Personalized Treatments Based on Laryngopharyngeal Reflux Patient Profiles: A Narrative Review
Abstract
1. Introduction
2. Physiology
2.1. Gastroduodenal Enzymes
2.2. Diet and Lifestyle Habits
2.3. Autonomic Nerve Dysfunction
- -
- Laryngopharyngeal symptoms and findings may be due to the inflammation of the mucosa, which is related to pepsin and/or bile acidic toxicity.
- -
- Pepsin is mainly active in acidic or weakly acidic environment, whereas bile acids may be active in acidic, weakly acidic or alkaline environment.
- -
- The consideration of pepsin and bile salt saliva concentrations should indicate a more personalized treatment, combining antiacids and over-the-counter drugs.
- -
- The composition of foods and beverages may influence the gastroesophageal motility and sphincter tonicity and, consequently, the occurrence of pharyngeal reflux events and deposition of enzymes into the upper aerodigestive tract mucosa.
- -
- Depression, anxiety, stress, and the related autonomic nerve dysfunction are more commonly found in patients with symptoms and findings of LPR, which suggests a key role of the autonomic nervous system in the physiology of LPR.
- -
- The patients’ baseline diet, personality, lifestyle, and potential triggers of autonomic nerve dysfunction need to be considered to propose a more personalized short-to-long-term treatment without medication as much as possible.
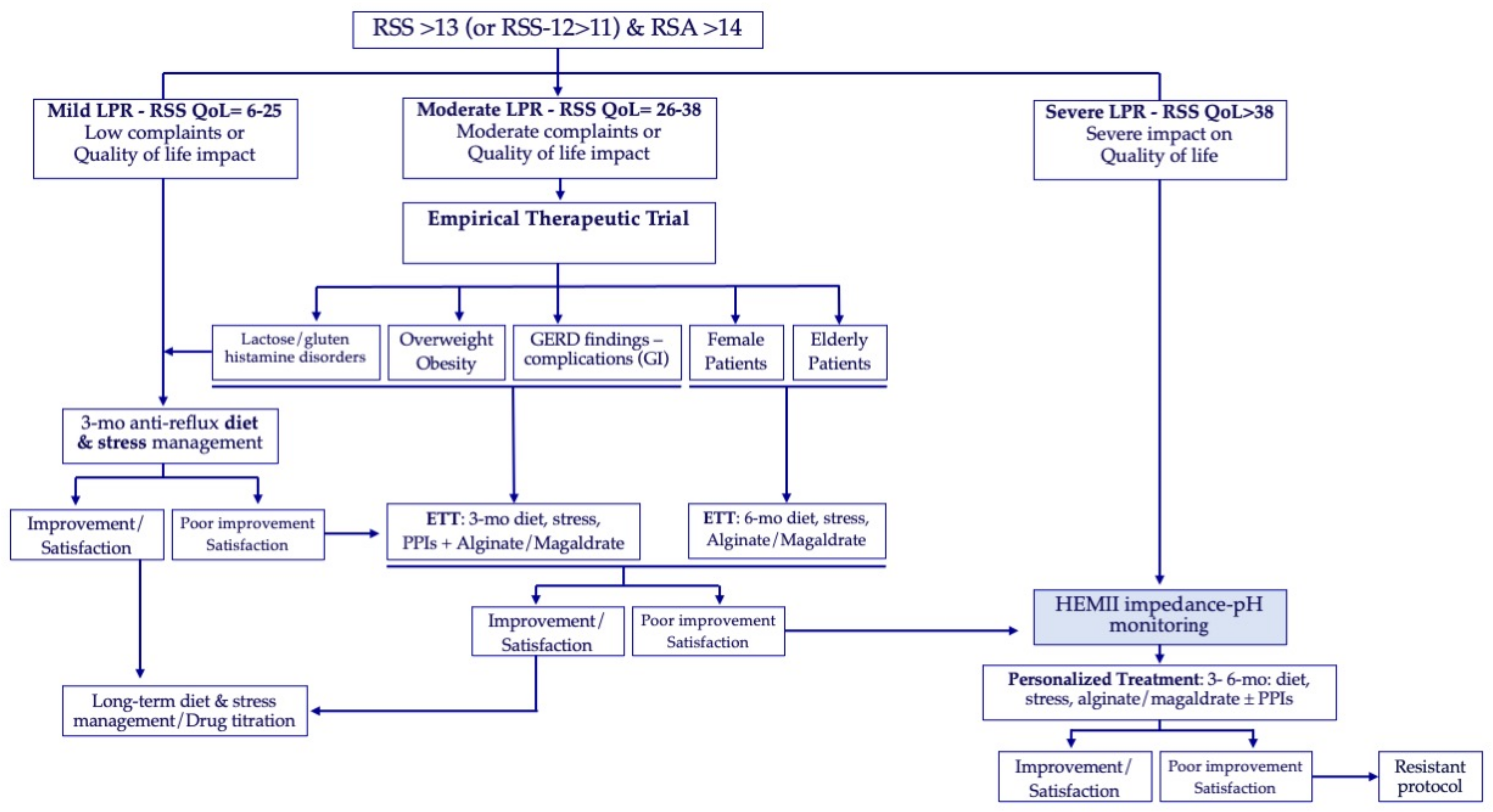
3. Patient Features
3.1. Gender
3.2. Age
3.3. Overweight
3.4. Medical and Surgical Conditions
- -
- The severity of laryngopharyngeal symptoms and findings may be influenced by the age, gender, or body mass index of the patient.
- -
- Elderly and female patients may require more time to see symptom relief because the symptoms will continue to improve from until 6 months posttreatment, while the symptoms of responder males commonly improve by 3-months posttreatment.
- -
- Elderly patients may report lower baseline LPR and GERD symptom scores than younger patients, while they may have silent esophageal complications of GERD.
- -
- Some conditions may favor the development of LPR or recalcitrant symptoms and findings, including gluten sensitivity, lactose intolerance, or histamine sensitivity. These conditions need to be considered for the duration of treatment and throughout the follow-up of patients.
- -
- According to the IFOS classification [51], LPR may present as acute, recurrent, or chronic disease. To date, the influence of age, gender, overweight/obesity, or other contributing factors remains unknown. The identification of epidemiological factors contributing to both the recurrence of symptoms or the chronic course of the disease makes sense regarding the cost burden of LPR in Western populations [78].
4. Additional Examination Features
4.1. The Impedance-pH Monitoring Profile
4.2. Oropharyngeal and Nasopharyngeal pH Monitoring
4.3. High-Resolution Manometry
4.4. Gastrointestinal Endoscopy
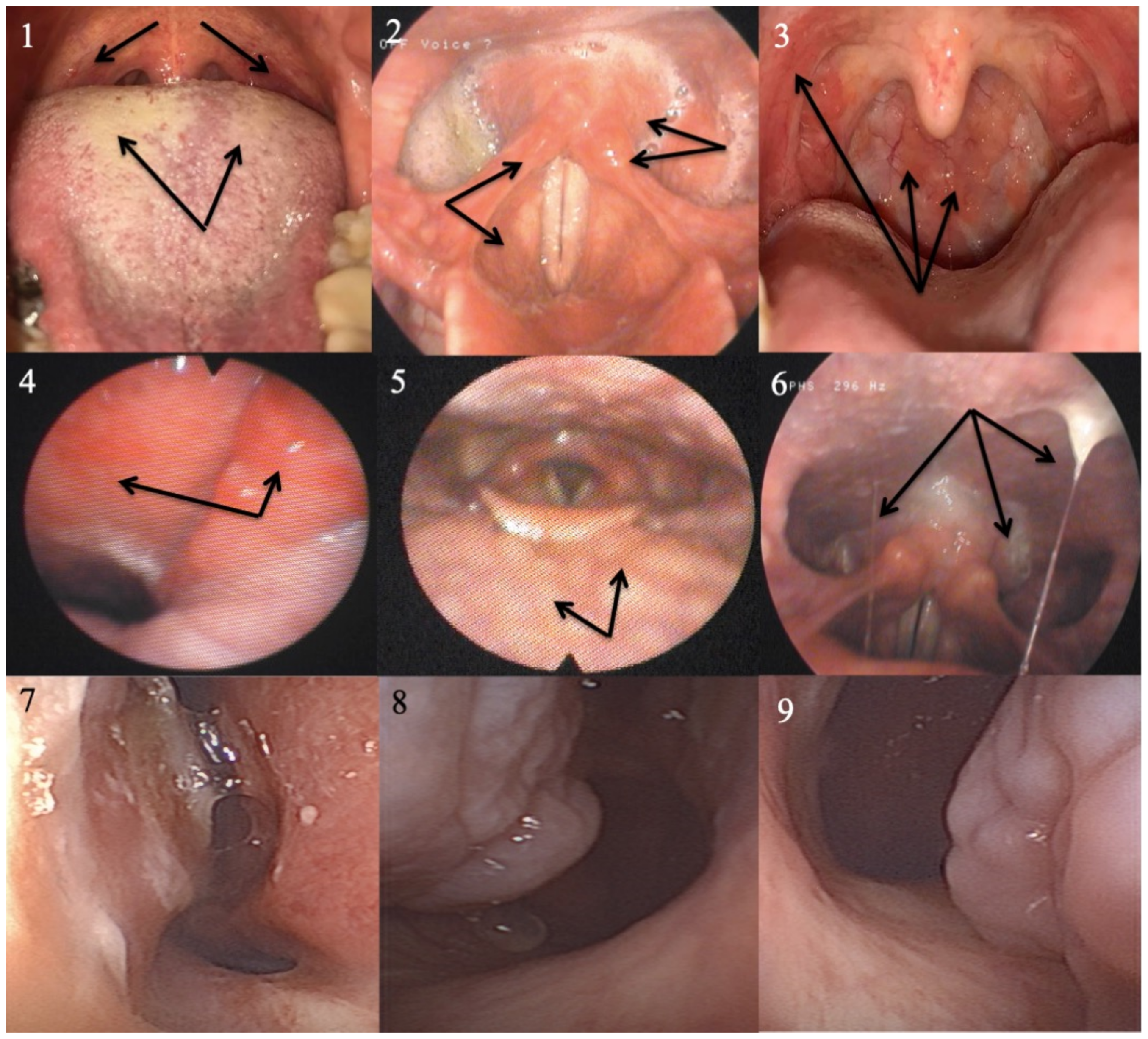
4.5. Pepsin Saliva Concentration
- -
- The HEMII-pH tracing may specify the profile of LPR disease regarding pH, composition, and time of occurrence of pharyngeal reflux events, which may orientate the personalized treatment.
- -
- High-resolution manometry may be advised in patients with a history of esophageal motility disorder or those with therapeutic resistance.
- -
- GI endoscopy is recommended for patients with a history of GERD complications, patients resistant to treatment, or elderly individuals.
- -
- Pepsin tests are sensitive but not specific and could be considered in oral diseases associated with LPR.
5. Treatment
5.1. Proton Pump Inhibitors
5.2. Alginate and Magaldrate
5.3. Surgery
5.4. Long-Term Management
- -
- The management of LPR etiologies, e.g., diet and lifestyle, is the primary therapeutic step, while the use of medication may just control the LPR consequences, such as symptoms and the associated conditions.
- -
- The medical and surgical histories, lifestyle, diet, and medications of patients, especially the elderly, need to be considered in the selection of drugs, especially PPIs.
- -
- In patients with HEMII-pH findings, the medical treatment may be personalized according to the features of the esophageal and pharyngeal reflux events through a combination of PPIs and alginate or magaldrate.
- -
- In patients without HEMII-pH findings, the personalized approach may be focused on patient characteristics rather than the HEMII-pH tracing and may consider alginate/magaldrate with or without PPIs.
- -
- Fundoplicature may be proposed in patients with troublesome or recalcitrant GERD symptoms or complications, although the surgery effectiveness for LPR symptoms and findings remains unpredictable.
- -
- Most patients may be weaned from all medications but the etiological factors of LPR need to be controlled over the long-term, which may be difficult for patients with chronic autonomic nerve dysfunction or a high sensitivity to a refluxogenic diet.
6. Conclusions and Future Directions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A. Antireflux Diet
| Lifestyle Habits | Foods to Favor | Foods to Avoid |
| 1. Stress control | 1. Meat, fish, chicken, eggs | 1. Meat, fish, chicken, eggs |
| 2. Reduce in tobacco and other addiction(s) | Fresh and thin fish | Fatty fish, fish oil (sardines, cods, herrings) |
| 3. Reduce size of meals | Shrimps, lobster, shellfish | Fatty chicken |
| 4. Hot lunch in place of hot diner | Chicken fillet (without skin) | High-fat meat |
| 5. Eat slowly | Turkey (without skin and fat) | -Kidney |
| 6. Do not talk while eating | Duck (without skin and fat) | -Bacon |
| 7. Avoid tight clothing | Low-fat meat | -Ground meat |
| 8. If possible, avoid the following drugs: | -Veal cutlet | -Pâté |
| Non-steroidal anti-inflammatory drugs, | -Rindless, fatless, cooked ham | -Tripe |
| corticosteroids, aspirin, theophylline, | -Steak, fillet, striploin | -Lamb |
| progesterone, iron supplementation, | -Roast veal, veal chop | -Lamb chops, shoulder, or legs |
| calcium channel blockers, | -Pork tenderloin | -Ribs, rib steak |
| nitro-derivatives, anticholinergic drugs | -Horse | -Pork chops, roast, and shoulder |
| Remove fat from meat | -Foie gras | |
| Egg white | -Deli meats, sausage, salami | |
| Other: | Other: | |
| If heartburn occurs | 2. Dairy products | 2. Dairy products |
| 1. Reduce weight | Low-fat cheese | Chocolate, ice cream |
| 2. Elevate the head of the bed | Skim milk | Hard cheese, full-fat cheese |
| Other: | -Goat cheese, cheddar, Roquefort, | |
| -Fontina, gruyere, parmesan, munster, etc. | ||
| Whole milk | ||
| Other: | ||
| Laryngopharyngeal reflux treatment | 3. Cereals and starches | 3. Cereals and starches |
| Drug: | Oat | Chocolate cookies |
| Wheat | Peanuts | |
| To take: before—during—after | Crackers | French fries and fried foods |
| Pasta | Nuts, cashews, hazelnuts | |
| Meals (circle the adequate response): | Whole meal bread | White bread |
| Brown bread | Other: | |
| -Breakfast | Boiled potatoes | |
| Rice, brown rice | ||
| -Lunch | Other: | |
| 4. Fruits and vegetables | 4. Fruits and vegetables | |
| -Dinner | Agave | Shallot |
| Asparagus | Spicy vegetables | |
| Drug: | Banana, melon, peach | Onion |
| Broccoli | Chili | |
| To take: before—during—after | Celery | Tomato (sauce or raw tomato) |
| Cooked mushroom | Aspartame | |
| Meals (circle the adequate response): | Cauliflower | Beet/cane sugar |
| Fennel | Rhubarb | |
| -Breakfast | Ginger, spirulina | Blueberry |
| Green bean, lentil, chickpea | Garlic | |
| -Lunch | Turnip, parsley, tofu | |
| Other: | ||
| -Dinner | Preparation: | |
| Cook by steaming or boiling in water | Other: | |
| Drug: | 5. Beverages | 5. Beverages |
| Chamomile | Strong alcohol, red and rosé wines | |
| To take: before—during—after | Water, alkaline water | Sparkling beverages (water, soda, beer, etc.) |
| Appel/pear juices (no sugar added) | Coffee, tea | |
| Meals (circle the adequate response): | Melon/banana juices (no sugar added) | Citrus juices (orange, grapefruit) |
| Other: | Other: | |
| -Breakfast | 6. Greasy substances | 6. Greasy substances |
| Olive oil | Butter, spicy oils, pizza | |
| -Lunch | Other: | Sauces (mayonnaise, mustard, ketchup, etc.) |
| Other: | ||
| -Dinner | 7. Sugar | 7. Sugar |
| Honey | Sweets |
References
- Lechien, J.R.; Akst, L.M.; Hamdan, A.L.; Schindler, A.; Karkos, P.D.; Barillari, M.R.; Calvo-Henriquez, C.; Crevier-Buchman, L.; Finck, C.; Eun, Y.-G.; et al. Evaluation and Management of Laryngopharyngeal Reflux Disease: State of the Art Review. Otolaryngol. Head Neck Surg. 2019, 160, 762–782. [Google Scholar] [CrossRef] [PubMed]
- Samuels, T.L.; Johnston, N. Pepsin in gastroesophageal and extraesophageal reflux: Molecular pathophysiology and diagnostic utility. Curr. Opin. Otolaryngol. Head Neck Surg. 2020, 28, 401–409. [Google Scholar] [CrossRef] [PubMed]
- Klimara, M.J.; Randall, D.R.; Allen, J.; Figueredo, E.; Johnston, N. Proximal reflux: Biochemical mediators, markers, therapeutic targets, and clinical correlations. Ann. N. Y. Acad. Sci. 2020, 1481, 127–138. [Google Scholar] [CrossRef]
- Kotby, M.N.; Hassan, O.; El-Makhzangy, A.M.; Farahat, M.; Shadi, M.; Milad, P. Gastroesophageal reflux/laryngopharyngeal reflux disease: A critical analysis of the literature. Eur. Arch. Otorhinolaryngol. 2010, 267, 171–179. [Google Scholar] [CrossRef] [PubMed]
- Snow, G.; Dhar, S.I.; Akst, L.M. How to Understand and Treat Laryngopharyngeal Reflux. Gastroenterol. Clin. N. Am. 2021, 50, 871–884. [Google Scholar] [CrossRef]
- Jamieson, J.R.; Stein, H.J.; DeMeester, T.R.; Bonavina, L.; Schwizer, W.; Hinder, R.A.; Albertucci, M. Ambulatory 24-h esophageal pH monitoring: Normal values, optimal thresholds, specificity, sensitivity, and reproducibility. Am. J. Gastroenterol. 1992, 87, 1102–1111. [Google Scholar]
- Lechien, J.R.; Allen, J.E.; Barillari, M.R.; Karkos, P.D.; Jia, H.; Ceccon, F.P.; Imamura, R.; Metwaly, O.; Chiesa-Estomba, C.M.; Bock, J.M.; et al. Management of Laryngopharyngeal Reflux Around the World: An International Study. Laryngoscope 2021, 131, E1589–E1597. [Google Scholar] [CrossRef]
- Lechien, J.R. Do Otolaryngologists Over- or Underestimate Laryngopharyngeal RefluxSymptoms and Findings in Clinical Practice? A Comparison Study between the True Prevalence and the Otolaryngologist-Estimated Prevalence of Symptoms and Findings. J. Clin. Med. 2022, 11, 5192. [Google Scholar] [CrossRef]
- Lechien, J.R.; Bock, J.M.; Carroll, T.L.; Akst, L.M. Is empirical treatment a reasonable strategy for laryngopharyngeal reflux? A contemporary review. Clin. Otolaryngol. 2020, 45, 450–458. [Google Scholar] [CrossRef]
- Lechien, J.R.; Saussez, S.; Schindler, A.; Karkos, P.D.; Hamdan, A.L.; Harmegnies, B.; De Marrez, L.G.; Finck, C.; Journe, F.; Paesmans, M.; et al. Clinical outcomes of laryngopharyngeal reflux treatment: A systematic review and meta-analysis. Laryngoscope 2019, 129, 1174–1187. [Google Scholar] [CrossRef]
- Liu, Z.; Zhang, C.; Wang, X.; Zhang, J.; Liu, L.; Wang, J.; Zhao, J.; Zou, S.; Ma, X.; Li, J. Characteristics of Laryngopharyngeal Reflux in Patients of Different Gendersand Ages. J. Voice 2022, in press. [Google Scholar] [CrossRef]
- Lechien, J.R.; Bobin, F.; Muls, V.; Saussez, S.; Hans, S. Laryngopharyngeal Reflux Disease is More Severe in Obese Patients: A Prospective Multicenter Study. Laryngoscope 2021, 131, E2742–E2748. [Google Scholar] [CrossRef] [PubMed]
- Borges, L.F.; Chan, W.W.; Carroll, T.L. Dual pH Probes Without Proximal Esophageal and Pharyngeal Impedance May Be Deficient in Diagnosing LPR. J. Voice 2019, 33, 697–703. [Google Scholar] [CrossRef] [PubMed]
- Jeon, S.Y.; Park, J.M.; Lee, Y.C.; Ko, S.G.; Eun, Y.G. Differences in Diagnostic Rates After Hypopharyngeal-esophageal Impedance-pH Monitoring of Laryngopharyngeal Reflux Patients by Age and Sex. J. Voice 2022, in press. [Google Scholar] [CrossRef]
- Kwon, S.K.; Park, S.J.; Chung, E.J.; Sohn, J.H.; Sun, D.I.; Jin, S.M.; Lee, B.J.; Park, I.S.; Cho, J.G.; Park, Y.H. Predictors of Early and Late Response to Esomezol and Lifestyle Modification in Adults with Laryngopharyngeal Reflux Disease: A Prospective, Multicenter, Open-Label Cohort Study. Clin. Exp. Otorhinolaryngol. 2023, 16, 259–274. [Google Scholar] [CrossRef]
- Lechien, J.R.; Saussez, S.; Harmegnies, B.; Finck, C.; Burns, J.A. Laryngopharyngeal Reflux and Voice Disorders: A Multifactorial Model of Etiology and Pathophysiology. J. Voice 2017, 31, 733–752. [Google Scholar] [CrossRef]
- Sereg-Bahar, M.; Jerin, A.; Jansa, R.; Stabuc, B.; Hocevar-Boltezar, I. Pepsin and bile acids in saliva in patients with laryngopharyngeal reflux—A prospective comparative study. Clin. Otolaryngol. 2015, 40, 234–239. [Google Scholar] [CrossRef]
- De Corso, E.; Baroni, S.; Salonna, G.; Marchese, M.; Graziadio, M.; Di Cintio, G.; Paludetti, G.; Costamagna, G.; Galli, J. Impact of bile acids on the severity of laryngo-pharyngeal reflux. Clin. Otolaryngol. 2021, 46, 189–195. [Google Scholar] [CrossRef]
- Doukas, P.G.; Vageli, D.P.; Judson, B.L. The Role of PARP-1 and NF-κB in Bile-Induced DNA Damage and Oncogenic Profile in Hypopharyngeal Cells. Laryngoscope 2023, 133, 1146–1155. [Google Scholar] [CrossRef]
- Johnston, N.; Dettmar, P.W.; Bishwokarma, B.; Lively, M.O.; Koufman, J.A. Activity/stability of human pepsin: Implications for reflux attributed laryngeal disease. Laryngoscope 2007, 117, 1036–1039. [Google Scholar] [CrossRef] [PubMed]
- Chouhdry, H.; Villwock, J. Patient Perspective on Adherence to Reflux Lifestyle Modifications: A Qualitative Study. J. Prim. Care Community Health 2023, 14, 21501319231207320. [Google Scholar] [CrossRef]
- Ciqual Table de Composition Nutritionnelle des Aliments. Available online: https://ciqual.anses.fr/ (accessed on 30 July 2019).
- Becker, D.J.; Sinclair, J.; Castell, D.O.; Wu, W.C. A comparison of high and low fat meals on postprandial esophageal acid exposure. Am. J. Gastroenterol. 1989, 84, 782–786. [Google Scholar]
- Hills, J.M.; Aaronson, P.I. The mechanism of action of peppermint oil on gastrointestinal smooth muscle. An analysis using patch clamp electrophysiology and isolated tissue pharmacology in rabbit and guinea pig. Gastroenterology 1991, 101, 55–65. [Google Scholar] [CrossRef] [PubMed]
- Murphy, D.W.; Castell, D.O. Chocolate and heartburn: Evidence of increased esophageal acid exposure after chocolate ingestion. Am. J. Gastroenterol. 1988, 83, 633–636. [Google Scholar] [PubMed]
- Nebel, O.T.; Castell, D.O. Lower esophageal sphincter pressure changes after food ingestion. Gastroenterology 1972, 63, 778–783. [Google Scholar] [CrossRef]
- Sutphen, J.L.; Dillard, V.L. Dietary caloric density and osmolality influence gastroesophageal reflux in infants. Gastroenterology 1989, 97, 601–604. [Google Scholar] [CrossRef]
- Wright, L.E.; Castell, D.O. The adverse effect of chocolate on lower esophageal sphincter pressure. Am. J. Dig Dis. 1975, 20, 703–707. [Google Scholar] [CrossRef] [PubMed]
- Hamoui, N.; Lord, R.V.; Hagen, J.A.; Theisen, J.; Demeester, T.R.; Crookes, P.F. Response of the lower esophageal sphincter to gastric distention by carbonated beverages. J. Gastrointest. Surg. 2006, 10, 870–877. [Google Scholar] [CrossRef]
- Thomas, F.B.; Steinbaugh, J.T.; Fromkes, J.J.; Mekhjian, H.S.; Caldwell, J.H. Inhibitory effect of coffee on lower esophageal sphincter pressure. Gastroenterology 1980, 79, 1262–1266. [Google Scholar] [CrossRef]
- Chang, C.-H.; Wu, C.-P.; Wang, J.-D.; Lee, S.-W.; Chang, C.-S.; Yeh, H.-Z.; Ko, C.-W.; Lien, H.-C. Alcohol and tea consumption are associated with asymptomatic erosive esophagitis in Taiwanese men. PLoS ONE 2017, 12, e0173230. [Google Scholar] [CrossRef]
- Niu, C.-Y.; Zhou, Y.-L.; Yan, R.; Mu, N.-L.; Gao, B.-H.; Wu, F.-X.; Luo, J.-Y. Incidence of gastroesophageal reflux disease in Uygur and Han Chinese adults in Urumqi. World J. Gastroenterol. 2012, 18, 7333–7340. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, I.; Nightingale, P.; Trudgill, N.J. Risk factors for gastro-oesophageal reflux disease symptoms: A community study. Aliment. Pharmacol. Ther. 2005, 21, 821–827. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.-H.; Luo, J.-Y.; Dong, L.; Gong, J.; Tong, M. Epidemiology of gastroesophageal reflux disease: A general population-based study in Xi’an of Northwest China. World J. Gastroenterol. 2004, 10, 1647–1651. [Google Scholar] [CrossRef]
- O’Leary, C.; McCarthy, J.; Humphries, M.; Shanahan, F.; Quigley, E. The prophylactic use of a proton pump inhibitor before food and alcohol. Aliment. Pharmacol. Ther. 2003, 17, 683–686. [Google Scholar] [CrossRef]
- Cao, H.; Huang, X.; Zhi, X.; Han, C.; Li, L.; Li, Y. Association between tea consumption and gastroesophageal reflux disease: A meta-analysis. Medicine 2019, 98, e14173. [Google Scholar] [CrossRef]
- Pollock, B.G.; Wylie, M.; Stack, J.A.; Sorisio, D.A.; Thompson, D.S.; Kirshner, M.A.; Folan, M.M.; Condifer, K.A.; Msn, J.A.S.; Bs, D.A.S.; et al. Inhibition of caffeine metabolism by estrogen replacement therapy in postmenopausal women. J. Clin. Pharmacol. 1999, 39, 936–940. [Google Scholar] [CrossRef] [PubMed]
- Abernethy, D.R.; Todd, E.L. Impairment of caffeine clearance by chronic use of low-dose oestrogen-containing oral contraceptives. Eur. J. Clin. Pharmacol. 1985, 28, 425–428. [Google Scholar] [CrossRef]
- Yeoh, K.G.; Ho, K.Y.; Guan, R.; Kang, J.Y. How does chili cause upper gastrointestinal symptoms? A correlation study with esophageal mucosal sensitivity and esophageal motility. J. Clin. Gastroenterol. 1995, 21, 87–90. [Google Scholar] [CrossRef]
- Milke, P.; Diaz, A.; Valdovinos, M.A.; Moran, S. Gastroesophageal reflux in healthy subjects induced by two different species of chilli (Capsicum annum). Dig Dis. 2006, 24, 184–188. [Google Scholar] [CrossRef]
- Richter, J.E. Gastroesophageal reflux disease in the older patient: Presentation, treatment, and complications. Am. J. Gastroenterol. 2000, 95, 368–373. [Google Scholar] [CrossRef]
- De Bortoli, N.; Guidi, G.; Martinucci, I.; Savarino, E.; Imam, H.; Bertani, L.; Russo, S.; Franchi, R.; Macchia, L.; Furnari, M.; et al. Voluntary and controlled weight loss can reduce symptoms and proton pump inhibitor use and dosage in patients with gastroesophageal reflux disease: A comparative study. Dis. Esophagus 2016, 29, 197–204. [Google Scholar] [CrossRef] [PubMed]
- Allen, M.L.; Mellow, M.H.; Robinson, M.G.; Orr, W.C. The effect of raw onions on acid reflux and reflux symptoms. Am. J. Gastroenterol. 1990, 85, 377–380. [Google Scholar]
- Kaltenbach, T.; Crockett, S.; Gerson, L.B. Are lifestyle measures effective in patients with gastroesophageal reflux disease? An evidence-based approach. Arch. Intern. Med. 2006, 166, 965–971. [Google Scholar] [CrossRef] [PubMed]
- Bulat, R.; Fachnie, E.; Chauhan, U.; Chen, Y.; Tougas, G. Lack of effect of spearmint on lower oesophageal sphincter function and acid reflux in healthy volunteers. Aliment. Pharmacol. Ther. 1999, 13, 805–812. [Google Scholar] [CrossRef] [PubMed]
- Lechien, J.R.; Crevier-Buchman, L.; Distinguin, L.; Iannella, G.; Maniaci, A.; De Marrez, L.G.; Saussez, S.; Hans, S. Is Diet Sufficient as Laryngopharyngeal Reflux Treatment? A Cross-Over Observational Study. Laryngoscope 2022, 132, 1916–1923. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.J.; Shu, C.H.; Chou, K.T.; Wang, Y.F.; Hsu, Y.B.; Ho, C.Y.; Lan, M.Y. Evaluating the autonomic nervous system in patients with laryngopharyngeal reflux. Otolaryngol. Head Neck Surg. 2013, 148, 997–1002. [Google Scholar] [CrossRef]
- Wang, A.M.; Wang, G.; Huang, N.; Zheng, Y.Y.; Yang, F.; Qiu, X.; Chen, X.M. Association between laryngopharyngeal reflux disease and autonomic nerve dysfunction. Eur. Arch. Otorhinolaryngol. 2019, 276, 2283–2287. [Google Scholar] [CrossRef] [PubMed]
- Joo, Y.H.; Song, Y.S.; Pae, C.U. Relationship between Depression and Laryngopharyngeal Reflux. Psychiatry Investig. 2017, 14, 226–229. [Google Scholar] [CrossRef][Green Version]
- Heading, R.C.; Mönnikes, H.; Tholen, A.; Schmitt, H. Prediction of response to PPI therapy and factors influencing treatment outcome in patients with GORD: A prospective pragmatic trial using pantoprazole. BMC Gastroenterol. 2011, 11, 52. [Google Scholar] [CrossRef]
- Lechien, J.R.; Lisan, Q.; Eckley, C.A.; Hamdan, A.L.; Eun, Y.G.; Hans, S.; Saussez, S.; Akst, L.M.; Carroll, T.L. Acute, Recurrent, and Chronic Laryngopharyngeal Reflux: The IFOS Classification. Laryngoscope 2023, 133, 1073–1080. [Google Scholar] [CrossRef]
- Pisaryuk, A.S.; Povalyaev, N.M.; Poletaev, A.V.; Shibeko, A.M. Systems Biology Approach for Personalized Hemostasis Correction. J. Pers. Med. 2022, 12, 1903. [Google Scholar] [CrossRef] [PubMed]
- Duong-Quy, S.; Nguyen-Huu, H.; Hoang-Chau-Bao, D.; Tran-Duc, S.; Nguyen-Thi-Hong, L.; Nguyen-Duy, T.; Tang-Thi-Thao, T.; Phan, C.; Bui-Diem, K.; Vu-Tran-Thien, Q.; et al. Personalized Medicine and Obstructive Sleep Apnea. J. Pers. Med. 2022, 12, 2034. [Google Scholar] [CrossRef] [PubMed]
- Lefèvre, N.; Corazza, F.; Valsamis, J.; Delbaere, A.; De Maertelaer, V.; Duchateau, J.; Casimir, G. The number of X chromosomes influences inflammatory cytokine production following toll-like receptor stimulation. Front. Immunol. 2019, 10, 1052. [Google Scholar] [CrossRef] [PubMed]
- Lefèvre, N.; Corazza, F.; Valsamis, J.; Delbaere, A.; De Maertelaer, V.; Duchateau, J.; Casimir, G. Chronic inflammatory diseases in children are more severe in girls. Shock 2010, 34, 23–26. [Google Scholar] [CrossRef]
- Baumann, I.; Blumenstock, G.; Zalaman, I.M.; Praetorius, M.; Klingmann, C.; Sittel, C.; Plinkert, P.K.; Piccirillo, J.F. Impact of gen- der, age, and comorbidities on quality of life in patients with chronic rhinosinusitis. Rhinology 2007, 45, 268–272. [Google Scholar]
- Lechien, J.R.; Carroll, T.L.; Bobin, F.; Muls, V.; Rodriguez, A.; Horoi, M.; Dequanter, D.; Crevier-Buchman, L.; Hans, S.; Saussez, S. Influence of Age and Sex on Clinical and Therapeutic Features of Laryngopharyngeal Reflux. Otolaryngol. Head Neck Surg. 2022, 166, 468–476. [Google Scholar] [CrossRef]
- Lechien, J.R.; Huet, K.; Khalife, M.; Fourneau, A.-F.; Finck, C.; Delvaux, V.; Piccaluga, M.; Harmegnies, B.; Saussez, S. Gender differences in the presentation of dysphonia related to laryngopharyngeal reflux disease: A case-control study. Eur. Arch. Otorhinolaryngol. 2018, 275, 1513–1524. [Google Scholar] [CrossRef]
- Lechien, J.R.; Huet, K.; Finck, C.; Khalife, M.; Fourneau, A.-F.; Harmegnies, B.; Saussez, S. Clinical and acoustical voice quality evolutions throughout empirical treatment for laryngo- pharyngeal reflux disease according to gender: A preliminary study. Folia Phoniatr. Logop. 2020, 72, 257–266. [Google Scholar] [CrossRef]
- Akyildiz, S.; Ogut, F.; Varis, A.; Kirazli, T.; Bor, S. Impact of laryn- geal findings on acoustic parameters of patients with laryngo- pharyngeal reflux. ORL J. Otorhinolaryngol. Relat. Spec. 2012, 74, 215–219. [Google Scholar] [CrossRef]
- Qi, Z.; Zhang, Y.; Su, R.; Niu, R.; Liu, C. The influence of age on the score of reflux symptom index scale and reflux finding score scale in the diagnosis of pharyngeal and laryngeal reflux. J. Clin. Otorhinolaryngol. Head Neck Surg. 2020, 34, 170–172. [Google Scholar] [CrossRef]
- Pilotto, A.; Maggi, S.; Noale, M.; Franceschi, M.; Parisi, G.; Crepaldi, G. Association of upper gastro- intestinal symptoms with functional and clinical charateristics in elderly. World J. Gastroenterol. 2011, 17, 3020–3026. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.C.; Lee, J.S.; Kim, S.W.; Kwon, K.H.; Eun, Y.G. Influence of age on treatment with proton pump inhibitors in patients with laryngopharyngeal reflux disease: A prospective multicenter study. JAMA Otolaryngol. Head Neck Surg. 2013, 139, 1291–1295. [Google Scholar] [CrossRef] [PubMed]
- Thornton, F.J.; Barbul, A. Healing in the gastrointestinal tract. Surg. Clin. N. Am. 1997, 77, 549–573. [Google Scholar] [CrossRef] [PubMed]
- Ruhl, C.E.; Everhart, J.E. Overweight, but not high dietary fat intake, increases risk of gastroesophageal reflux disease hospitalization: The NHANES I Epidemiologic Follow-up Study. Ann. Epidemiol. 1999, 9, 424–425. [Google Scholar] [CrossRef] [PubMed]
- Halum, S.L.; Postma, G.N.; Johnston, C.; Belafsky, P.C.; Koufman, J.A. Patients with isolated laryngopharyngeal reflux are not obese. Laryngoscope 2005, 115, 1042–1045. [Google Scholar] [CrossRef]
- Balouch, B.; Melley, L.E.; Yeakel, H.; Ranjbar, P.A.; Tong, J.; Eichorn, D.; Alnouri, G.; Brennan, M.; Tran, Q.; Sataloff, R.T. Gluten Sensitivity Underlying Resistant “Laryngopharyngeal Reflux” Symptoms and Signs. J. Voice 2023, in press. [Google Scholar] [CrossRef]
- Lechien, J.R.; Muls, V.; Dapri, G.; Mouawad, F.; Eisendrath, P.; Schindler, A.; Nacci, A.; Barillari, M.R.; Finck, C.; Saussez, S.; et al. The management of suspected or confirmed laryngopharyngeal reflux patients with recalcitrant symptoms: A contemporary review. Clin. Otolaryngol. 2019, 44, 784–800. [Google Scholar] [CrossRef] [PubMed]
- Pérez Lara, F.J.; Hernández Gonzalez, J.M.; Doblas Fernández, J.; Corrales Valero, E.; Oehling de Los Reyes, H. Prospective Study of Lactose Intolerance as a Potential Cause of Gas Bloat Syndrome in Patients Treated Surgically for Gastroesophageal Reflux. Surg. Innov. 2020, 27, 160–164. [Google Scholar] [CrossRef]
- Wu, W.Y.; Chang, S.C.; Hsu, J.T.; Yeh, T.S.; Liu, K.H. Gastroesophageal Reflux Disease Symptoms after Laparoscopic Sleeve Gastrectomy: A Retrospective Study. J. Pers. Med. 2022, 12, 1795. [Google Scholar] [CrossRef]
- Alnouri, G.; Cha, N.; Sataloff, R.T. Histamine Sensitivity: An Uncommon Recognized Cause of Living Laryngopharyngeal Reflux Symptoms and Signs-A Case Report. Ear Nose Throat J. 2022, 101, NP155–NP157. [Google Scholar] [CrossRef]
- De La Chapa, J.S.; Harryman, C.J.; McGarey, P.O.; Daniero, J.J. Clinical Characteristics of the Cervical Inlet Patch: A Case Series. OTO Open 2023, 7, e24. [Google Scholar] [CrossRef] [PubMed]
- Sikavi, D.R.; Cai, J.X.; Leung, R.; Carroll, T.L.; Chan, W.W. Impaired Proximal Esophageal Contractility Predicts Pharyngeal Reflux in Patients with Laryngopharyngeal Reflux Symptoms. Clin. Transl. Gastroenterol. 2021, 12, e00408. [Google Scholar] [CrossRef] [PubMed]
- Hoppo, T.; Sanz, A.F.; Nason, K.S.; Carroll, T.L.; Rosen, C.; Normolle, D.P.; Shaheen, N.J.; Luketich, J.D.; Jobe, B.A. How much pharyngeal exposure is “normal”? Normative data for laryngopharyngeal reflux events using hypopharyngeal multichannel intraluminal impedance (HMII). J. Gastrointest. Surg. 2012, 16, 16–25. [Google Scholar] [CrossRef]
- Nakagawa, K.; Sawada, A.; Hoshikawa, Y.; Nikaki, K.; Sonmez, S.; Woodland, P.; Yazaki, E.; Sifrim, D. Persistent Postprandial Regurgitation vs Rumination in Patients with Refractory Gastroesophageal Reflux Disease Symptoms: Identification of a Distinct Rumination Pattern Using Ambulatory Impedance-pH Monitoring. Am. J. Gastroenterol. 2019, 114, 1248–1255. [Google Scholar] [CrossRef] [PubMed]
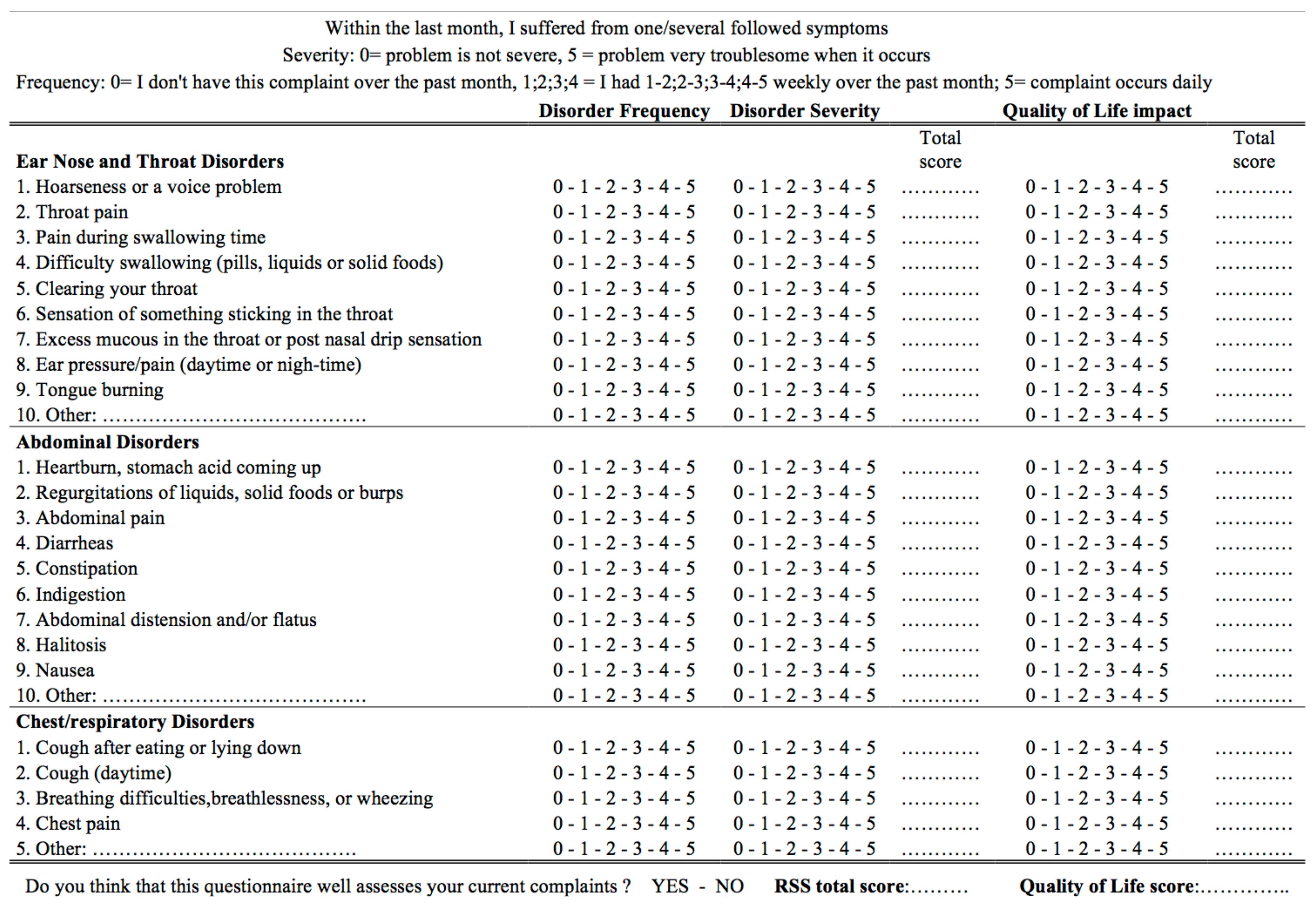
- Lechien, J.R.; Bobin, F.; Muls, V.; Thill, M.P.; Horoi, M.; Ostermann, K.; Huet, K.; Harmegnies, B.; Dequanter, D.; Dapri, G.; et al. Validity and reliability of the reflux symptom score. Laryngoscope 2020, 130, E98–E107. [Google Scholar] [CrossRef] [PubMed]
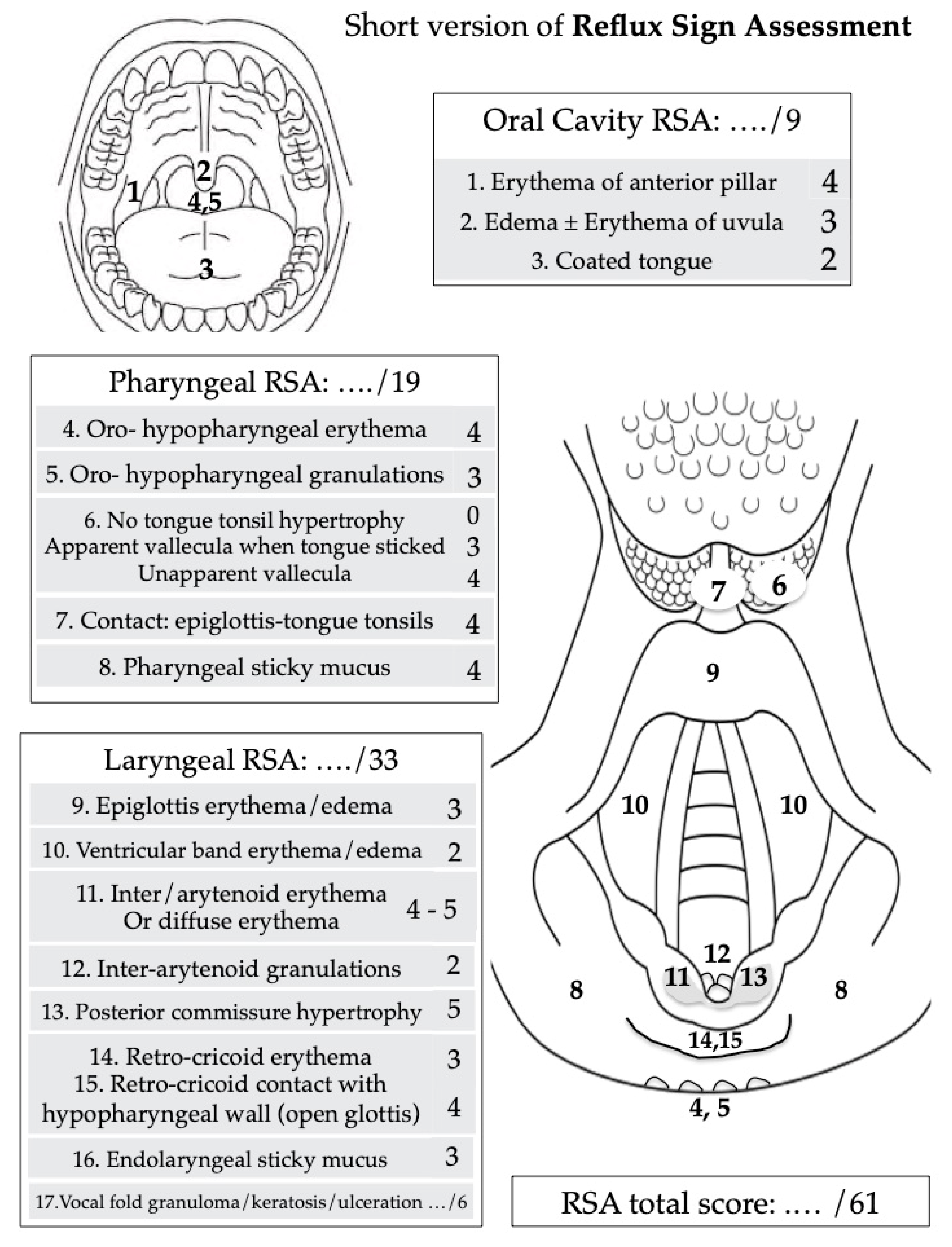
- Lechien, J.R.; Rodriguez Ruiz, A.; Dequanter, D.; Bobin, F.; Mouawad, F.; Muls, V.; Huet, K.; Harmegnies, B.; Remacle, S.; Finck, C.; et al. Validity and Reliability of the Reflux Sign Assessment. Ann. Otol. Rhinol. Laryngol. 2020, 129, 313–325. [Google Scholar] [CrossRef]
- Francis, D.O.; Rymer, J.A.; Slaughter, J.C.; Choksi, Y.; Jiramongkolchai, P.; Ogbeide, E.; Tran, C.; Goutte, M.; Garrett, C.G.; Hagaman, D.; et al. High economic burden of caring for patients with suspected extraesophageal reflux. Am. J. Gastroenterol. 2013, 108, 905–911. [Google Scholar] [CrossRef]
- Lechien, J.R.; Lisan, Q.; Eckley, C.A.; Hamdan, A.L.; Eun, Y.G.; Hans, S.; Saussez, S.; Akst, L.M.; Carroll, T.L. The Dubai Definition and Diagnostic Criteria of Laryngopharyngeal Reflux: The IFOS Consensus. Laryngoscope 2023. [Google Scholar]
- Lechien, J.R.; Bobin, F.; Dapri, G.; Eisendrath, P.; Salem, C.; Mouawad, F.; Horoi, M.; Thill, M.P.; Dequanter, D.; Rodriguez, A.; et al. Hypopharyngeal-Esophageal Impedance-pH Monitoring Profiles of Laryngopharyngeal Reflux Patients. Laryngoscope 2021, 131, 268–276. [Google Scholar] [CrossRef]
- Muderris, T.; Gokcan, M.K.; Yorulmaz, I. The clinical value of pharyngeal pH monitoring using a double-probe, triple-sensor catheter in patients with laryngopharyngeal reflux. Arch. Otolaryngol. Head Neck Surg. 2009, 135, 163–167. [Google Scholar] [CrossRef]
- Kang, J.W.; Lee, Y.C.; Ko, S.G.; Eun, Y.G. The key timing of pharyngeal reflux in patients with laryngopharyngeal reflux. Auris Nasus Larynx 2023, 50, 565–570. [Google Scholar] [CrossRef] [PubMed]
- Lechien, J.R. Clinical Update Findings about pH-Impedance Monitoring Features in Laryngopharyngeal Reflux Patients. J. Clin. Med. 2022, 11, 3158. [Google Scholar] [CrossRef] [PubMed]
- Lechien, J.R.; Bobin, F.; Muls, V.; Mouawad, F.; Dequanter, D.; Horoi, M.; Thill, M.P.; Rodriguez Ruiz, A.; Saussez, S. The efficacy of a personalised treatment depending on the characteristics of reflux at multichannel intraluminal impedance-pH monitoring in patients with acid, non-acid and mixed laryngopharyngeal reflux. Clin. Otolaryngol. 2021, 46, 602–613. [Google Scholar] [CrossRef]
- Ayazi, S.; Lipham, J.C.; Hagen, J.A.; Tang, A.L.; Zehetner, J.; Leers, J.M.; Oezcelik, A.; Abate, E.; Banki, F.; DeMeester, S.R.; et al. A new technique for measurement of pharyngeal pH: Normal values and discriminating pH threshold. J. Gastrointest. Surg. 2009, 13, 1422–1429. [Google Scholar] [CrossRef]
- Lechien, J.R.; Chan, W.W.; Akst, L.M.; Hoppo, T.; Jobe, B.A.; Chiesa-Estomba, C.M.; Muls, V.; Bobin, F.; Saussez, S.; Carroll, T.L.; et al. Normative Ambulatory Reflux Monitoring Metrics for Laryngopharyngeal Reflux: A Systematic Review of 720 Healthy Individuals. Otolaryngol. Head Neck Surg. 2021, 166, 802–819. [Google Scholar] [CrossRef]
- Bonini, S.; Di Zazzo, A.; Surico, P.L.; Balzamino, B.O.; Luccarelli, V.; Niutta, M.; Coassin, M.; Micera, A. Inflammation and Dry Eye-like Symptoms as Concomitant Manifestations of Laryngo-Pharyngeal Reflux. Curr. Eye Res. 2023, 48, 724–730. [Google Scholar] [CrossRef]
- Javorská, Z.; Zeleník, K.; Lukáčová, K.; Taimrová, R.; Vrtková, A.; Hránková, V.; Lubojacký, J.; Formánek, M.; Tedla, M. Mulberry Posterior Inferior Nasal Turbinate Is Associated with a Lower Pharyngeal pH Environment. Laryngoscope 2023. [Google Scholar] [CrossRef]
- Zhen, Z.; Zhao, T.; Wang, Q.; Zhang, J.; Zhong, Z. Laryngopharyngeal reflux as a potential cause of Eustachian tubedysfunction in patients with otitis media with effusion. Front. Neurol. 2022, 13, 1024743. [Google Scholar] [CrossRef] [PubMed]
- Al-Saab, F.; Manoukian, J.J.; Al-Sabah, B.; Almot, S.; Nguyen, L.H.; Tewfik, T.L.; Daniel, S.J.; Schloss, M.D.; Hamid, Q.A. Linking laryngopharyngeal reflux to otitis media with effusion: Pepsinogen study of adenoid tissue and middle ear fluid. J. Otolaryngol. Head Neck Surg. 2008, 37, 565–571. [Google Scholar]
- Weitzendorfer, M.; Antoniou, S.A.; Schredl, P.; Witzel, K.; Weitzendorfer, I.C.; Majerus, A.; Emmanuel, K.; Koch, O.O. Pepsin and oropharyngeal pH monitoring to diagnose patients with laryngopharyngeal reflux. Laryngoscope 2020, 130, 1780–1786. [Google Scholar] [CrossRef]
- Sikavi, D.R.; Cai, J.X.; Carroll, T.L.; Chan, W.W. Prevalence and clinical significance of esophageal motility disorders in patients with laryngopharyngeal reflux symptoms. J. Gastroenterol. Hepatol. 2021, 36, 2076–2082. [Google Scholar] [CrossRef]
- Tseng, W.H.; Hsu, W.C.; Hsiao, T.Y.; Wu, J.F.; Lee, H.C.; Wang, H.P.; Wu, M.S.; Tseng, P.H. Anatomical and physiological characteristics in patients with Laryngopharyngeal Reflux Symptoms: A case-control study utilizing high-resolution impedance manometry. J. Formos. Med. Assoc. 2022, 121, 1034–1043. [Google Scholar] [CrossRef]
- Van Daele, D.J. Esophageal Manometry, pH Testing, Endoscopy, and Videofluoroscopy in Patients with Globus Sensation. Laryngoscope 2020, 130, 2120–2125. [Google Scholar] [CrossRef] [PubMed]
- Borges, L.F.; Salgado, S.; Hathorn, K.E.; Feldman, N.; Carroll, T.L.; Chan, W.W. Failed Swallows on High-Resolution Manometry Independently Correlates with Severity of LPR Symptoms. J. Voice 2022, 36, 832–837. [Google Scholar] [CrossRef]
- Vakil, N.; van Zanten, S.V.; Kahrilas, P.; Dent, J.; Jones, R.; Global Consensus Group. The Montreal definition and classification of gastroesophageal reflux disease: A global evidence-based consensus. Am. J. Gastroenterol. 2006, 101, 1900–1920; quiz 1943. [Google Scholar] [CrossRef]
- Reichel, O.; Issing, W.J. Should patients with pH-documented laryngopharyngeal reflux routinely undergo oesophagogastroduodenoscopy? A retrospective analysis. J. Laryngol. Otol. 2007, 121, 1165–1169. [Google Scholar] [CrossRef] [PubMed]
- Zelenik, K.; Kajzrlikova, I.M.; Vitek, P.; Urban, O.; Hanousek, M.; Kominek, P. There is no correlation between signs of reflux laryngitis and reflux oesophagitis in patients with gastro-oesophageal reflux disease symptoms. Acta Otorhinolaryngol. Ital. 2017, 37, 401–405. [Google Scholar] [CrossRef]
- Calvo-Henríquez, C.; Ruano-Ravina, A.; Vaamonde, P.; Martínez-Capoccioni, G.; Martín-Martín, C. Is Pepsin a Reliable Marker of Laryngopharyngeal Reflux? A Systematic Review. Otolaryngol. Head Neck Surg. 2017, 157, 385–391. [Google Scholar] [CrossRef]
- Guo, Z.; Jiang, J.; Wu, H.; Zhu, J.; Zhang, S.; Zhang, C. Salivary peptest for laryngopharyngeal reflux and gastroesophageal refluxdisease: A systemic review and meta-analysis. Medicine 2021, 100, e26756. [Google Scholar] [CrossRef] [PubMed]
- Russo, M.; Crafa, P.; Guglielmetti, S.; Franzoni, L.; Fiore, W.; Di Mario, F. Burning Mouth Syndrome Etiology: A Narrative Review. J. Gastrointestin. Liver Dis. 2022, 31, 223–228. [Google Scholar] [CrossRef]
- Kitasako, Y.; Tanabe, T.; Koeda, M.; Momma, E.; Hoshikawa, Y.; Hoshino, S.; Kawami, N.; Ikeda, M.; Iwakiri, K. Patients with gastroesophageal reflux disease (both reflux oesophagitis and non-erosive reflux disease): Prevalence and severity of erosive tooth wear and saliva properties. J. Oral Rehabil. 2023. [Google Scholar] [CrossRef] [PubMed]
- Howard, J.P.; Howard, L.J.; Geraghty, J.; Leven, A.J.; Ashley, M. Gastrointestinal conditions related to tooth wear. Br. Dent. J. 2023, 234, 451–454. [Google Scholar] [CrossRef] [PubMed]
- Casciaro, S.; Gelardi, M.; Giancaspro, R.; Quaranta, V.N.; Porro, G.; Sterlicchio, B.; Abbinante, A.; Corsalini, M. Dental Disorders and Salivary Changes in Patients with LaryngopharyngealReflux. Diagnostics 2022, 12, 153. [Google Scholar] [CrossRef]
- Pizzorni, N.; Ambrogi, F.; Eplite, A.; Rama, S.; Robotti, C.; Lechien, J.; Schindler, A. Magnesium alginate versus proton pump inhibitors for the treatment of laryngopharyngeal reflux: A non-inferiority randomized controlled trial. Eur. Arch. Otorhinolaryngol. 2022, 279, 2533–2542. [Google Scholar] [CrossRef]
- Thjodleifsson, B. Treatment of acid-related diseases in the elderly with emphasis on the use of proton pump inhibitors. Drugs Aging 2002, 19, 911–927. [Google Scholar] [CrossRef]
- Calabrese, C.; Fabbri, A.; Di Febo, G. Long-term management of GERD in the elderly with pantoprazole. Clin. Interv. Aging. 2007, 2, 85–92. [Google Scholar] [CrossRef] [PubMed]
- Desilets, A.R.; Asal, N.J.; Dunican, K.C. Considerations for the use of proton-pump inhibitors in older adults. Consult. Pharm. 2012, 27, 114–120. [Google Scholar] [CrossRef]
- Flouvat, B.; Delhotal-Landes, B.; Cournot, A.; Dellatolas, F. Single and multiple dose pharmacokinetics of lansoprazole in elderly subjects. Br. J. Clin. Pharmacol. 1993, 36, 467–469. [Google Scholar] [CrossRef]
- Huber, R.; Hartmann, M.; Bliesath, H.; Lühmann, R.; Steinijans, V.W.; Zech, K. Pharmacokinetics of pantoprazole in man. Int. J. Clin. Pharmacol. Ther. 1996, 34, 185–194. [Google Scholar]
- Park, W.; Hicks, D.M.; Khandwala, F.; Richter, J.E.; Abelson, T.I.; Milstein, C.; Vaezi, M.F. Laryngopharyngeal reflux: Prospective cohort study evaluating optimal dose of proton-pump inhibitor therapy and pretherapy predictors of response. Laryngoscope 2005, 115, 1230–1238. [Google Scholar] [CrossRef]
- Woodland, P.; Batista-Lima, F.; Lee, C.; Preston, S.L.; Dettmar, P.; Sifrim, D. Topical protection of human esophageal mucosal integrity. Am. J. Physiol. Gastrointest. Liver Physiol. 2015, 308, G975–G980. [Google Scholar] [CrossRef] [PubMed]
- Zentilin, P.; Dulbecco, P.; Savarino, E.; Parodi, A.; Iiritano, E.; Bilardi, C.; Reglioni, S.; Vigneri, S.; Savarino, V. An evaluation of the antireflux properties of sodium alginate by means of combined multichannel intraluminal impedance and pH-metry. Aliment. Pharmacol. Ther. 2005, 21, 29–34.e118. [Google Scholar] [CrossRef]
- Krause, A.J.; Walsh, E.H.; Weissbrod, P.A.; Taft, T.H.; Yadlapati, R. An update on current treatment strategies for laryngopharyngeal reflux symptoms. Ann. N. Y. Acad. Sci. 2022, 1510, 5–17. [Google Scholar] [CrossRef]
- Verhasselt, M.; Rodriguez, A.; Dequanter, D.; Lechien, J.R. Chronic Course, Weaning, and Awareness of Patients with Reflux Toward Proton Pump Inhibitor Therapy. J. Voice 2021, 37, 577–585. [Google Scholar] [CrossRef]
- Lin, R.J.; Sridharan, S.; Smith, L.J.; Young, V.N.; Rosen, C.A. Weaning of proton pump inhibitors in patients with suspected laryngopharyngeal reflux disease. Laryngoscope 2018, 128, 133–137. [Google Scholar] [CrossRef]
- Beaver, M.E.; Stasney, C.R.; Weitzel, E.; Stewart, M.G.; Donovan, D.T.; Parke, R.B., Jr.; Rodriguez, M. Diagnosis of laryngopharyngeal reflux disease with digital imaging. Otolaryngol. Head Neck Surg. 2003, 128, 103–108. [Google Scholar] [CrossRef]
- Rameau, A.; Andreadis, K.; Bayoumi, A.; Kaufman, M.; Belafsky, P. Side Effects of Proton Pump Inhibitors: What Are Patients’ Concerns? J. Voice 2020, 35, 809.e15–809.e20. [Google Scholar] [CrossRef]
- Lanas-Gimeno, A.; Hijos, G.; Lanas, Á. Proton pump inhibitors, adverse events and increased risk of mortality. Expert Opin. Drug. Saf. 2019, 18, 1043–1053. [Google Scholar] [CrossRef]
- Chapman, D.B.; Rees, C.J.; Lippert, D.; Sataloff, R.T.; Wright, S.C., Jr. Adverse effects of long-term proton pump inhibitor use: A review for the otolaryngologist. J. Voice 2011, 25, 236–240. [Google Scholar] [CrossRef] [PubMed]
- Kojima, T.; Hasebe, K.; Fujimura, S.; Okanoue, Y.; Kagoshima, H.; Taguchi, A.; Yamamoto, H.; Shoji, K.; Hori, R. A New iPhone Application for Voice Quality Assessment Based on the GRBAS Scale. Laryngoscope 2021, 131, 580–582. [Google Scholar] [CrossRef] [PubMed]
- Adamian, N.; Naunheim, M.R.; Jowett, N. An Open-Source Computer Vision Tool for Automated Vocal Fold Tracking from Videoendoscopy. Laryngoscope 2021, 131, E219–E225. [Google Scholar] [CrossRef] [PubMed]
- Mondol, S.I.M.M.R.; Kim, H.J.; Kim, K.S.; Lee, S. Machine Learning-Based Hearing Aid Fitting Personalization Using Clinical Fitting Data. J. Healthc. Eng. 2022, 2022, 1667672. [Google Scholar] [CrossRef] [PubMed]
- Wiedermann, C.J.; Mahlknecht, A.; Piccoliori, G.; Engl, A. Redesigning Primary Care: The Emergence of Artificial-Intelligence-Driven Symptom Diagnostic Tools. J. Pers. Med. 2023, 13, 1379. [Google Scholar] [CrossRef]
- Poalelungi, D.G.; Musat, C.L.; Fulga, A.; Neagu, M.; Neagu, A.I.; Piraianu, A.I.; Fulga, I. Advancing Patient Care: How Artificial Intelligence Is Transforming Healthcare. J. Pers. Med. 2023, 13, 1214. [Google Scholar] [CrossRef] [PubMed]
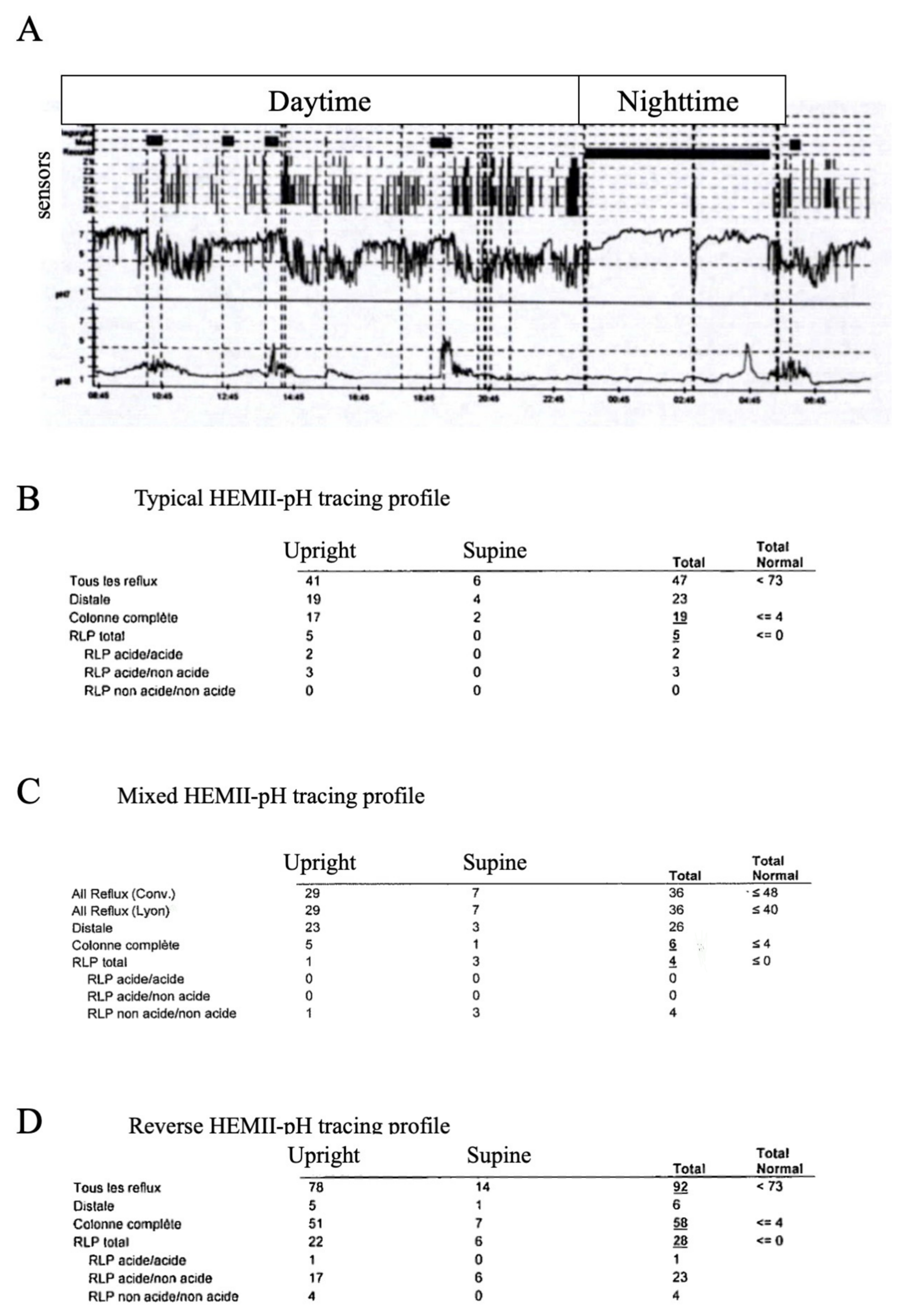
| Hypopharyngeal–Esophageal Multichannel Intraluminal Impedance pH-Monitoring Outcomes and Diagnosis Criteria | |
|---|---|
| 1 | Single-channel (esophageal) or dual-channel (esophageal-esophageal) pH probes are useful for diagnosing GERD but not adequate for diagnosing LPR |
| because of the lack of a pharyngeal sensor and lack of consideration of non-acidic events. | |
| 2 | If HEMII-pH is unavailable, an empirical treatment covering acidic, weakly acidic, and nonacidic LPR may be prescribed and evaluated at 3 months. |
| Treatment success of LPR should be based on improvement of the patient’s LPR symptoms. | |
| 3 | The HEMII-pH results may provide guidance as to the appropriate nature, dosing, and timing of medications for the specific patient according to |
| the type of LPR (acidic, weakly acidic, nonacidic) and time of occurrence (upright and daytime and/or nighttime) | |
| 4 | Triple-channel (dual esophageal and pharyngeal) pH-only studies may detect acidic pharyngeal reflux events but they are not sufficient to rule out |
| LPR disease as they may miss weakly acidic and nonacidic pharyngeal events. | |
| 5 | HEMII-pH monitoring has to respect the following placement characteristics: |
| (1) Proximal pH sensor in the hypopharyngeal cavity at 0.5 cm to 1 cm above upper esophageal sphincter or within the sphincter. | |
| (2) Distal pH sensor in the esophagus as close to 5 cm above lower esophageal sphincter as possible. | |
| (3) At least 2 impedance sensor pairs in the esophagus. | |
| (4) At least 1 impedance sensor pair in the pharyngeal cavity. It is recommended to control the placement of the upper pH sensor using flexible laryngoscopic | |
| or manometric guidance. The recommended duration of the examination is 24 h. During the 24 h testing, the patient should continue their normal | |
| diet and activities. | |
| 6 | Based on HEMII-pH, a hypopharyngeal acidic event consists of an event with a pH < 4.0. A hypopharyngeal weakly acidic reflux event consists of |
| an event with a pH between 4.0 and 7.0. A hypopharyngeal alkaline reflux event consists of an event with pH > 7.0. | |
| 7 | The analysis of the 24 h recording must respect the following: |
| (1) Exclusion of reflux events during meals; | |
| (2) Pharyngeal reflux event diagnosed only when a reflux event originating from the distal most impedance channel reaches the pharyngeal channels in | |
| a retrograde fashion; | |
| (3) Manual analysis to identify reflux events that the computer may have reported incorrectly. | |
| 8 | The severity of LPR seen using HEMII-pH or oropharyngeal pH monitoring is not necessarily correlated with the severity of symptoms and findings. |
| 9 | While HEMII-pH is promising as an objective tool for diagnosing LPR, the correlation between its findings and treatment outcomes remains limited. |
| Controlled studies are needed to validate the value of this technology in predicting treatment outcomes. | |
| 10 | Reflux monitoring for LPR, whether using HEMII-pH, MII-pH, or pH measurements, should be performed off acid suppression medications, beginning |
| at least 7 days prior to the study. | |
| 11 | The LPR diagnosis may not be confirmed with esophageal catheters that are configured with two esophageal pH sensors and without impedance or |
| pH sensors in the pharynx because (1) the proximal esophageal reflux events may not reach the hypopharynx and (2) the presence of reflux events near | |
| the UES may be altered by swallowing saliva. | |
| 12 | Hypopharyngeal–esophageal multichannel intraluminal impedance pH monitoring (HEMII-pH) is an objective tool to identify esophago-pharyngeal |
| reflux events (acidic, weakly acidic, or nonacidic) and can suggest a diagnosis of LPR when there is >1 hypopharyngeal reflux event in 24 h. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lechien, J.R. Personalized Treatments Based on Laryngopharyngeal Reflux Patient Profiles: A Narrative Review. J. Pers. Med. 2023, 13, 1567. https://doi.org/10.3390/jpm13111567
Lechien JR. Personalized Treatments Based on Laryngopharyngeal Reflux Patient Profiles: A Narrative Review. Journal of Personalized Medicine. 2023; 13(11):1567. https://doi.org/10.3390/jpm13111567
Chicago/Turabian StyleLechien, Jerome R. 2023. "Personalized Treatments Based on Laryngopharyngeal Reflux Patient Profiles: A Narrative Review" Journal of Personalized Medicine 13, no. 11: 1567. https://doi.org/10.3390/jpm13111567
APA StyleLechien, J. R. (2023). Personalized Treatments Based on Laryngopharyngeal Reflux Patient Profiles: A Narrative Review. Journal of Personalized Medicine, 13(11), 1567. https://doi.org/10.3390/jpm13111567






