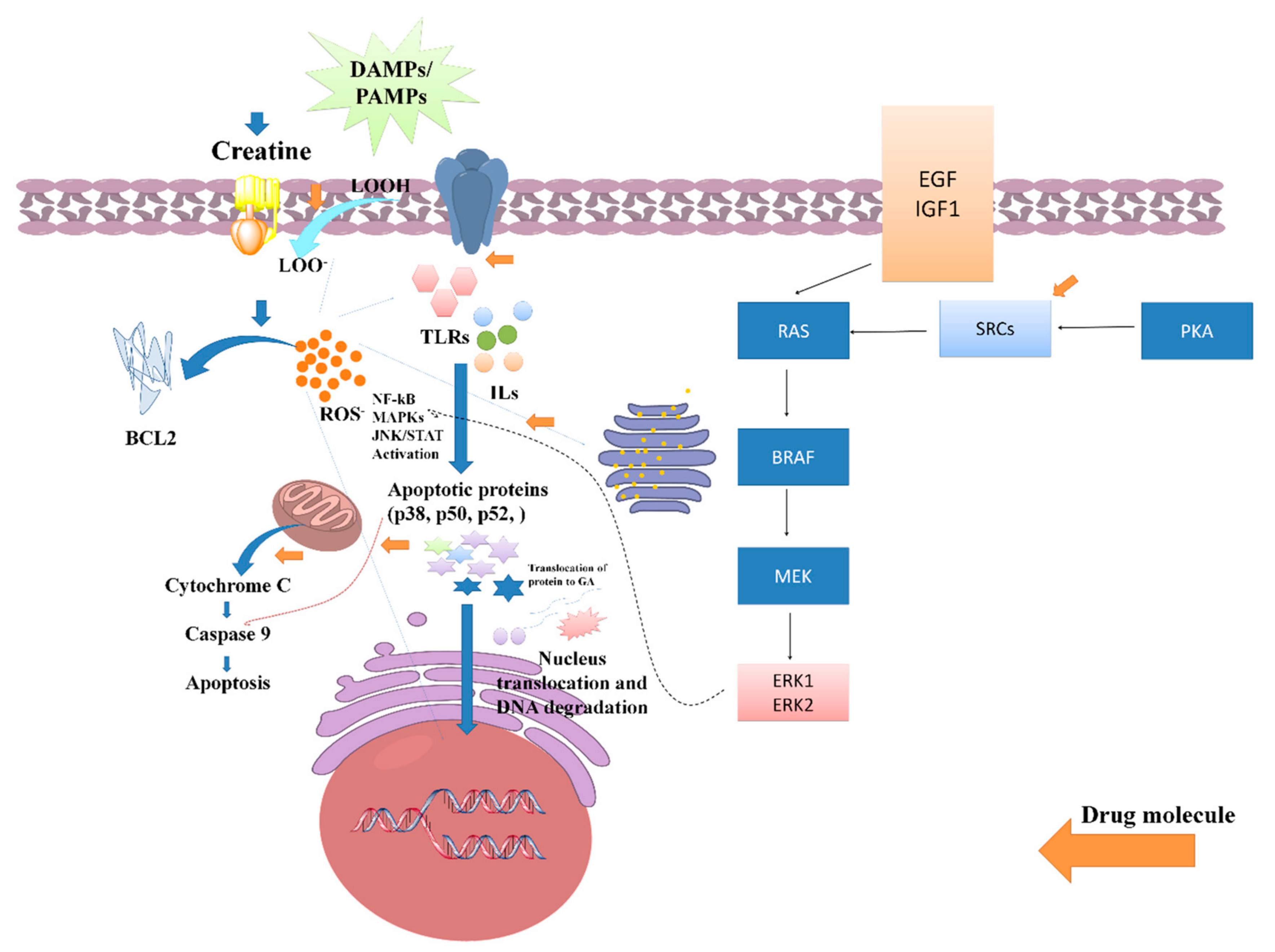
3.4. Oxidative Stress-Induced CKD
3.5. Inflammatory Stress-Induced CKD
3.6. Safety Concerns of LC
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Khan, M.U.; Gaurav; Zahiruddin, S.; Basist, P.; Krishnan, A.; Parveen, R.; Ahmad, S. Nephroprotective Potential of Sharbat-e-Bazoori Motadil (Sugar-Free) in HEK-293 Cells and Wistar Rats against Cisplatin Induced Nephrotoxicity. J. King Saud Univ. Sci. 2022, 34, 101839. [Google Scholar] [CrossRef]
- Rapa, S.F.; Di Iorio, B.R.; Campiglia, P.; Heidland, A.; Marzocco, S. Inflammation and Oxidative Stress in Chronic Kidney Disease—Potential Therapeutic Role of Minerals, Vitamins and Plant-Derived Metabolites. Int. J. Mol. Sci. 2020, 21, 263. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention (CDCP). Chronic Kidney Disease in the United States, 2021; Centers for Disease Control and Prevention (CDCP): Atlanta, GA, USA, 2021. [Google Scholar]
- Gautam, G.; Parveen, B.; Umar Khan, M.; Sharma, I.; Kumar Sharma, A.; Parveen, R.; Ahmad, S. A Systematic Review on Nephron Protective AYUSH Drugs as Constituents of NEERI-KFT (A Traditional Indian Polyherbal Formulation) for the Management of Chronic Kidney Disease. Saudi J. Biol. Sci. 2021, 28, 6441–6453. [Google Scholar] [CrossRef]
- Kumar, V.; Yadav, A.K.; Sethi, J.; Ghosh, A.; Sahay, M.; Prasad, N.; Varughese, S.; Parameswaran, S.; Gopalakrishnan, N.; Kaur, P.; et al. The Indian Chronic Kidney Disease (ICKD) Study: Baseline Characteristics. Clin. Kidney J. 2022, 15, 60–69. [Google Scholar] [CrossRef] [PubMed]
- Liyanage, T.; Toyama, T.; Hockham, C.; Ninomiya, T.; Perkovic, V.; Woodward, M.; Fukagawa, M.; Matsushita, K.; Praditpornsilpa, K.; Hooi, L.S.; et al. Prevalence of Chronic Kidney Disease in Asia: A Systematic Review and Analysis. BMJ Glob. Health 2022, 7, e007525. [Google Scholar] [CrossRef]
- Pugh, D.; Gallacher, P.J.; Dhaun, N. Management of Hypertension in Chronic Kidney Disease. Drugs 2019, 79, 365–379. [Google Scholar] [CrossRef]
- Hahr, A.J.; Molitch, M.E. Management of Diabetes Mellitus in Patients with Chronic Kidney Disease. Clin. Diabetes Endocrinol. 2015, 1, 2. [Google Scholar] [CrossRef]
- Shirazian, S. Depression in CKD: Understanding the Mechanisms of Disease. Kidney Int. Rep. 2019, 4, 189–190. [Google Scholar] [CrossRef]
- Gai, Z.; Wang, T.; Visentin, M.; Kullak-Ublick, G.A.; Fu, X.; Wang, Z. Lipid Accumulation and Chronic Kidney Disease. Nutrients 2019, 11, 722. [Google Scholar] [CrossRef]
- Snider, J.T.; Sullivan, J.; Van Eijndhoven, E.; Hansen, M.K.; Bellosillo, N.; Neslusan, C.; O’Brien, E.; Riley, R.; Seabury, S.; Kasiske, B.L. Lifetime Benefits of Early Detection and Treatment of Diabetic Kidney Disease. PLoS ONE 2019, 14, e0217487. [Google Scholar] [CrossRef]
- Matthew, B.D.; Katalin, S. The next Generation of Therapeutics for Chronic Kidney Disease. Nat. Rev. Drug Discov. 2016, 15, 568–588. [Google Scholar] [CrossRef]
- Kumela Goro, K.; Desalegn Wolide, A.; Kerga Dibaba, F.; Gashe Fufa, F.; Wakjira Garedow, A.; Edilu Tufa, B.; Mulisa Bobasa, E. Patient Awareness, Prevalence, and Risk Factors of Chronic Kidney Disease among Diabetes Mellitus and Hypertensive Patients at Jimma University Medical Center, Ethiopia. Biomed Res. Int. 2019, 2019, 2383508. [Google Scholar] [CrossRef]
- Peltzer, K.; Pengpid, S. The Use of Herbal Medicines among Chronic Disease Patients in Thailand: A Cross-Sectional Survey. J. Multidiscip. Healthc. 2019, 12, 573–582. [Google Scholar] [CrossRef]
- Mulder, S.; Hammarstedt, A.; Nagaraj, S.B.; Nair, V.; Ju, W.; Hedberg, J.; Greasley, P.J.; Eriksson, J.W.; Oscarsson, J.; Heerspink, H.J.L. A Metabolomics-Based Molecular Pathway Analysis of How the Sodium-Glucose Co-Transporter-2 Inhibitor Dapagliflozin May Slow Kidney Function Decline in Patients with Diabetes. Diabetes Obes. Metab. 2020, 22, 1157–1166. [Google Scholar] [CrossRef]
- Deng, X.; Jiang, N.; Guo, L.; Wang, C.; Li, J.; Liu, X.; Zhu, B.; Cai, R.; Chen, Y.; Wang, T.; et al. Protective Effects and Metabolic Regulatory Mechanisms of Shenyan Fangshuai Recipe on Chronic Kidney Disease in Rats. Evid.-Based Complement. Altern. Med. 2020, 2020, 5603243. [Google Scholar] [CrossRef]
- Şener, G.; Paskaloǧlu, K.; Şatiroglu, H.; Alican, I.; Kaçmaz, A.; Sakarcan, A. L-Carnitine Ameliorates Oxidative Damage Due to Chronic Renal Failure in Rats. J. Cardiovasc. Pharmacol. 2004, 43, 698–705. [Google Scholar] [CrossRef]
- Aydogdu, N.; Atmaca, G.; Yalcin, O.; Taskiran, R.; Tastekin, E.; Kaymak, K. Protective Effects of L-Carnitine on Myoglobinuric Acute Renal Failure in Rats. Clin. Exp. Pharmacol. Physiol. 2006, 33, 119–124. [Google Scholar] [CrossRef]
- Cao, Y.; Qu, H.J.; Li, P.; Wang, C.B.; Wang, L.X.; Han, Z.W. Single Dose Administration of L-Carnitine Improves Antioxidant Activities in Healthy Subjects. Tohoku J. Exp. Med. 2011, 22, 209–213. [Google Scholar] [CrossRef]
- Ribas, G.S.; Vargas, C.R.; Wajner, M. L-Carnitine Supplementation as a Potential Antioxidant Therapy for Inherited Neurometabolic Disorders. Gene 2014, 533, 469–476. [Google Scholar] [CrossRef]
- L-Carnitine Market Size, Share & Trends Analysis Report by Process (Bioprocess, Chemical Synthesis), by Product (Food & Pharmaceutical Grade, Feed Grade), by Application, by Region, and Segment Forecasts, 2021–2028; Grand View Research, Inc.: San Francisco, CA, USA, 2021.
- Nishioka, N.; Luo, Y.; Taniguchi, T.; Ohnishi, T.; Kimachi, M.; Ng, R.C.K.; Watanabe, N. Carnitine Supplements for People with Chronic Kidney Disease Requiring Dialysis. Cochrane Database Syst. Rev. 2020, 12, CD013601. [Google Scholar] [CrossRef]
- Cooper, R.; Naclerio, F.; Allgrove, J.; Jimenez, A. Creatine Supplementation with Specific View to Exercise/Sports Performance: An Update. J. Int. Soc. Sports Nutr. 2012, 9, 33. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.J.; Lin, J.S.; Lin, Y.C.; Lin, P.T. Effects of L-Carnitine Supplementation on Oxidative Stress and Antioxidant Enzymes Activities in Patients with Coronary Artery Disease: A Randomized, Placebo-Controlled Trial. Nutr. J. 2014, 13, 79. [Google Scholar] [CrossRef] [PubMed]
- Post, A.; Tsikas, D.; Bakker, S.J.L. Creatine Is a Conditionally Essential Nutrient in Chronic Kidney Disease: A Hypothesis and Narrative Literature Review. Nutrients 2019, 11, 1044. [Google Scholar] [CrossRef] [PubMed]
- Chaudhary, R.; Singh, R.; Verma, R.; Kumar, P.; Kumar, N.; Singh, L.; Kumar, S.S. Investigation on Protective Effect of Terminalia Bellirica (Roxb.) against Drugs Induced Cardiotoxicity in Wistar Albino Rats. J. Ethnopharmacol. 2020, 261, 113080. [Google Scholar] [CrossRef] [PubMed]
- Riesberg, L.A.; McDonald, T.L.; Wang, Y.; Chen, X.M.; Holzmer, S.W.; Tracy, S.M.; Drescher, K.M. Creatinine Downregulates TNF-α in Macrophage and T Cell Lines. Cytokine 2018, 110, 29–38. [Google Scholar] [CrossRef]
- Pritchard, N.R.; Kalra, P.A. Renal Dysfunction Accompanying Oral Creatine Supplements. Lancet 1998, 352, 233. [Google Scholar] [CrossRef]
- Poortmans, J.R.; Auquier, H.; Renaut, V.; Durussel, A.; Saugy, M.; Brisson, G.R. Effect of Short-Term Creatine Supplementation on Renal Responses in Men. Eur. J. Appl. Physiol. Occup. Physiol. 1997, 76, 566–567. [Google Scholar] [CrossRef]
- Harris, R.C.; Soderlund, K.; Hultman, E. Elevation of Creatine in Resting and Exercised Muscle of Normal Subjects by Creatine Supplementation. Clin. Sci. 1992, 83, 367–374. [Google Scholar] [CrossRef]
- Gülçin, I. Antioxidant and Antiradical Activities of L-Carnitine. Life Sci. 2006, 13, 343–351. [Google Scholar] [CrossRef]
- Nomura, A.; Zhang, M.; Sakamoto, T.; Ishii, Y.; Morishima, Y.; Mochizuki, M.; Kimura, T.; Uchida, Y.; Sekizawa, K. Anti-Inflammatory Activity of Creatine Supplementation in Endothelial Cells in Vitro. Br. J. Pharmacol. 2003, 89, 605–611. [Google Scholar] [CrossRef]
- Almeida, F.M.; Oliveira-Junior, M.C.; Souza, R.A.; Petroni, R.C.; Soto, S.F.; Soriano, F.G.; De Carvalho, P.T.C.; Albertini, R.; Damaceno-Rodrigues, N.R.; Lopes, F.D.T.Q.S.; et al. Creatine Supplementation Attenuates Pulmonary and Systemic Effects of Lung Ischemia and Reperfusion Injury. J. Hear. Lung Transplant. 2016, 35, 242–250. [Google Scholar] [CrossRef]
- De Souza e Silva, A.; Pertille, A.; Reis Barbosa, C.G.; Aparecida de Oliveira Silva, J.; de Jesus, D.V.; Ribeiro, A.G.S.V.; Baganha, R.J.; de Oliveira, J.J. Effects of Creatine Supplementation on Renal Function: A Systematic Review and Meta-Analysis. J. Ren. Nutr. 2019, 29, 480–489. [Google Scholar] [CrossRef]
- Abu Ahmad, N.; Armaly, Z.; Berman, S.; Jabour, A.; Aga-Mizrachi, S.; Mosenego-Ornan, E.; Avital, A. L-Carnitine Improves Cognitive and Renal Functions in a Rat Model of Chronic Kidney Disease. Physiol. Behav. 2016, 164, 182–188. [Google Scholar] [CrossRef]
- Sturm, M.; Herebian, D.; Mueller, M.; Laryea, M.D.; Spiekerkoetter, U. Functional Effects of Different Medium-Chain Acyl-CoA Dehydrogenase Genotypes and Identification of Asymptomatic Variants. PLoS ONE 2012, 2012, e45110. [Google Scholar] [CrossRef]
- Ikeda, M.; Takahashi, K.; Ohtake, T.; Imoto, R.; Kawakami, H.; Hayashi, M.; Takenoa, S. A Futile Metabolic Cycle of Fatty Acyl Coenzyme A (Acyl-CoA) Hydrolysis and Resynthesis in Corynebacterium Glutamicum and Its Disruption Leading to Fatty Acid Production. Appl. Environ. Microbiol. 2021, 87, e02469-20. [Google Scholar] [CrossRef]
- Celestino-Soper, P.B.S.; Violante, S.; Crawford, E.L.; Luo, R.; Lionel, A.C.; Delaby, E.; Cai, G.; Sadikovic, B.; Lee, K.; Lo, C.; et al. A Common X-Linked Inborn Error of Carnitine Biosynthesis May Be a Risk Factor for Nondysmorphic Autism. Proc. Natl. Acad. Sci. USA 2012, 109, 7974–7981. [Google Scholar] [CrossRef]
- Liepinsh, E.; Kuka, J.; Vilks, K.; Svalbe, B.; Stelfa, G.; Vilskersts, R.; Sevostjanovs, E.; Goldins, N.R.; Groma, V.; Grinberga, S.; et al. Low Cardiac Content of Long-Chain Acylcarnitines in TMLHE Knockout Mice Prevents Ischaemia-Reperfusion-Induced Mitochondrial and Cardiac Damage. Free Radic. Biol. Med. 2021, 177, 370–380. [Google Scholar] [CrossRef]
- Schlegel, G.; Keller, J.; Hirche, F.; Geißler, S.; Schwarz, F.J.; Ringseis, R.; Stangl, G.I.; Eder, K. Expression of Genes Involved in Hepatic Carnitine Synthesis and Uptake in Dairy Cows in the Transition Period and at Different Stages of Lactation. BMC Vet. Res. 2012, 8, 28. [Google Scholar] [CrossRef]
- Vinishdharma, T.; Don, K.R. Vitamin C as a Therapeutic Healing Agent. Drug Invent. Today 2019, 12, 554–558. [Google Scholar]
- Vaz, F.M.; Van Gool, S.; Ofman, R.; Ijlst, L.; Wanders, R.J.A. Carnitine Biosynthesis: Identification of the CDNA Encoding Human γ-Butyrobetaine Hydroxylase. Biochem. Biophys. Res. Commun. 1998, 250, 506–510. [Google Scholar] [CrossRef]
- Cenini, G.; Lloret, A.; Cascella, R. Oxidative Stress in Neurodegenerative Diseases: From a Mitochondrial Point of View. Oxid. Med. Cell. Longev. 2019, 2019, 2105607. [Google Scholar] [CrossRef] [PubMed]
- Daenen, K.; Andries, A.; Mekahli, D.; Van Schepdael, A.; Jouret, F.; Bammens, B. Oxidative Stress in Chronic Kidney Disease. Pediatr. Nephrol. 2019, 34, 975–991. [Google Scholar] [CrossRef] [PubMed]
- Duni, A.; Liakopoulos, V.; Roumeliotis, S.; Peschos, D.; Dounousi, E. Oxidative Stress in the Pathogenesis and Evolution of Chronic Kidney Disease: Untangling Ariadne’s Thread. Int. J. Mol. Sci. 2019, 20, 3711. [Google Scholar] [CrossRef] [PubMed]
- Crawford, D.R.; Davies, K.J.A. Adaptive Response and Oxidative Stress. Proc. Environ. Health Perspect. 1994, 102, 25–28. [Google Scholar]
- Ueno, N.; Takeya, R.; Miyano, K.; Kikuchi, H.; Sumimoto, H. The NADPH Oxidase Nox3 Constitutively Produces Superoxide in a P22 Phox-Dependent Manner: Its Regulation by Oxidase Organizers and Activators. J. Biol. Chem. 2005, 286, 4760–4771. [Google Scholar] [CrossRef]
- Cao, Z.; Xiong, J.; Takeuchi, M.; Kurama, T.; Goeddel, D.V. TRAF6 Is a Signal Transducer for Interleukin-1. Nature 1996, 383, 443–446. [Google Scholar] [CrossRef]
- Panday, A.; Sahoo, M.K.; Osorio, D.; Batra, S. NADPH Oxidases: An Overview from Structure to Innate Immunity-Associated Pathologies. Cell. Mol. Immunol. 2015, 12, 5–23. [Google Scholar] [CrossRef]
- Guo, C.Y.; Sun, L.; Chen, X.P.; Zhang, D.S. Oxidative Stress, Mitochondrial Damage and Neurodegenerative Diseases. Neural Regen. Res. 2013, 8, 2003. [Google Scholar] [CrossRef]
- Rayego-Mateos, S.; Goldschmeding, R.; Ruiz-Ortega, M. Inflammatory and Fibrotic Mediators in Renal Diseases. Mediators Inflamm. 2019, 2019, 7025251. [Google Scholar] [CrossRef]
- Kaneko, N.; Kurata, M.; Yamamoto, T.; Morikawa, S.; Masumoto, J. The Role of Interleukin-1 in General Pathology. Inflamm. Regen. 2019, 39, 12. [Google Scholar] [CrossRef]
- He, X.; Li, C.; Wei, Z.; Wang, J.; Kou, J.; Liu, W.; Shi, M.; Yang, Z.; Fu, Y. Protective Role of Apigenin in Cisplatin-Induced Renal Injury. Eur. J. Pharmacol. 2016, 789, 215–221. [Google Scholar] [CrossRef]
- De Cesare, D.; Jacquot, S.; Hanauer, A.; Sassone-Corsi, P. Rsk-2 Activity Is Necessary for Epidermal Growth Factor-Induced Phosphorylation of CREB Protein and Transcription of c-Fos Gene. Proc. Natl. Acad. Sci. USA 1998, 95, 1818–1822. [Google Scholar] [CrossRef]
- Cargnello, M.; Roux, P.P. Activation and Function of the MAPKs and Their Substrates, the MAPK-Activated Protein Kinases. Microbiol. Mol. Biol. Rev. 2012, 75, 50–83. [Google Scholar] [CrossRef]
- Huang, D.; Ding, Y.; Luo, W.M.; Bender, S.; Qian, C.N.; Kort, E.; Zhang, Z.F.; VandenBeldt, K.; Duesbery, N.S.; Resau, J.H.; et al. Inhibition of MAPK Kinase Signaling Pathways Suppressed Renal Cell Carcinoma Growth and Angiogenesis in Vivo. Cancer Res. 2008, 68, 81–88. [Google Scholar] [CrossRef]
- Kumar, D.; Singla, S.K.; Puri, V.; Puri, S. The Restrained Expression of NF-KB in Renal Tissue Ameliorates Folic Acid Induced Acute Kidney Injury in Mice. PLoS ONE 2015, 10, e115947. [Google Scholar] [CrossRef]
- Song, N.; Thaiss, F.; Guo, L. NFκB and Kidney Injury. Front. Immunol. 2019, 10, 815. [Google Scholar] [CrossRef]
- Zhang, H.; Sun, S.C. NF-ΚB in Inflammation and Renal Diseases. Cell Biosci. 2015, 5, 63. [Google Scholar] [CrossRef]
- Lee, J.; An, J.N.; Hwang, J.H.; Lee, H.; Lee, J.P.; Kim, S.G. P38 MAPK Activity Is Associated with the Histological Degree of Interstitial Fibrosis in IgA Nephropathy Patients. PLoS ONE 2019, 14, e0213981. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, N.; Xu, Y.; Tan, H.Y.; Li, S.; Feng, Y. Molecular Mechanisms Involved in Oxidative Stress-Associated Liver Injury Induced by Chinese Herbal Medicine: An Experimental Evidence-Based Literature Review and Network Pharmacology Study. Int. J. Mol. Sci. 2018, 19, 2745. [Google Scholar] [CrossRef]
- Kurtzeborn, K.; Kwon, H.N.; Kuure, S. MAPK/ERK Signaling in Regulation of Renal Differentiation. Int. J. Mol. Sci. 2019, 20, 1779. [Google Scholar] [CrossRef]
- Chen, D.Q.; Hu, H.H.; Wang, Y.N.; Feng, Y.L.; Cao, G.; Zhao, Y.Y. Natural Products for the Prevention and Treatment of Kidney Disease. Phytomedicine 2018, 50, 50–60. [Google Scholar] [CrossRef] [PubMed]
- Moshapa, F.T.; Riches-Suman, K.; Palmer, T.M. Therapeutic Targeting of the Proinflammatory IL-6-JAK/STAT Signalling Pathways Responsible for Vascular Restenosis in Type 2 Diabetes Mellitus. Cardiol. Res. Pract. 2019, 2019, 9846312. [Google Scholar] [CrossRef] [PubMed]
- Matsui, F.; Meldrum, K.K. The Role of the Janus Kinase Family/Signal Transducer and Activator of Transcription Signaling Pathway in Fibrotic Renal Disease. J. Surg. Res. 2012, 178, 339–345. [Google Scholar] [CrossRef] [PubMed]
- Alunno, A.; Padjen, I.; Fanouriakis, A.; Boumpas, D.T. Pathogenic and Therapeutic Relevance of JAK/STAT Signaling in Systemic Lupus Erythematosus: Integration of Distinct Inflammatory Pathways and the Prospect of Their Inhibition with an Oral Agent. Cells 2019, 8, 898. [Google Scholar] [CrossRef] [PubMed]
- Antonio, J.; Candow, D.G.; Forbes, S.C.; Gualano, B.; Jagim, A.R.; Kreider, R.B.; Rawson, E.S.; Smith-Ryan, A.E.; VanDusseldorp, T.A.; Willoughby, D.S.; et al. Common Questions and Misconceptions about Creatine Supplementation: What Does the Scientific Evidence Really Show? J. Int. Soc. Sports Nutr. 2021, 18, 13. [Google Scholar] [CrossRef]
- Kreider, R.B.; Kalman, D.S.; Antonio, J.; Ziegenfuss, T.N.; Wildman, R.; Collins, R.; Candow, D.G.; Kleiner, S.M.; Almada, A.L.; Lopez, H.L. International Society of Sports Nutrition Position Stand: Safety and Efficacy of Creatine Supplementation in Exercise, Sport, and Medicine. J. Int. Soc. Sports Nutr. 2017, 7, 7. [Google Scholar] [CrossRef]
- Buford, T.W.; Kreider, R.B.; Stout, J.R.; Greenwood, M.; Campbell, B.; Spano, M.; Ziegenfuss, T.; Lopez, H.; Landis, J.; Antonio, J. International Society of Sports Nutrition Position Stand: Creatine Supplementation and Exercise. J. Int. Soc. Sports Nutr. 2007, 4, 6. [Google Scholar] [CrossRef]
- Wilborn, C.D.; Willoughby, D.S. The Role of Dietary Protein Intake and Resistance Training on Myosin Heavy Chain Expression. J. Int. Soc. Sports Nutr. 2004, 1, 27. [Google Scholar] [CrossRef]
- Gonzalez Guerrico, A.M.; Lieske, J.; Klee, G.; Kumar, S.; Lopez-Baez, V.; Wright, A.M.; Bobart, S.; Shevell, D.; Maldonado, M.; Troost, J.P.; et al. Urinary CD80 Discriminates Among Glomerular Disease Types and Reflects Disease Activity. Kidney Int. Rep. 2020, 5, 2021–2031. [Google Scholar] [CrossRef]
- Jayachandran, M.; Yuzhakov, S.V.; Kumar, S.; Larson, N.B.; Enders, F.T.; Milliner, D.S.; Rule, A.D.; Lieske, J.C. Specific Populations of Urinary Extracellular Vesicles and Proteins Differentiate Type 1 Primary Hyperoxaluria Patients without and with Nephrocalcinosis or Kidney Stones. Orphanet J. Rare Dis. 2020, 15, 319. [Google Scholar] [CrossRef]
- Keshani, M.; Alikiaii, B.; Askari, G.; Yahyapoor, F.; Ferns, G.A.; Bagherniya, M. The Effects of L-Carnitine Supplementation on Inflammatory Factors, Oxidative Stress, and Clinical Outcomes in Patients with Sepsis Admitted to the Intensive Care Unit (ICU): Study Protocol for a Double Blind, Randomized, Placebo-Controlled Clinical Trial. Trials 2022, 23, 170. [Google Scholar] [CrossRef]
- Basist, P.; Parveen, B.; Zahiruddin, S.; Gautam, G.; Parveen, R.; Khan, M.A.; Krishnan, A.; Shahid, M.; Ahmad, S. Potential Nephroprotective Phytochemicals: Mechanism and Future Prospects. J. Ethnopharmacol. 2022, 283, 114743. [Google Scholar] [CrossRef]
- Gholipur-Shahraki, T.; Feizi, A.; Mortazavi, M.; Badri, S. Effects of Carnitine on Nutritional Parameters in Patients with Chronic Kidney Disease: An Updated Systematic Review and Meta-Analysis. J. Res. Pharm. Pract. 2018, 10, 17. [Google Scholar] [CrossRef]
- Ahmad, S. L-Carnitine in Dialysis Patients. In Seminars in Dialysis; Blackwell Science Inc.: Boston, MA, USA, 2001; Volume 14, pp. 209–217. [Google Scholar] [CrossRef]
- Takashima, H.; Maruyama, T.; Abe, M. Significance of Levocarnitine Treatment in Dialysis Patients. Nutrients 2021, 13, 1219. [Google Scholar] [CrossRef]
- Salama, A.A.; Mostafa, R.; Elgohary, R. Effect of L-Carnitine on Potassium Dichromate-Induced Nephrotoxicity in Rats: Modulation of PI3K/AKT Signaling Pathway. Res. Pharm. Sci. 2022, 17, 153. [Google Scholar] [CrossRef]

| Report Attribute | Details |
|---|---|
| Base year for estimation | 2020 |
| Country scope | USA; Canada; Mexico; Germany; UK; France; Italy; China; India; Japan; South Korea; Brazil; Argentina; South Africa; Saudi Arabia |
| Customization scope | Free report customization (equivalent up to eight analyst working days) with purchase. Addition or alteration to country, regional, and segment scope. |
| Forecast period | 2021–2028 |
| Growth Rate | CAGR of 5.1% from 2021 to 2028 |
| Historical data | 2017–2019 |
| Key companies profiled | Lonza (Basel, Switzerland); Northeast Pharmaceutical Group Co., Ltd. (NEPG) (Shenyang, China); Biosint S.p.A. (Sermoneta, Italy); Cayman Chemical (Ann Arbor, MI, USA); Merck KGaA (Darmstadt, Germany); Tokyo Chemical Industry Co., Ltd. (Tokyo, Japan); Ceva (Marseille, France); Kaiyuan Hengtai Nutrition Co., Ltd. (Kaiyuan, China); ChengDa PharmaCeuticals Co., Ltd. (Jiaxing, China); Huanggang Huayang Pharmaceutical Co., Ltd. (Huanggang, China); HuBeiYuancheng SaichuangTechnology Co., Ltd. (Wuhan, China) |
| Market size value in 2021 | USD 194.0 million |
| Pricing and purchase options | Avail customized purchase options to meet exact research needs. Explore purchase options |
| Quantitative units | Volume in tons, revenue in USD thousand, CAGR from 2021 to 2028 |
| Regional scope | North America; Europe; Asia Pacific; Central and South America; Middle East and Africa |
| Report coverage | Volume forecast, revenue forecast, company ranking, competitive landscape, growth factors, and trends |
| Revenue forecast in 2028 | USD 276.0 million |
| Segments covered | Process, product, application, region |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sharma, B.; Yadav, D.K. L-Carnitine and Chronic Kidney Disease: A Comprehensive Review on Nutrition and Health Perspectives. J. Pers. Med. 2023, 13, 298. https://doi.org/10.3390/jpm13020298
Sharma B, Yadav DK. L-Carnitine and Chronic Kidney Disease: A Comprehensive Review on Nutrition and Health Perspectives. Journal of Personalized Medicine. 2023; 13(2):298. https://doi.org/10.3390/jpm13020298
Chicago/Turabian StyleSharma, Bharti, and Dinesh Kumar Yadav. 2023. "L-Carnitine and Chronic Kidney Disease: A Comprehensive Review on Nutrition and Health Perspectives" Journal of Personalized Medicine 13, no. 2: 298. https://doi.org/10.3390/jpm13020298
APA StyleSharma, B., & Yadav, D. K. (2023). L-Carnitine and Chronic Kidney Disease: A Comprehensive Review on Nutrition and Health Perspectives. Journal of Personalized Medicine, 13(2), 298. https://doi.org/10.3390/jpm13020298