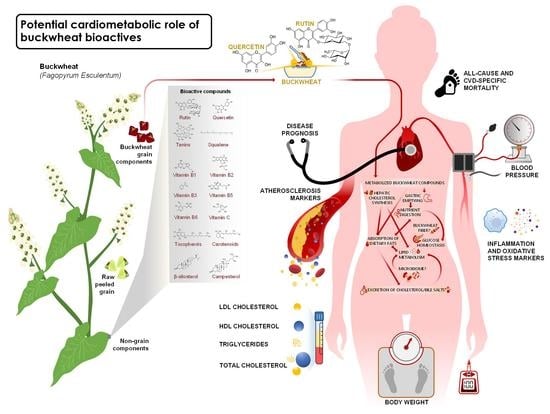
Buckwheat and Cardiometabolic Health: A Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Strategy and Study Selection
2.1.1. Inclusion Criteria
2.1.2. Exclusion Criteria
2.2. Screening, Data Extraction and Assessment of the Methodological Rigor of Included Studies
2.3. Statistical Analysis and Evidence Synthesis
3. Results
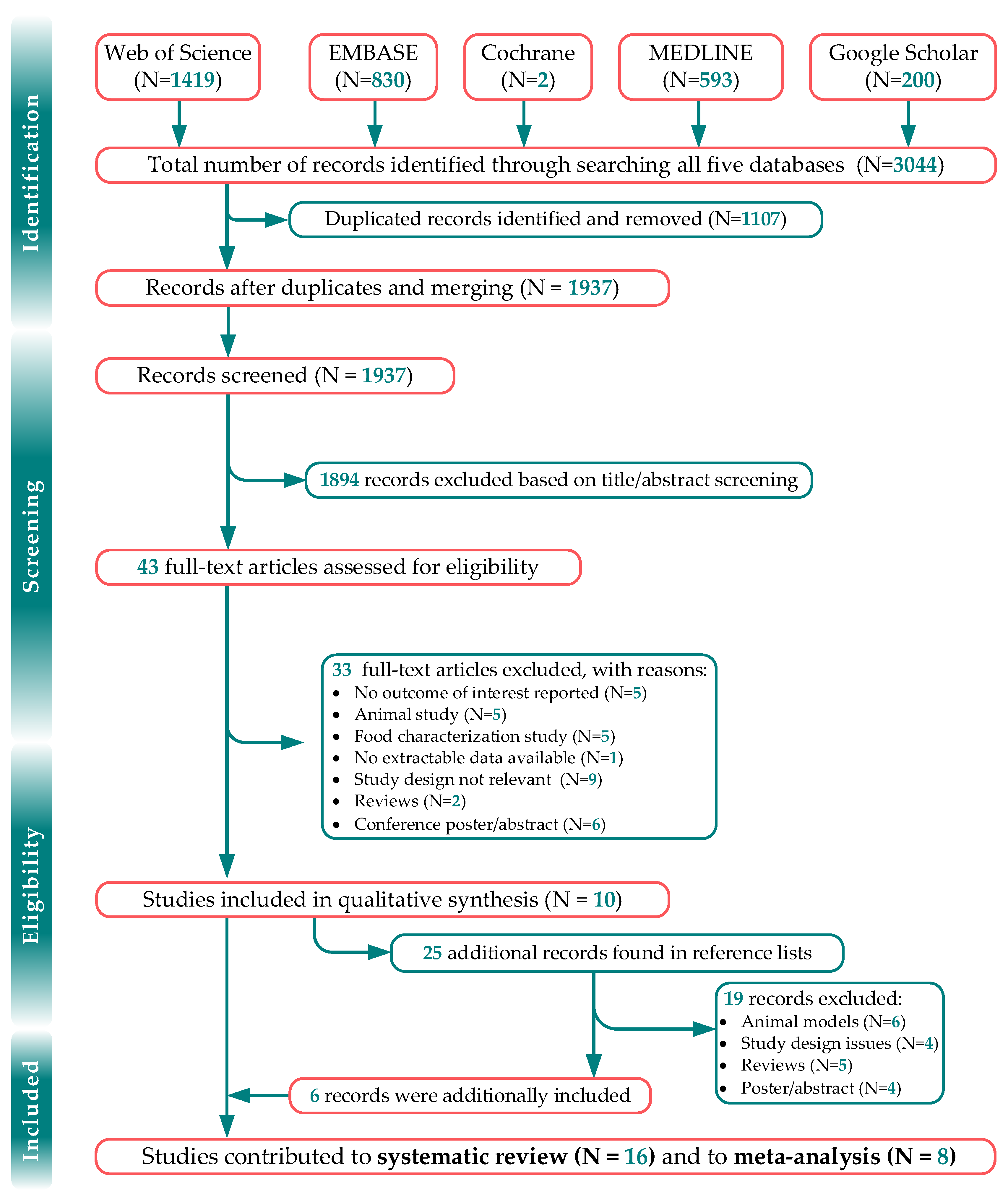
3.1. Included Studies
3.2. Qualitative Synthesis
3.2.1. Inflammation Markers
3.2.2. Metabolic and Body Morphology Markers
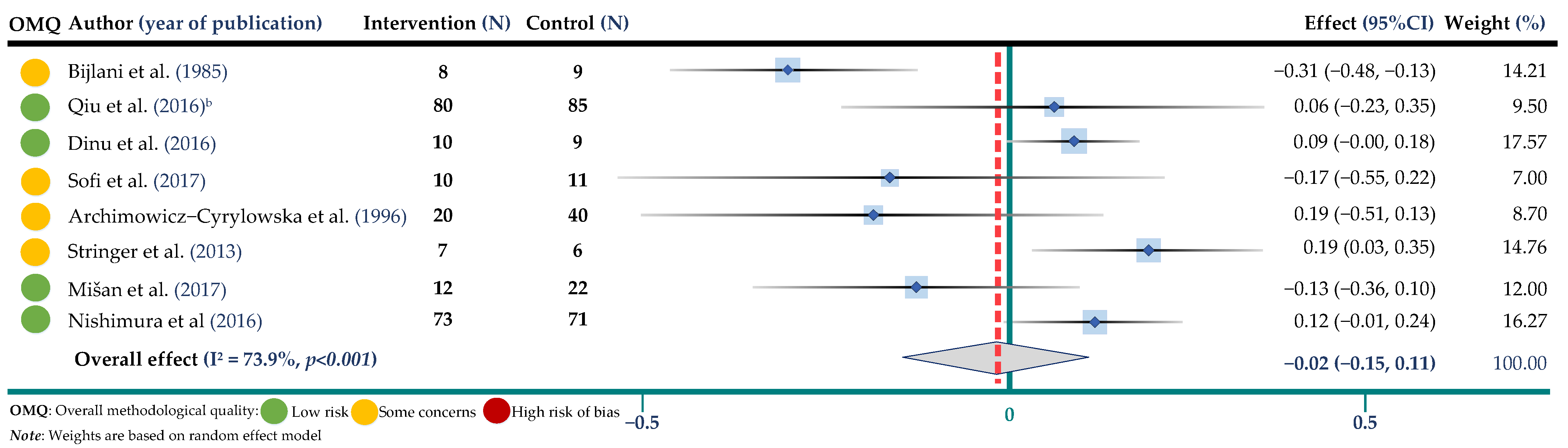
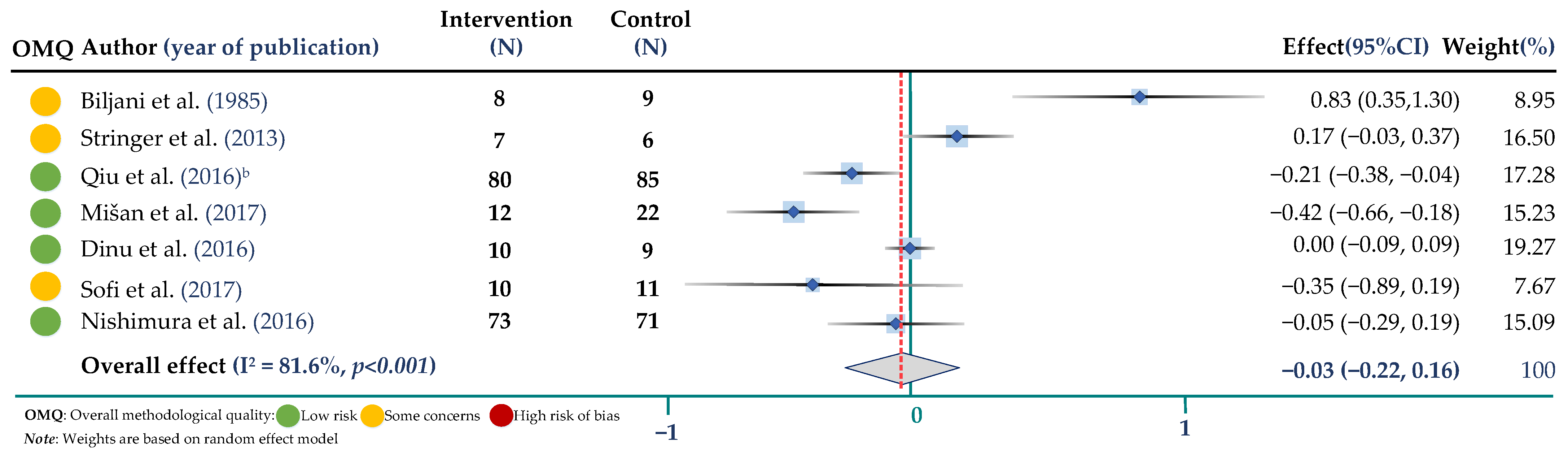
3.3. Meta-Analysis of Trials Assessing the Effect of Buckwheat Interventions on Lipid Profile
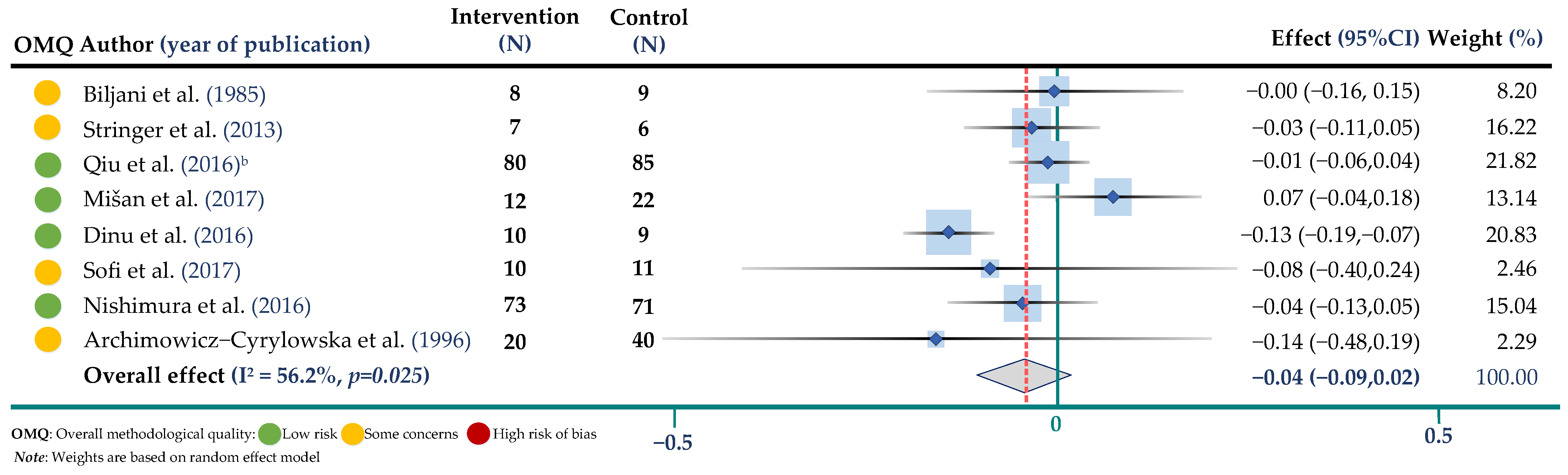
3.4. Meta-Analysis of Trials Assessing the Effect of Buckwheat Interventions on Body Weight and Glucose
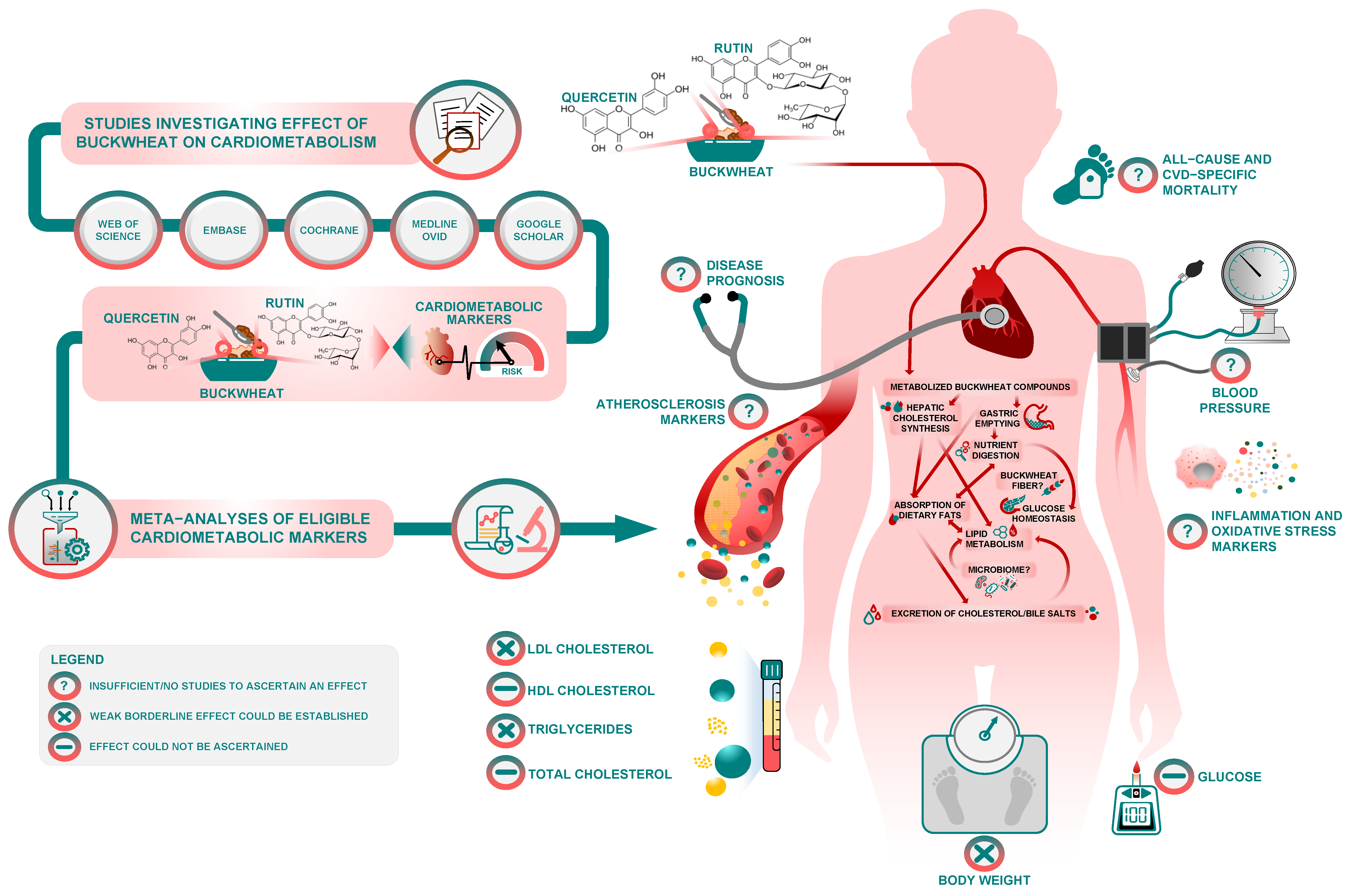
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, S.; Zhang, Q. Advances in the Development of Functional Foods from Buckwheat. Crit. Rev. Food Sci. Nutr. 2001, 41, 451–464. [Google Scholar] [CrossRef] [PubMed]
- Raguindin, P.F.; Itodo, O.A.; Stoyanov, J.; Dejanovic, G.M.; Gamba, M.; Asllanaj, E.; Minder, B.; Bussler, W.; Metzger, B.; Muka, T.; et al. A systematic review of phytochemicals in oat and buckwheat. Food Chem. 2020, 338, 127982. [Google Scholar] [CrossRef] [PubMed]
- Su Jeong, K.; Hwang Bae, S.; Jong Taek, S.; Geum Hee, K.; Su Young, H.; Dong Chil, C.; Kim, K.D.; Koo, B.C.; Kim, Y.H. Domestic and Overseas Status of Buckwheat Production and Future Trends. J. Korean Soc. Int. Agric. 2017, 29, 226–233. [Google Scholar]
- Zou, L.; Wu, D.; Ren, G.; Hu, Y.; Peng, L.; Zhao, J.; Garcia-Perez, P.; Carpena, M.; Prieto, M.A.; Cao, H.; et al. Bioactive compounds, health benefits, and industrial applications of Tartary buckwheat (Fagopyrum tataricum). Crit. Rev. Food Sci. Nutr. 2021, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Lietz, G.; Seal, C. Buckwheat and CVD risk markers: A systematic review and meta-analysis. Nutrients 2018, 10, 619. [Google Scholar] [CrossRef] [PubMed]
- Giménez-Bastida, J.A.; Zieliński, H. Buckwheat as a Functional Food and Its Effects on Health. J. Agric. Food Chem. 2015, 63, 7896–7913. [Google Scholar] [CrossRef] [PubMed]
- Kreft, M. Buckwheat phenolic metabolites in health and disease. Nutr. Res. Rev. 2016, 29, 30–39. [Google Scholar] [CrossRef] [PubMed]
- Riley, R.D. Multivariate meta-analysis: The effect of ignoring within-study correlation. J. R. Stat. Soc. Ser. A Stat. Soc. 2009, 172, 789–811. [Google Scholar] [CrossRef]
- Muka, T.; Glisic, M.; Milic, J.; Verhoog, S.; Bohlius, J.; Bramer, W.; Chowdhury, R.; Franco, O.H. A 24-step guide on how to design, conduct, and successfully publish a systematic review and meta-analysis in medical research. Eur. J. Epidemiol. 2020, 35, 49–60. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; Group, P. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS ONE Med. 2009, 6, e1000097. [Google Scholar]
- Harzing, A.W. Publish or Perish. 2007. Available online: https://harzing.com/resources/publish-or-perish (accessed on 17 November 2022).
- Bramer, W.M.; Rethlefsen, M.L.; Mast, F.; Kleijnen, J. Evaluation of a new method for librarian-mediated literature searches for systematic reviews. J. Res. Synth. Methods 2018, 9, 510–520. [Google Scholar] [CrossRef] [PubMed]
- Pixelruler. PixelRuler—The Screen ruler for graphic artists and web designers. 2022. Available online: https://www.pixelruler.de/e/index.htm (accessed on 17 November 2022).
- Sterne, J.A.C.; Savović, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ 2019, 366, 14898. [Google Scholar] [CrossRef] [PubMed]
- Sterne, J.A.C.; Hernán, M.A.; Reeves, B.C.; Savović, J.; Berkman, N.D.; Viswanathan, M.; Henry, D.; Altman, D.G.; Ansari, M.T.; Boutron, I.; et al. ROBINS-I: A tool for assessing risk of bias in non-randomised studies of interventions. BMJ 2016, 355, i4919. [Google Scholar] [CrossRef] [PubMed]
- Hozo, S.P.; Djulbegovic, B.; Hozo, I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med. Res. Methodol. 2005, 5, 13. [Google Scholar] [CrossRef]
- Dinu, M.; Macchia, D.; Pagliai, G.; Gori, A.M.; Cesari, F.; Marcucci, R.; Sofi, F.; Casini, A. Symptomatic efficacy of buckwheat products in Non-Celiac Gluten Sensitivity (NCGS). Asia Pac. J. Clin. Nutr. 2017, 26, 630–636. [Google Scholar]
- Dinu, M.; Ghiselli, L.; Whittaker, A.; Pagliai, G.; Cesari, F.; Fiorillo, C.; Becatti, M.; Marcucci, R.; Benedettelli, S.; Sofi, F. Consumption of buckwheat products and cardiovascular risk profile: A randomized, single-blinded crossover trial. J. Nutr. Food Sci. 2016, 6, 1000501. [Google Scholar] [CrossRef]
- Stokić, E.; Mandić, A.; Sakač, M.; Mišan, A.; Pestorić, M.; Šimurina, O.; Jambrec, D.; Jovanov, P.; Nedeljković, N.; Milovanović, I.; et al. Quality of buckwheat-enriched wheat bread and its antihyperlipidemic effect in statin treated patients. LWT-Food Sci. Technol. 2015, 63, 556–561. [Google Scholar] [CrossRef]
- Archimowicz-Cyrylowska, B.; Adamek, B.; Droździk, M.; Samochowiec, L.; Wójcicki, J. Clinical effect of buckwheat herb, Ruscus extract and troxerutin on retinopathy and lipids in diabetic patients. Phytother Res. 1996, 10, 659–662. [Google Scholar] [CrossRef]
- Wieslander, G.; Fabjan, N.; Vogrincic, M.; Kreft, I.; Janson, C.; Spetz-Nyström, U.; Vombergar, B.; Tagesson, C.; Leanderson, P.; Norbäck, D.; et al. Eating buckwheat cookies is associated with the reduction in serum levels of myeloperoxidase and cholesterol: A double blind crossover study in day-care centre staffs. Tohoku J. Exp. Med. 2011, 225, 123–130. [Google Scholar] [CrossRef]
- Qiu, J.; Li, Z.; Qin, Y.; Yue, Y.; Liu, Y. Protective effect of tartary buckwheat on renal function in type 2 diabetics: A randomized controlled trial. Clin. Risk Manag. 2016, 12, 1721–1727. [Google Scholar] [CrossRef]
- Qiu, J.; Liu, Y.; Yue, Y.; Qin, Y.; Li, Z. Dietary tartary buckwheat intake attenuates insulin resistance and improves lipid profiles in patients with type 2 diabetes: A randomized controlled trial. Nutr. Res. 2016, 36, 1392–1401. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Guan, L. Effects of buckwheat flour eating for 8 weeks on blood glucose, serum lipids levels and blood pressure. Chin. J. Clin. Rehabil. 2003, 7, 2231. [Google Scholar]
- Nishimura, M.; Ohkawara, T.; Sato, Y.; Satoh, H.; Suzuki, T.; Ishiguro, K.; Noda, T.; Morishita, T.; Nishihira, J. Effectiveness of rutin-rich Tartary buckwheat (Fagopyrum tataricum Gaertn.) ‘Manten-Kirari’ in body weight reduction related to its antioxidant properties: A randomised, double-blind, placebo-controlled study. J. Funct. Foods 2016, 26, 460–469. [Google Scholar] [CrossRef]
- Huang, G.; Huang, M.; Chen, W.; Huang, Y.; Yang, Z.; You, Y. Clinical effects of tartary buckwheat mixture on the treatment of early diabetic and nephropathy. J. Chin. Med. Mater. 2009, 32, 1932–1935. [Google Scholar]
- Zheng, G.; Pan, X.; An, Z.; Wang, Y. Preliminary observation of lipid-decreasing effects of compound Tartary buckwheat flour on niddm patients. Beijing Med. J. 1991, 5, 280–281. [Google Scholar]
- Bijlani, R.L.; Gandhi, B.M.; Gupta, M.C.; Manocha, S.; Tandon, B.N. Effect of whole buckwheat (Fagopyrum esculentum) flour supplementation on lipid profile & glucose tolerance. Indian J. Med. Res. 1985, 81, 162–168. [Google Scholar]
- Bijlani, R.L.; Sud, S.; Sahi, A.; Gandhi, B.M.; Tandon, B.N. Effect of sieved buckwheat (Fagopyrum esculentum) flour supplementation on lipid profile and glucose tolerance. Indian J. Physiol. Pharmacol. 1984, 29, 69–74. [Google Scholar]
- Shakib, M.; Gabrial, S.; Gabrial, G. Buckwheat consumption improved lipid profile, fasting and postprandial blood glucose in hypercholesterolemic and type 2 diabetic patients. Int. J. Acad. Res. 2011, 3, 132–139. [Google Scholar]
- Stringer, D.M.; Taylor, C.G.; Appah, P.; Blewett, H.; Zahradka, P. Consumption of buckwheat modulates the post-prandial response of selected gastrointestinal satiety hormones in individuals with type 2 diabetes mellitus. Metab. Clin. Exp. 2013, 62, 1021–1031. [Google Scholar] [CrossRef]
- Mišan, A.; Petelin, A.; Stubelj, M.; Mandić, A.; Šimurina, O.; Pojić, M.; Milovanović, I.; Jakus, T.; Filipčev, B.; Pražnikar, Z.J. Buckwheat—Enriched instant porridge improves lipid profile and reduces inflammation in participants with mild to moderate hypercholesterolemia. J. Funct. Foods. 2017, 36, 186–194. [Google Scholar] [CrossRef]
- Dinu, M.; Ghiselli, L.; Whittaker, A.; Pagliai, G.; Cesari, F.; Fiorillo, C.; Becatti, M.; Marcucci, R.; Benedettelli, S.; Sofi, F. Consumption of buckwheat products and cardiovascular risk profile: A randomized single-blinded crossover trial. Nutr. Metab. Cardiovasc. Dis. 2017, 27, e20–e21. [Google Scholar] [CrossRef]
- He, J.; Klag, M.J.; Whelton, P.K.; Mo, J.P.; Chen, J.Y.; Qian, M.C.; Mo, P.S.; He, G.Q. Oats and buckwheat intakes and cardiovascular disease risk factors in an ethnic minority of China. Am. J. Clin. Nutr. 1995, 61, 366–372. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.-W.; Zhang, Y.-H.; Lu, M.-J.; Tong, W.-J.; Cao, G.-W. Comparison of hypertension, dyslipidaemia and hyperglycaemia between buckwheat seed-consuming and non-consuming Mongolian-Chinese populations in Inner Mongolia, China. Clin. Exp. Pharmacol. Physiol. 2007, 34, 838–844. [Google Scholar] [CrossRef] [PubMed]
- Luthar, Z.; Golob, A.; Germ, M.; Vombergar, B.; Kreft, I. Tartary Buckwheat in Human Nutrition. Plants 2021, 10, 700. [Google Scholar] [CrossRef] [PubMed]
- Zhu, F. Chemical composition and health effects of Tartary buckwheat. Food Chem. 2016, 203, 231–245. [Google Scholar] [CrossRef]
- Brown, L.; Rosner, B.; Willett, W.W.; Sacks, F.M. Cholesterol-lowering effects of dietary fiber: A meta-analysis. Am. J. Clin. Nutr. 1999, 69, 30–42. [Google Scholar] [CrossRef]
- Fernandez, M.-L. Soluble fiber and nondigestible carbohydrate effects on plasma lipids and cardiovascular risk. Curr. Opin. Lipidol. 2001, 12, 35–40. [Google Scholar] [CrossRef]
- Roy, S.; Vega-Lopez, S.; Fernandez, M.L. Gender and Hormonal Status Affect the Hypolipidemic Mechanisms of Dietary Soluble Fiber in Guinea Pigs. J. Nutr. 2000, 130, 600–607. [Google Scholar] [CrossRef]
- Gill, S.K.; Rossi, M.; Bajka, B.; Whelan, K. Dietary fibre in gastrointestinal health and disease. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 101–116. [Google Scholar] [CrossRef]



| Parameter | Criterion |
|---|---|
| Population | Human adults (≥18 years) |
| Interventions/exposures | Diet supplementation with buckwheat, rutin, quercetin and/or other buckwheat related bioactives |
| Comparisons | Placebo, no buckwheat or other comparison |
| Outcomes | Serum lipid profile, type 2 diabetes and glucose homeostasis parameters, inflammatory and oxidative stress markers, body morphology parameters, blood pressure, all-cause and cardiovascular mortality, cardiovascular disease severity and/or clinical progression, markers of vasoconstriction/vasodilatation and/or markers of atherosclerosis, such as atherosclerotic plaque, arterial wall thickness, coronary artery calcification, intima media thickness, etc. |
| Study design | Prospective cohort studies, case-cohort, nested-case control studies, randomized and non-randomized clinical trials |
| No. | Study (Year) | Location | Study Design | Sample (N) | Females (%) | Study Population | Mean Age (SD) * | Duration (Weeks) | Washout Information | Intervention | Control | Primary Aim of the Study | Risk of Bias ** | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Bijlani et al. (1985) |  India |  | 8 |  0% | Healthy | 18–34 | 12 | 2 wks WO | Wholegrain BW flatbread | Breakfast cereals | Lipid profile and glucose tolerance | Some concerns | [26] |
| 2 | Bijlani et al. (1984) |  India India |  | 12 |  0% | Healthy | 18–22 | 4 | No WO | Sieved BW flatbread | Staple lunch cereal | Lipid profile and glucose tolerance | High | [27] |
| 3 | Stringer et al. (2013) |  Canada Canada |  | 13 |  15% | Healthy/T2D | 49 (11.5) | 1 | No WO | Wholegrain BW crackers | Rice cracker | Food intake and glucose homeostatsis | Some concerns | [29] |
| 4 | Dinu et al. (2017) |  Italy Italy |  | 19 |  95% | NCGS | 45.3 (10) | 6 | No WO | BW-enriched pasta, tacks, biscuits and flakes | Gluten-free diet | Gastrointestinal health | Low | [15] |
| 5 | Zheng et al. (1991) |  China China |  | 19 |  47% | NIDDM | 53.8 (29–64) | 12 | NA | Tartary BW flour | No control | Lipid profile | Moderate risk | [25] |
| 6 | Stokić et al. (2015) |  Serbia Serbia |  | 20 |  65% | At risk of CVDs | 59.5 (12.6) | 4 | NA | Wholegrain BW-enriched wheat bread | Wheat bread | Lipid profile | Moderate risk | [17] |
| 7 | Shakib et al. (2011) |  Egypt |  | 20 |  40% | HC and NIDDM | R: 30–50 | 6 | NA | BW-yogurt mixture | No control | Lipid profile and glucose homeostatsis | Serious risk | [28] |
| 8 | Sofi et al. (2016) |  Italy Italy |  | 21 |  52% | At risk of CVDs | 51.3 (13.4) | 8 | 8 wks WO | BW-based bread, pasta, biscuits and crackers | Wheat-based bread, pasta, biscuits and crackers | CVD risk markers | Some concerns | [16] |
| 9 | Mišan et al. (2017) |  Serbia Serbia |  | 34 |  62% | HC | 46 (8.2) | 5 | 3 wks WO | BW-enriched instant porridge | Maize instant porridge | Lipid profile and inflamation | Low | [30] |
| 10 | Archimowicz-Cyrylowska et al. (1996) |  Poland Poland |  | 60 |  53% | T2D | 20–75 | 12 | NA | BW herb mixture | Troxerutin or Ruscus mixture | Retinopathy and lipid profile | Some concerns | [18] |
| 11 | Zhao and Guan (2003) |  China |  | 60 |  50% | T2D | R: 26–67 | 8 | NA | BW flour | No control | CVD risk profile | Moderate risk | [22] |
| 12 | Wieslander et al. (2011) |  Sweden Sweden |  | 62 |  100% | Healthy | 46 (10) | 4 | 2 wks WO | Tatary BW cookies | Common BW cookies | Lipid profile and inflamation | High risk | [19] |
| 13 | Huang et al. (2009) |  China |  | 70 |  50% | T2D | 53 (8.2) | 8 | NA | BW mixture | Control drug | Diabetic nephropathy | Some concerns | [24] |
| 14 | Qiu et al. (2016a) |  China China |  | 104 |  39% | T2D | 58.8 (9.4) | 4 | NA | Tartary BW foods | Diet plan and nutritional education | Lipid profile and glucose homeostatsis | Low | [20] |
| 15 | Nishimura et al. (2016) |  Japan |  | 144 |  70% | Healthy | 54.1 (8.9) | 12 | NA | Rutin-rich Tartary BW noodles and cookies | Wheat-based noodles and cookies | Antioxidant effects | Some concerns | [23] |
| 16 | Qiu et al. (2016b) |  China China |  | 165 |  41% | T2D | 56.9 (10.4) | 4 | NA | Tartary BW-rich foods | Refined cereals (i.e., rice or wheat flour) | Renal function | Low | [21] |
 —based on the sample size range of included studies.
—based on the sample size range of included studies.  Parallel RCT study design;
Parallel RCT study design;  crossover RCT study design;
crossover RCT study design;  dietary intervention study (e.g., before-after intervention, etc.); NA: not applicable.
dietary intervention study (e.g., before-after intervention, etc.); NA: not applicable.Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Llanaj, E.; Ahanchi, N.S.; Dizdari, H.; Taneri, P.E.; Niehot, C.D.; Wehrli, F.; Khatami, F.; Raeisi-Dehkordi, H.; Kastrati, L.; Bano, A.; et al. Buckwheat and Cardiometabolic Health: A Systematic Review and Meta-Analysis. J. Pers. Med. 2022, 12, 1940. https://doi.org/10.3390/jpm12121940
Llanaj E, Ahanchi NS, Dizdari H, Taneri PE, Niehot CD, Wehrli F, Khatami F, Raeisi-Dehkordi H, Kastrati L, Bano A, et al. Buckwheat and Cardiometabolic Health: A Systematic Review and Meta-Analysis. Journal of Personalized Medicine. 2022; 12(12):1940. https://doi.org/10.3390/jpm12121940
Chicago/Turabian StyleLlanaj, Erand, Noushin Sadat Ahanchi, Helga Dizdari, Petek Eylul Taneri, Christa D. Niehot, Faina Wehrli, Farnaz Khatami, Hamidreza Raeisi-Dehkordi, Lum Kastrati, Arjola Bano, and et al. 2022. "Buckwheat and Cardiometabolic Health: A Systematic Review and Meta-Analysis" Journal of Personalized Medicine 12, no. 12: 1940. https://doi.org/10.3390/jpm12121940
APA StyleLlanaj, E., Ahanchi, N. S., Dizdari, H., Taneri, P. E., Niehot, C. D., Wehrli, F., Khatami, F., Raeisi-Dehkordi, H., Kastrati, L., Bano, A., Glisic, M., & Muka, T. (2022). Buckwheat and Cardiometabolic Health: A Systematic Review and Meta-Analysis. Journal of Personalized Medicine, 12(12), 1940. https://doi.org/10.3390/jpm12121940