Imaging Features of Main Hepatic Resections: The Radiologist Challenging
Abstract
1. Introduction
Type of Resection
2. Anatomic Liver Resection
3. Non Anatomic Liver Resection
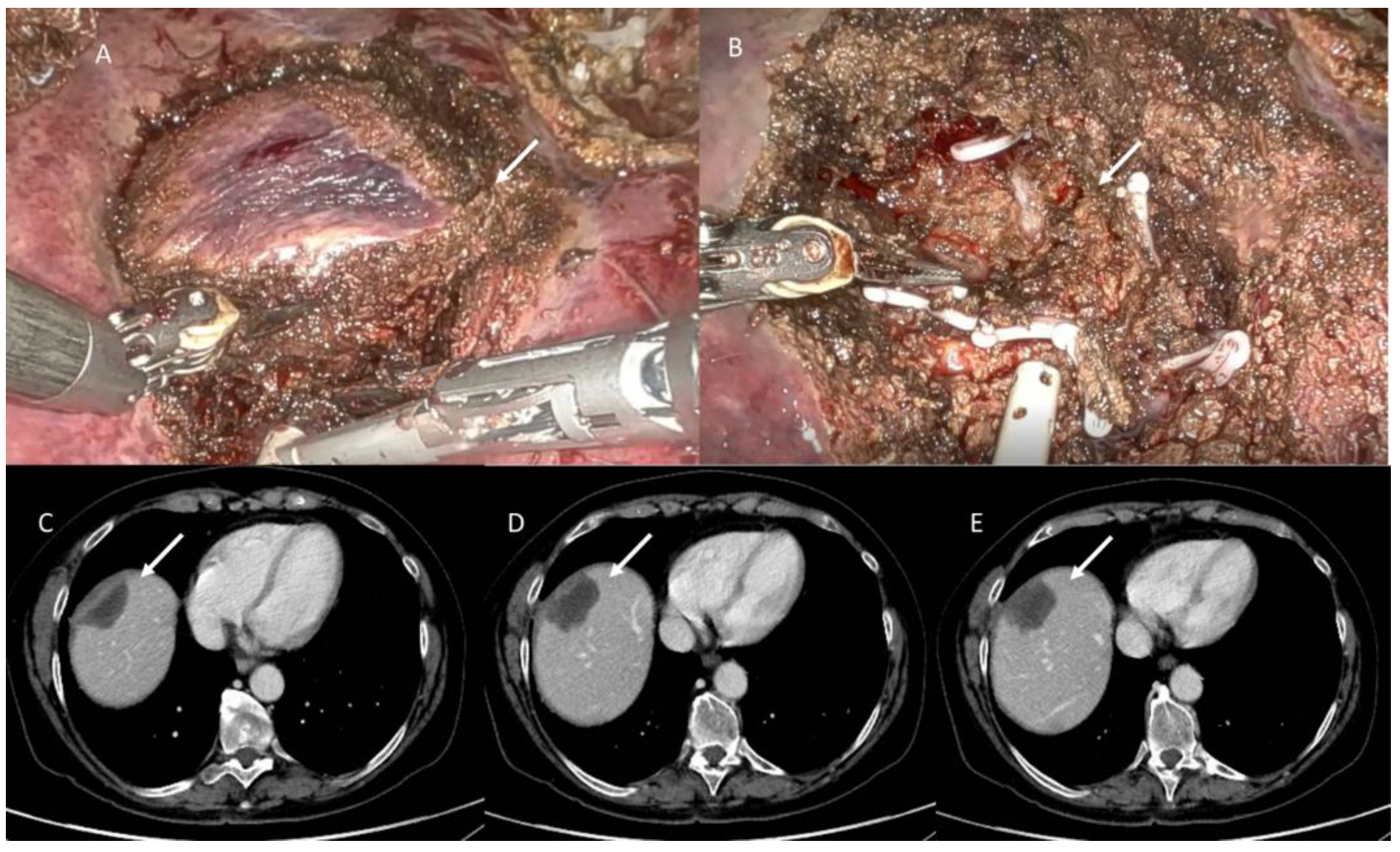
3.1. Two Stage Hepatectomy and ALLPS
3.2. Treatment and Imaging Assessment
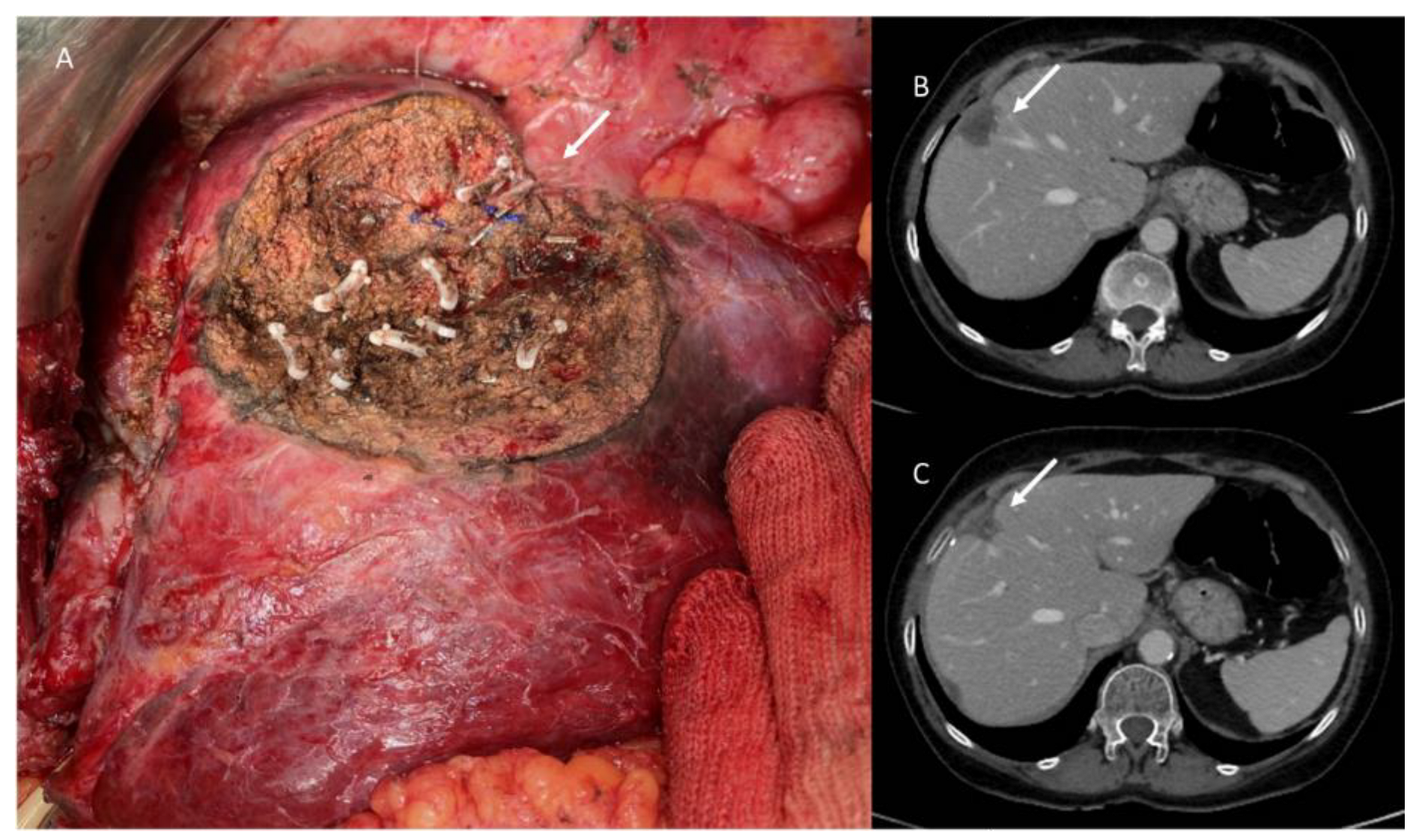
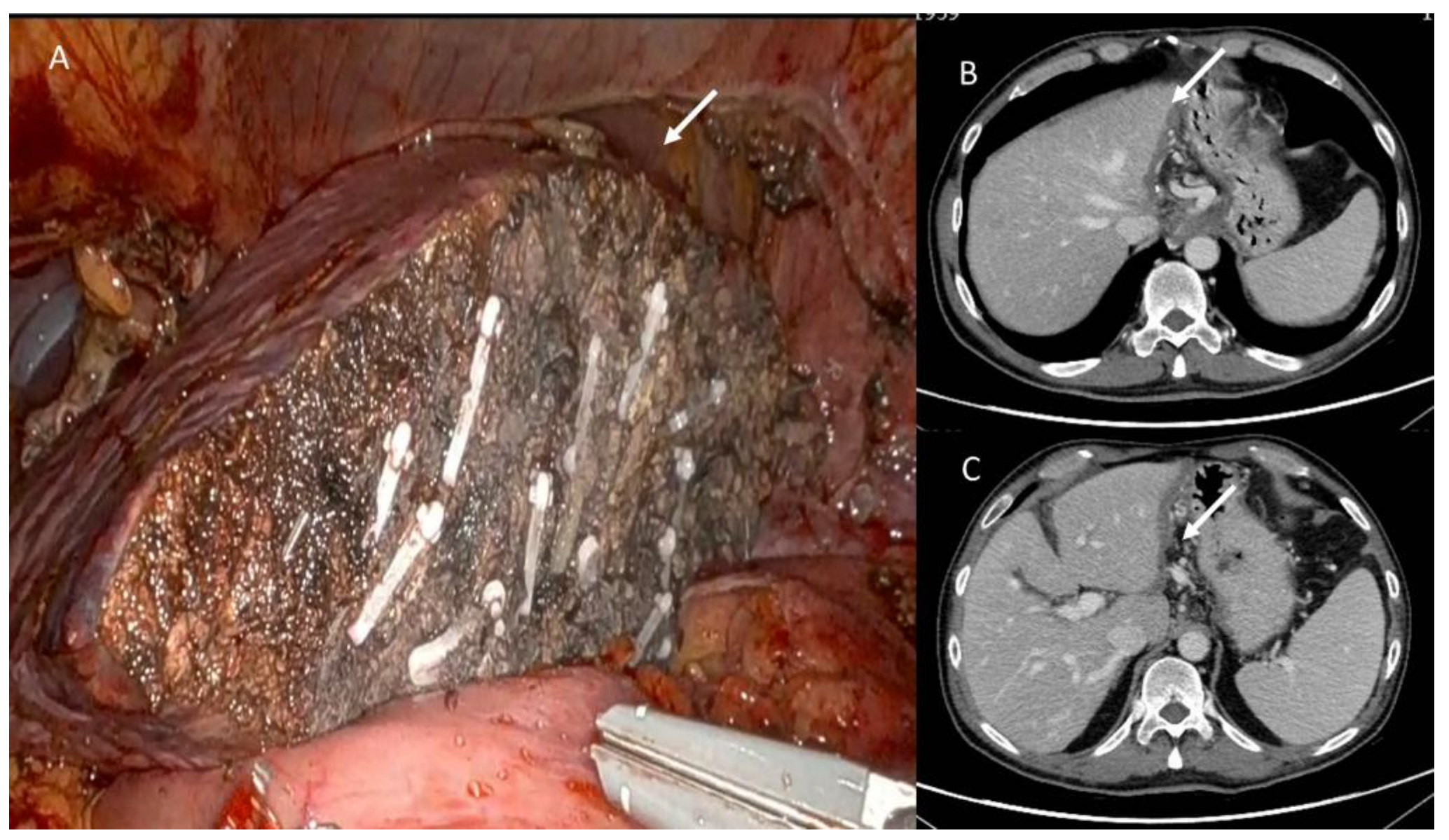
3.3. Post-Surgical Imaging Findings
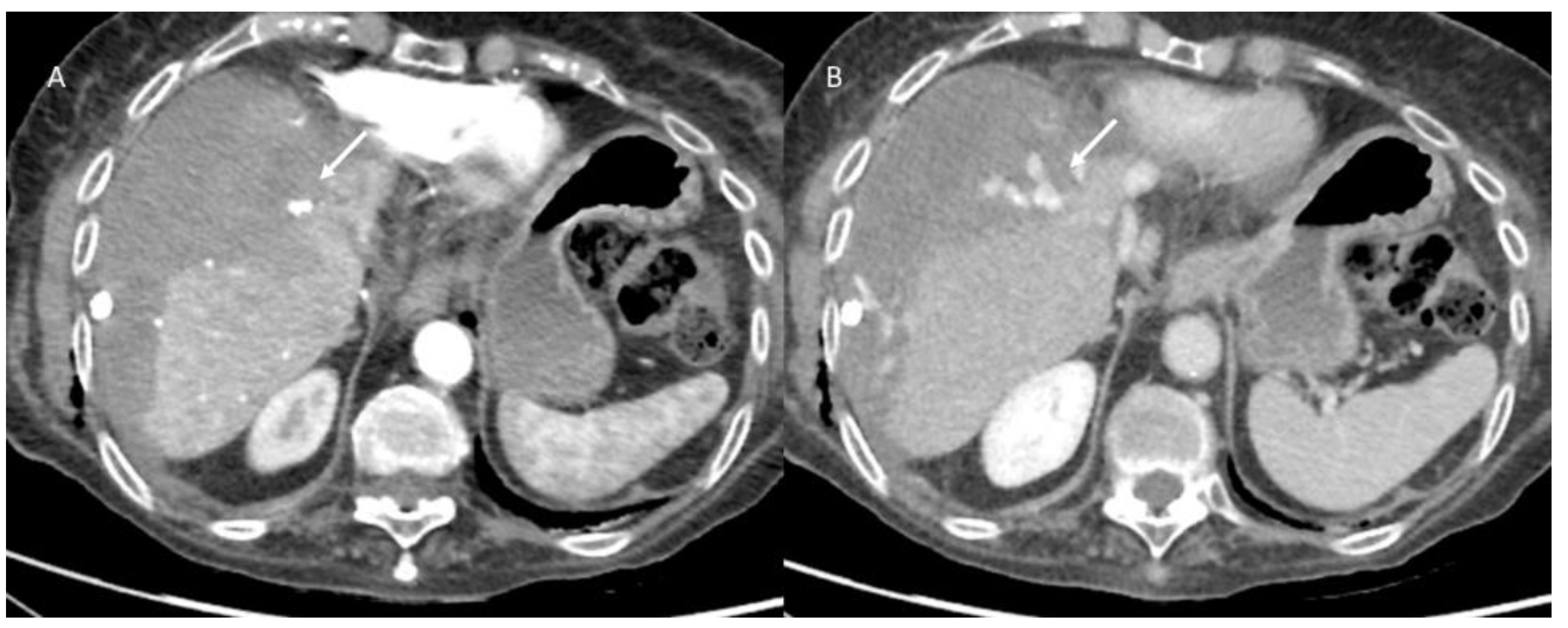
3.4. Discharge Assessment
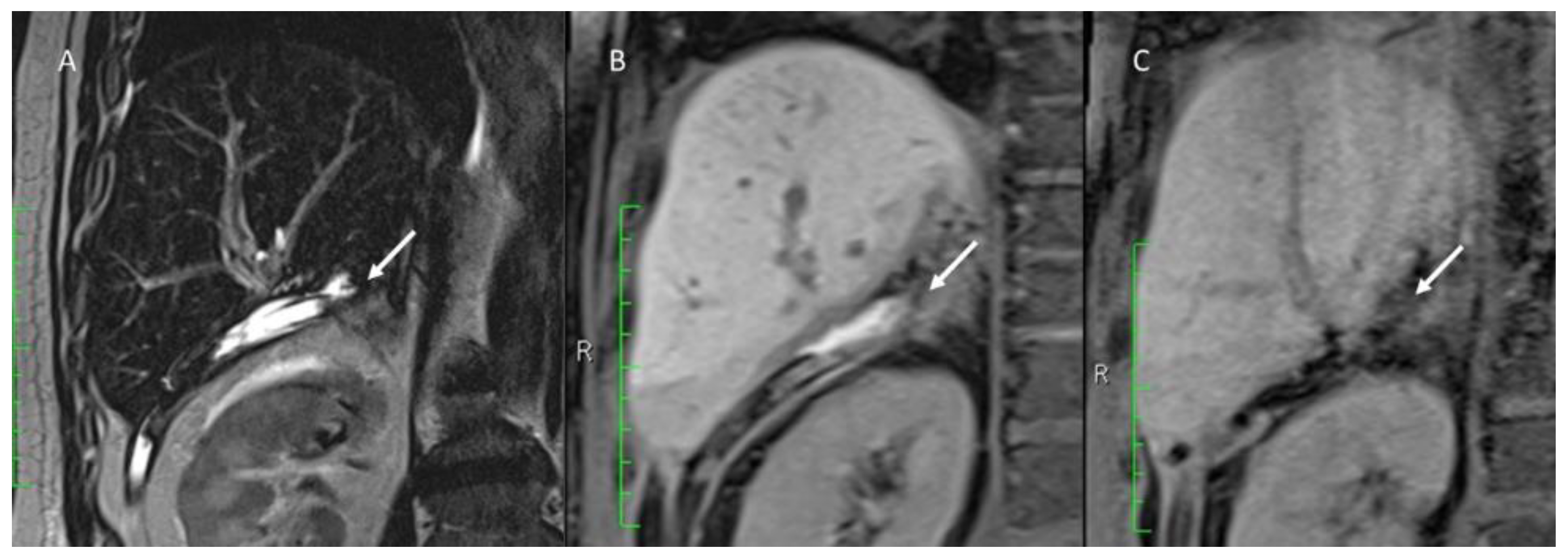
3.5. Follow-Up Assessment
3.6. Two Stage Hepatectomy and ALLPS Assessment
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lafaro, K.J.; Stewart, C.; Fong, A.; Fong, Y. Robotic Liver Resection. Surg. Clin. N. Am. 2020, 100, 265–281. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, V.; Divatia, J.V. Enhanced recovery after surgery in liver resection: Current concepts and controversies. Korean J. Anesthesiol. 2019, 72, 119–129. [Google Scholar] [CrossRef] [PubMed]
- Izzo, F.; Granata, V.; Grassi, R.; Fusco, R.; Palaia, R.; Delrio, P.; Carrafiello, G.; Azoulay, D.; Petrillo, A.; Curley, S.A. Radiof-requency Ablation and Microwave Ablation in Liver Tumors: An Update. Oncologist 2019, 24, e990–e1005. [Google Scholar] [CrossRef] [PubMed]
- Granata, V.; Grassi, R.; Fusco, R.; Setola, S.V.; Belli, A.; Ottaiano, A.; Nasti, G.; La Porta, M.; Danti, G.; Cappabianca, S.; et al. Intrahepatic cholangiocarcinoma and its differential diagnosis at MRI: How radiologist should assess MR features. Radiol. Med. 2021, 126, 1584–1600. [Google Scholar] [CrossRef]
- Ruan, S.M.; Huang, H.; Cheng, M.Q.; Lin, M.X.; Hu, H.T.; Huang, Y.; Li, M.D.; Lu, M.D.; Wang, W. Shear-wave elastography combined with contrast-enhanced ultrasound algorithm for noninvasive characterization of focal liver lesions. Radiol. Med. 2022. [Google Scholar] [CrossRef]
- Granata, V.; Fusco, R.; Avallone, A.; Catalano, O.; Piccirillo, M.; Palaia, R.; Nasti, G.; Petrillo, A.; Izzo, F. A radiologist’s point of view in the presurgical and intraoperative setting of colorectal liver metastases. Future Oncol. 2018, 14, 2189–2206. [Google Scholar] [CrossRef] [PubMed]
- Hyodo, R.; Takehara, Y.; Naganawa, S. 4D Flow MRI in the portal venous system: Imaging and analysis methods, and clinical applications. Radiol. Med. 2022, 127, 1181–1198. [Google Scholar] [CrossRef] [PubMed]
- Granata, V.; Fusco, R.; Catalano, O.; Piccirillo, M.; De Bellis, M.; Izzo, F.; Petrillo, A. Percutaneous ablation therapy of hepa-tocellular carcinoma with irreversible electroporation: MRI findings. AJR Am. J. Roentgenol. 2015, 204, 1000–1007. [Google Scholar] [CrossRef]
- Granata, V.; Fusco, R.; Catalano, O.; Avallone, A.; Palaia, R.; Botti, G.; Tatangelo, F.; Granata, F.; Cascella, M.; Izzo, F.; et al. Diagnostic accuracy of magnetic resonance, computed tomography and contrast enhanced ultrasound in radiological mul-timodality assessment of peribiliary liver metastases. PLoS ONE 2017, 12, e0179951. [Google Scholar] [CrossRef]
- Terminology Committee of the International Hepato-Pancreato-Biliary Association. Terminology of liver anatomy and re-sections. HPB 2000, 2, 333–339. [Google Scholar]
- Wakabayashi, G.; Cherqui, D.; Geller, D.A.; Abu Hilal, M.; Berardi, G.; Ciria, R.; Abe, Y.; Aoki, T.; Asbun, H.J.; Chan, A.C.Y.; et al. The Tokyo 2020 terminology of liver anatomy and resections: Updates of the Brisbane 2000 system. J. Hepato-Biliary-Pancreat. Sci. 2022, 29, 6–15. [Google Scholar] [CrossRef] [PubMed]
- Curley, S.A.; Izzo, F.; Abdalla, I.; Vauthey, J.N. Surgical treatment of colorectal cancer metastasis. Cancer Metastasis Rev. 2004, 23, 165–182. [Google Scholar] [CrossRef]
- Sarpel, U.; Bonavia, A.S.; Grucela, A.; Roayaie, S.; Schwartz, M.E.; Labow, D.M. Does Anatomic Versus Nonanatomic Resection Affect Recurrence and Survival in Patients Undergoing Surgery for Colorectal Liver Metastasis? Ann. Surg. Oncol. 2009, 16, 379–384. [Google Scholar] [CrossRef]
- Kokudo, N.; Tada, K.; Seki, M.; Ohta, H.; Azekura, K.; Ueno, M.; Matsubara, T.; Takahashi, T.; Nakajima, T.; Muto, T. Anatomical major resection versus nonanatomical limited resection for liver metastases from colorectal carcinoma. Am. J. Surg. 2001, 181, 153–159. [Google Scholar] [CrossRef]
- Hosokawa, I.; Allard, M.-A.; Mirza, D.F.; Kaiser, G.; Barroso, E.; Lapointe, R.; Laurent, C.; Ferrero, A.; Miyazaki, M.; Adam, R. Outcomes of parenchyma-preserving hepatectomy and right hepatectomy for solitary small colorectal liver metastasis: A LiverMetSurvey study. Surgery 2017, 162, 223–232. [Google Scholar] [CrossRef]
- Alvarez, F.A.; Sanchez Claria, R.; Oggero, S.; de Santibanes, E. Parenchymal-sparing liver surgery in patients with colorectal carcinoma liver metastases. World J. Gastrointest. Surg. 2016, 8, 407–423. [Google Scholar] [CrossRef]
- Hamady, Z.Z.; Lodge JP, A.; Welsh, F.K.; Toogood, G.J.; White, A.; John, T.; Rees, M. One-millimeter cancer-free margin is curative for colorectal liver metastases: A pro-pensity score case-match approach. Ann. Surg. 2014, 259, 543–548. [Google Scholar] [CrossRef]
- De Haas, R.J.; Wicherts, D.A.; Flores, E.; Azoulay, D.; Castaing, D.; Adam, R. R1 resection by necessity for colorectal liver metastases: Is it still a contraindication to surgery? Ann. Surg. 2008, 248, 626–637. [Google Scholar] [CrossRef] [PubMed]
- Ayez, N.; Lalmahomed, Z.S.; Eggermont, A.M.M.; Ijzermans, J.N.M.; de Jonge, J.; van Montfort, K.; Verhoef, C. Outcome of Microscopic Incomplete Resection (R1) of Colorectal Liver Metastases in the Era of Neoadjuvant Chemotherapy. Ann. Surg. Oncol. 2012, 19, 1618–1627. [Google Scholar] [CrossRef] [PubMed]
- De Re, V.; Caggiari, L.; De Zorzi, M.; Repetto, O.; Zignego, A.L.; Izzo, F.; Tornesello, M.L.; Buonaguro, F.M.; Mangia, A.; Sansonno, D.; et al. Genetic Diversity of the KIR/HLA System and Susceptibility to Hepatitis C Virus-Related Diseases. PLoS ONE 2015, 10, e0117420. [Google Scholar] [CrossRef]
- Torzilli, G.; Viganò, L.; Fontana, A.; Procopio, F.; Terrone, A.; Cimino, M.M.; Donadon, M.; Del Fabbro, D. Oncological outcome of R1 vascular margin for mass-forming cholangiocarcinoma. A single center observational cohort analysis. HPB 2019, 22, 570–577. [Google Scholar] [CrossRef] [PubMed]
- Torzilli, G.; Garancini, M.; Donadon, M.; Cimino, M.; Procopio, F.; Montorsi, M. Intraoperative ultrasonographic detection of communicating veins between adjacent hepatic veins during hepatectomy for tumours at the hepatocaval confluence. Br. J. Surg. 2010, 97, 1867–1873. [Google Scholar] [CrossRef]
- Knowles, S.A.; Bertens, K.A.; Croome, K.P.; Hernandez-Alejandro, R. The current role of intraoperative ultrasound during the resection of colorectal liver metastases: A retrospective cohort study. Int. J. Surg. 2015, 20, 101–106. [Google Scholar] [CrossRef] [PubMed]
- Torzilli, G. Ultrasound-Guided Liver Surgery: An Atlas, 1st ed.; Springer: Milan, Italy, 2014. [Google Scholar]
- Torzilli, G.; Procopio, F.; Costa, G. Adjuncts to hepatic resection—Ultrasound and emerging guidance systems. In Blumgart’s Surgery of the Liver, Pancreas, and Biliary Tract, 6th ed.; Jarnagin, W.R., Ed.; Elsevier Saunders: Philadelphia, PA, USA, 2012. [Google Scholar]
- Torzilli, G.; Donadon, M.; Marconi, M.; Botea, F.; Palmisano, A.; Del Fabbro, D.; Procopio, F.; Montorsi, M. Systematic extended right posterior sectionectomy: A safe and effective alternative to right hepatectomy. Ann. Surg. 2008, 247, 603–611. [Google Scholar] [CrossRef]
- Torzilli, G.; Palmisano, A.; Procopio, F.; Cimino, M.; Botea, F.; Donadon, M.; Del Fabbro, D.; Montorsi, M. A new systematic small for size resection for liver tumors invading the middle hepatic vein at its caval confluence: Mini-mesohepatectomy. Ann. Surg. 2010, 251, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Capone, F.; Costantini, S.; Guerriero, E.; Calemma, R.; Napolitano, M.; Scala, S.; Izzo, F.; Castello, G. Serum cytokine levels in patients with hepatocellular carcinoma. Eur. Cytokine Netw. 2010, 21, 99–104. [Google Scholar] [CrossRef] [PubMed]
- Viganò, L.; Costa, G.; Procopio, F.; Donadon, M.; Cimino, M.; Del Fabbro, D.; Fabbro, A.; Gatti, A.; Torzilli, G. Parenchyma-sparing liver surgery for large segment 1 tumors: Ultrasound-guided lateral and superior approaches as safe alternatives to major hepa-tectomy. J. Am. Coll. Surg. 2015, 221, e65–e73. [Google Scholar] [CrossRef]
- Torzilli, G.; Procopio, F.; Viganò, L.; Costa, G.; Fontana, A.; Cimino, M.; Donadon, M.; Del Fabbro, D. The liver tunnel: Inten-tion-to-treat validation of a new type of hepatectomy. Ann. Surg. 2019, 269, 331. [Google Scholar] [CrossRef]
- Makuuchi, M.; Hasegawa, H.; Yamazaki, S.; Takayasu, K. Four new hepatectomy procedures for resection of the right hepatic vein and preservation of the inferior right hepatic vein. Surg. Gynecol. Obstet. 1987, 164, 68–72. [Google Scholar]
- Clavien, P.-A.; Petrowsky, H.; DeOliveira, M.L.; Graf, R. Strategies for Safer Liver Surgery and Partial Liver Transplantation. N. Engl. J. Med. 2007, 356, 1545–1559. [Google Scholar] [CrossRef]
- Narita, M.; Oussoultzoglou, E.; Ikai, I.; Bachellier, P.; Jaeck, D. Right Portal Vein Ligation Combined with In Situ Splitting Induces Rapid Left Lateral Liver Lobe Hypertrophy Enabling 2-Staged Extended Right Hepatic Resection in Small-for-Size Settings. Ann. Surg. 2012, 256, e7–e8. [Google Scholar] [CrossRef]
- Schadde, E.; Ardiles, V.; Robles-Campos, R.; Malago, M.; Machado, M.; Hernandez-Alejandro, R.; Soubrane, O.; Schnitzbauer, A.A.; Raptis, D.; Tschuor, C.; et al. Early survival and safety of ALPPS: First report of the International ALPPS Registry. Ann. Surg. 2014, 260, 829–836. [Google Scholar] [CrossRef]
- Cutolo, C.; De Muzio, F.; Fusco, R.; Simonetti, I.; Belli, A.; Patrone, R.; Grassi, F.; Dell’Aversana, F.; Pilone, V.; Petrillo, A.; et al. Imaging Features of Post Main Hepatectomy Complications: The Radiologist Challenging. Diagnostics 2022, 12, 1323. [Google Scholar] [CrossRef] [PubMed]
- Granata, V.; Fusco, R.; De Muzio, F.; Cutolo, C.; Setola, S.V.; Simonetti, I.; Dell’Aversana, F.; Grassi, F.; Bruno, F.; Belli, A.; et al. Complications Risk Assessment and Imaging Findings of Thermal Ablation Treatment in Liver Cancers: What the Radiologist Should Expect. J. Clin. Med. 2022, 11, 2766. [Google Scholar] [CrossRef] [PubMed]
- De Muzio, F.; Cutolo, C.; Dell’Aversana, F.; Grassi, F.; Ravo, L.; Ferrante, M.; Danti, G.; Flammia, F.; Simonetti, I.; Palumbo, P.; et al. Complications after Thermal Ablation of Hepatocellular Carcinoma and Liver Metastases: Imaging Findings. Diagnostics 2022, 12, 1151. [Google Scholar] [CrossRef] [PubMed]
- Fusco, R.; Setola, S.V.; Raiano, N.; Granata, V.; Cerciello, V.; Pecori, B.; Petrillo, A. Analysis of a monocentric computed tomography dosimetric database using a radiation dose index monitoring software: Dose levels and alerts before and after the implementation of the adaptive statistical iterative reconstruction on CT images. Radiol. Med. 2022, 127, 733–742. [Google Scholar] [CrossRef]
- Granata, V.; Fusco, R.; De Muzio, F.; Cutolo, C.; Setola, S.V.; Dell’Aversana, F.; Belli, A.; Romano, C.; Ottaiano, A.; Nasti, G.; et al. Magnetic Resonance Features of Liver Mucinous Colorectal Metastases: What the Radiologist Should Know. J. Clin. Med. 2022, 11, 2221. [Google Scholar] [CrossRef] [PubMed]
- Cutolo, C.; Dell’Aversana, F.; Fusco, R.; Grazzini, G.; Chiti, G.; Simonetti, I.; Bruno, F.; Palumbo, P.; Pierpaoli, L.; Valeri, T.; et al. Combined Hepatocellular-Cholangiocarcinoma: What the Multidisciplinary Team Should Know. Diagnostics 2022, 12, 890. [Google Scholar] [CrossRef]
- Acanfora, C.; Grassi, E.; Giacobbe, G.; Ferrante, M.; Granata, V.; Barile, A.; Cappabianca, S. Post-Procedural Follow-Up of the Interventional Radiology’s Management of Osteoid Osteomas and Osteoblastomas. J. Clin. Med. 2022, 11, 1987. [Google Scholar] [CrossRef]
- Granata, V.; Fusco, R.; Setola, S.V.; De Muzio, F.; Aversana, F.D.; Cutolo, C.; Faggioni, L.; Miele, V.; Izzo, F.; Petrillo, A. CT-Based Radiomics Analysis to Predict Histopathological Outcomes Following Liver Resection in Colorectal Liver Metastases. Cancers 2022, 14, 1648. [Google Scholar] [CrossRef]
- Bruno, F.; Granata, V.; Bellisari, F.C.; Sgalambro, F.; Tommasino, E.; Palumbo, P.; Arrigoni, F.; Cozzi, D.; Grassi, F.; Brunese, M.C.; et al. Advanced Magnetic Resonance Imaging (MRI) Techniques: Technical Principles and Applications in Nanomedicine. Cancers 2022, 14, 1626. [Google Scholar] [CrossRef]
- Granata, V.; Fusco, R.; De Muzio, F.; Cutolo, C.; Setola, S.V.; Grassi, R.; Grassi, F.; Ottaiano, A.; Nasti, G.; Tatangelo, F.; et al. Radiomics textural features by MR imaging to assess clinical outcomes following liver resection in colorectal liver metastases. Radiol. Med. 2022, 127, 461–470. [Google Scholar] [CrossRef] [PubMed]
- Granata, V.; Fusco, R.; De Muzio, F.; Cutolo, C.; Setola, S.V.; Dell’Aversana, F.; Ottaiano, A.; Nasti, G.; Grassi, R.; Pilone, V.; et al. EOB-MR Based Radiomics Analysis to Assess Clinical Outcomes following Liver Resection in Colorectal Liver Metastases. Cancers 2022, 14, 1239. [Google Scholar] [CrossRef] [PubMed]
- Granata, V.; Fusco, R.; De Muzio, F.; Cutolo, C.; Setola, S.V.; Aversana, F.D.; Ottaiano, A.; Avallone, A.; Nasti, G.; Grassi, F.; et al. Contrast MR-Based Radiomics and Machine Learning Analysis to Assess Clinical Outcomes following Liver Resection in Colorectal Liver Metastases: A Preliminary Study. Cancers 2022, 14, 1110. [Google Scholar] [CrossRef]
- Granata, V.; Fusco, R.; Setola, S.V.; Simonetti, I.; Cozzi, D.; Grazzini, G.; Grassi, F.; Belli, A.; Miele, V.; Izzo, F.; et al. An update on radiomics techniques in primary liver cancers. Infect. Agents Cancer 2022, 17, 6. [Google Scholar] [CrossRef] [PubMed]
- De Muzio, F.; Cutolo, C.; Granata, V.; Fusco, R.; Ravo, L.; Maggialetti, N.; Brunese, M.C.; Grassi, R.; Grassi, F.; Bruno, F.; et al. CT study protocol optimization in acute non-traumatic abdominal settings. Eur. Rev. Med. Pharmacol. Sci. 2022, 26, 860–878. [Google Scholar] [CrossRef] [PubMed]
- Park, S.H.; Kim, Y.S.; Choi, J. Dosimetric analysis of the effects of a temporary tissue expander on the radiotherapy technique. Radiol. Med. 2020, 126, 437–444. [Google Scholar] [CrossRef] [PubMed]
- Bozkurt, M.; Eldem, G.; Bozbulut, U.B.; Bozkurt, M.F.; Kılıçkap, S.; Peynircioğlu, B.; Çil, B.; Ergün, E.L.; Volkan-Salanci, B. Factors affecting the response to Y-90 microsphere therapy in the cholangiocarcinoma patients. Radiol. Med. 2020, 126, 323–333. [Google Scholar] [CrossRef]
- Patrone, R.; Izzo, F.; Palaia, R.; Granata, V.; Nasti, G.; Ottaiano, A.; Pasta, G.; Belli, A. Minimally invasive surgical treatment of intrahepatic cholangiocarcinoma: A systematic review. World J. Gastrointest. Oncol. 2021, 13, 2203–2215. [Google Scholar] [CrossRef]
- Granata, V.; Fusco, R.; Bicchierai, G.; Cozzi, D.; Grazzini, G.; Danti, G.; De Muzio, F.; Maggialetti, N.; Smorchkova, O.; D’Elia, M.; et al. Diagnostic protocols in oncology: Workup and treatment planning. Part 1: The optimitation of CT protocol. Eur. Rev. Med. Pharmacol. Sci. 2021, 25, 6972–6994. [Google Scholar] [CrossRef]
- Merlotti, A.; Bruni, A.; Borghetti, P.; Ramella, S.; Scotti, V.; Trovò, M.; Chiari, R.; Lohr, F.; Ricardi, U.; Bria, E.; et al. Sequential chemo-hypofractionated RT versus concurrent standard CRT for locally advanced NSCLC: GRADE recommendation by the Italian Association of Radiotherapy and Clinical Oncology (AIRO). Radiol. Med. 2021, 126, 1117–1128. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.; Cheng, J.; Gu, D.; Chai, F.; Hong, N.; Wang, Y.; Tian, J. Deep learning-based radiomics predicts response to chemotherapy in colorectal liver metastases. Med. Phys. 2021, 48, 513–522. [Google Scholar] [CrossRef] [PubMed]
- Giurazza, F.; Cionfoli, N.; Paladini, A.; Vallone, M.; Corvino, F.; Teodoli, L.; Moramarco, L.; Quaretti, P.; Catalano, C.; Niola, R.; et al. PHIL® (precipitating hydrophobic injectable liquid): Retrospective multicenter experience on 178 patients in peripheral embolizations. Radiol. Med. 2022, 127, 1303–1312. [Google Scholar] [CrossRef] [PubMed]
- Falcinelli, L.; Mendichi, M.; Chierchini, S.; Tenti, M.V.; Bellavita, R.; Saldi, S.; Ingrosso, G.; Reggioli, V.; Bini, V.; Aristei, C. Pulmonary function in stereotactic body radiotherapy with helical tomotherapy for primary and metastatic lung lesions. Radiol. Med. 2020, 126, 163–169. [Google Scholar] [CrossRef] [PubMed]
- Arslan, A.; Aktas, E.; Sengul, B.; Tekin, B. Dosimetric evaluation of left ventricle and left anterior descending artery in left breast radiotherapy. Radiol. Med. 2021, 126, 14–21. [Google Scholar] [CrossRef]
- Barra, S.; Guarnieri, A.; Bastia, M.B.D.M.E.; Marcenaro, M.; Tornari, E.; Belgioia, L.; Magrini, S.M.; Ricardi, U.; Corvò, R. Short fractionation radiotherapy for early prostate cancer in the time of COVID-19: Long-term excellent outcomes from a multicenter Italian trial suggest a larger adoption in clinical practice. Radiol. Med. 2021, 126, 142–146. [Google Scholar] [CrossRef]
- Cellini, F.; Di Franco, R.; Manfrida, S.; Borzillo, V.; Maranzano, E.; Pergolizzi, S.; Morganti, A.G.; Fusco, V.; Deodato, F.; Santarelli, M.; et al. Palliative radiotherapy indications during the COVID-19 pandemic and in future complex logistic settings: The NORMALITY model. Radiol. Med. 2021, 126, 1619–1656. [Google Scholar] [CrossRef]
- Lancellotta, V.; Del Regno, L.; Di Stefani, A.; Fionda, B.; Marazzi, F.; Rossi, E.; Balducci, M.; Pampena, R.; Morganti, A.G.; Mangoni, M.; et al. The role of stereotactic radiotherapy in addition to immunotherapy in the management of melanoma brain metastases: Results of a systematic review. Radiol. Med. 2022, 127, 773–783. [Google Scholar] [CrossRef]
- Ahmed, M.; Solbiati, L.; Brace, C.L.; Breen, D.J.; Callstrom, M.R.; Charboneau, J.W.; Chen, M.-H.; Choi, B.I.; de Baère, T.; Dodd, G.D.; et al. Image-Guided Tumor Ablation: Standardization of Terminology and Reporting Criteria—A 10-Year Update. J. Vasc. Interv. Radiol. 2014, 25, 1691–1705.e4. [Google Scholar] [CrossRef]
- Available online: https://www.assessurgery.com/clavien-dindo-classification/ (accessed on 17 September 2022).
- Arrigoni, F.; Bianchi, G.; Formiconi, F.; Palumbo, P.; Zugaro, L.; Gravina, G.L.; Barile, A.; Masciocchi, C. CT-guided cryo-ablation for management of bone metastases: A single center experience and review of the literature. Radiol. Med. 2022, 127, 199–205. [Google Scholar] [CrossRef]
- Hewitt, D.B.; Pawlik, T.M.; Cloyd, J.M. Who Will Benefit? Using Radiomics to Predict Response to Oxaliplatin-Based Chemotherapy in Patients with Colorectal Liver Metastases. Ann. Surg. Oncol. 2021, 28, 2931–2933. [Google Scholar] [CrossRef]
- Barile, A.; Bruno, F.; Arrigoni, F.; Splendiani, A.; Di Cesare, E.; Zappia, M.; Guglielmi, G.; Masciocchi, C. Emergency and Trauma of the Ankle. Semin. Musculoskelet. Radiol. 2017, 21, 282–289. [Google Scholar] [CrossRef] [PubMed]
- Hussein, M.A.M.; Cafarelli, F.P.; Paparella, M.T.; Rennie, W.J.; Guglielmi, G. Phosphaturic mesenchymal tumors: Radiological aspects and suggested imaging pathway. Radiol. Med. 2021, 126, 1609–1618. [Google Scholar] [CrossRef]
- Danti, G.; Flammia, F.; Matteuzzi, B.; Cozzi, D.; Berti, V.; Grazzini, G.; Pradella, S.; Recchia, L.; Brunese, L.; Miele, V. Gastrointestinal neuroendocrine neoplasms (GI-NENs): Hot topics in morphological, functional, and prognostic imaging. Radiol. Med. 2021, 126, 1497–1507. [Google Scholar] [CrossRef] [PubMed]
- Granata, V.; Bicchierai, G.; Fusco, R.; Cozzi, D.; Grazzini, G.; Danti, G.; De Muzio, F.; Maggialetti, N.; Smorchkova, O.; D’Elia, M.; et al. Diagnostic protocols in oncology: Workup and treatment planning. Part 2: Abbreviated MR protocol. Eur. Rev. Med. Pharmacol. Sci. 2021, 25, 6499–6528. [Google Scholar] [CrossRef] [PubMed]
- Petralia, G.; Zugni, F.; Summers, P.E.; Colombo, A.; Pricolo, P.; Grazioli, L.; Colagrande, S.; Giovagnoni, A.; Padhani, A.R. On behalf of the Italian Working Group on Magnetic Resonance Whole-body magnetic resonance imaging (WB-MRI) for cancer screening: Recommendations for use. Radiol. Med. 2021, 126, 1434–1450. [Google Scholar] [CrossRef] [PubMed]
- Granata, V.; Faggioni, L.; Grassi, R.; Fusco, R.; Reginelli, A.; Rega, D.; Maggialetti, N.; Buccicardi, D.; Frittoli, B.; Rengo, M.; et al. Structured reporting of computed tomography in the staging of colon cancer: A Delphi consensus proposal. Radiol. Med. 2022, 127, 21–29. [Google Scholar] [CrossRef]
- Granata, V.; Grassi, R.; Fusco, R.; Belli, A.; Cutolo, C.; Pradella, S.; Grazzini, G.; La Porta, M.; Brunese, M.C.; De Muzio, F.; et al. Diagnostic evaluation and ablation treatments assessment in hepatocellular carcinoma. Infect. Agents Cancer 2021, 16, 53. [Google Scholar] [CrossRef]
- Granata, V.; Grassi, R.; Fusco, R.; Belli, A.; Palaia, R.; Carrafiello, G.; Miele, V.; Grassi, R.; Petrillo, A.; Izzo, F. Local ablation of pancreatic tumors: State of the art and future perspectives. World J. Gastroenterol. 2021, 27, 3413–3428. [Google Scholar] [CrossRef]
- Granata, V.; Fusco, R.; Barretta, M.L.; Picone, C.; Avallone, A.; Belli, A.; Patrone, R.; Ferrante, M.; Cozzi, D.; Grassi, R.; et al. Radiomics in hepatic metastasis by colorectal cancer. Infect. Agents Cancer 2021, 16, 39. [Google Scholar] [CrossRef]
- Granata, V.; Fusco, R.; Salati, S.; Petrillo, A.; Di Bernardo, E.; Grassi, R.; Palaia, R.; Danti, G.; La Porta, M.; Cadossi, M.; et al. A Systematic Review about Imaging and Histopathological Findings for Detecting and Evaluating Electroporation Based Treatments Response. Int. J. Environ. Res. Public Health 2021, 18, 5592. [Google Scholar] [CrossRef]
- Fusco, R.; Granata, V.; Sansone, M.; Rega, D.; Delrio, P.; Tatangelo, F.; Romano, C.; Avallone, A.; Pupo, D.; Giordano, M.; et al. Validation of the standardized index of shape tool to analyze DCE-MRI data in the assessment of neo-adjuvant therapy in locally advanced rectal cancer. Radiol. Med. 2021, 126, 1044–1054. [Google Scholar] [CrossRef] [PubMed]
- Colombo, E.; Fick, T.; Esposito, G.; Germans, M.; Regli, L.; van Doormaal, T. Segmentation techniques of brain arteriovenous malformations for 3D visualization: A systematic review. Radiol. Med. 2022, 127, 1333–1341. [Google Scholar] [CrossRef] [PubMed]
- Granata, V.; Grassi, R.; Fusco, R.; Setola, S.; Belli, A.; Piccirillo, M.; Pradella, S.; Giordano, M.; Cappabianca, S.; Brunese, L.; et al. Abbreviated MRI Protocol for the Assessment of Ablated Area in HCC Patients. Int. J. Environ. Res. Public Health 2021, 18, 3598. [Google Scholar] [CrossRef] [PubMed]
- Patrone, R.; Granata, V.; Belli, A.; Palaia, R.; Albino, V.; Piccirillo, M.; Fusco, R.; Tatangelo, F.; Nasti, G.; Avallone, A.; et al. The safety and efficacy of Glubran 2 as biliostatic agent in liver resection. Infect. Agents Cancer 2021, 16, 19. [Google Scholar] [CrossRef] [PubMed]
- Fusco, R.; Granata, V.; Mazzei, M.A.; Di Meglio, N.; Del Roscio, D.; Moroni, C.; Monti, R.; Cappabianca, C.; Picone, C.; Neri, E.; et al. Quantitative imaging decision support (QIDSTM) tool consistency evaluation and radiomic analysis by means of 594 metrics in lung carcinoma on chest CT scan. Cancer Control 2021, 28, 1073274820985786. [Google Scholar] [CrossRef]
- Granata, V.; Fusco, R.; Avallone, A.; De Stefano, A.; Ottaiano, A.; Sbordone, C.; Brunese, L.; Izzo, F.; Petrillo, A. Radiomics-Derived Data by Contrast Enhanced Magnetic Resonance in RAS Mutations Detection in Colorectal Liver Metastases. Cancers 2021, 13, 453. [Google Scholar] [CrossRef] [PubMed]
- Granata, V.; Grassi, R.; Fusco, R.; Setola, S.V.; Palaia, R.; Belli, A.; Miele, V.; Brunese, L.; Grassi, R.; Petrillo, A.; et al. Assessment of Ablation Therapy in Pancreatic Cancer: The Radiologist’s Challenge. Front. Oncol. 2020, 10, 560952. [Google Scholar] [CrossRef]
- Nakamura, Y.; Higaki, T.; Honda, Y.; Tatsugami, F.; Tani, C.; Fukumoto, W.; Narita, K.; Kondo, S.; Akagi, M.; Awai, K. Advanced CT techniques for assessing hepatocellular carcinoma. Radiol. Med. 2021, 126, 925–935. [Google Scholar] [CrossRef]
- Granata, V.; Fusco, R.; Avallone, A.; Cassata, A.; Palaia, R.; Delrio, P.; Grassi, R.; Tatangelo, F.; Grazzini, G.; Izzo, F.; et al. Abbreviated MRI protocol for colorectal liver metastases: How the radiologist could work in pre surgical setting. PLoS ONE 2020, 15, e0241431. [Google Scholar] [CrossRef]
- Alvaro, D.; Hassan, C.; Cardinale, V.; Carpino, G.; Fabris, L.; Gringeri, E.; Granata, V.; Mutignani, M.; Morement, H.; Giuliante, F.; et al. Italian Clinical Practice Guidelines on Cholangiocarcinoma—Part II: Treatment. Dig. Liver Dis. 2020, 52, 1430–1442. [Google Scholar] [CrossRef]
- Alvaro, D.; Hassan, C.; Cardinale, V.; Carpino, G.; Fabris, L.; Gringeri, E.; Granata, V.; Mutignani, M.; Morement, H.; Giuliante, F.; et al. Italian Clinical Practice Guidelines on Cholangiocarcinoma—Part I: Classification, diagnosis and staging. Dig. Liver Dis. 2020, 52, 1282–1293. [Google Scholar] [CrossRef] [PubMed]
- Granata, V.; Fusco, R.; Amato, D.M.; Albino, V.; Patrone, R.; Izzo, F.; Petrillo, A. Beyond the Vascular Profile: Conventional DWI, IVIM and Kurtosis in the Assessment of Hepatocellular Carcinoma. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 7284–7293. [Google Scholar] [CrossRef] [PubMed]
- Granata, V.; Fusco, R.; Maio, F.; Avallone, A.; Nasti, G.; Palaia, R.; Albino, V.; Grassi, R.; Izzo, F.; Petrillo, A. Qualitative assessment of EOB-GD-DTPA and Gd-BT-DO3A MR contrast studies in HCC patients and colorectal liver metastases. Infect. Agents Cancer 2019, 14, 40. [Google Scholar] [CrossRef] [PubMed]
- Granata, V.; Fusco, R.; Setola, S.V.; Castelguidone, E.D.L.D.; Camera, L.; Tafuto, S.; Avallone, A.; Belli, A.; Incollingo, P.; Palaia, R.; et al. The multidisciplinary team for gastroenteropancreatic neuroendocrine tumours: The radiologist’s challenge. Radiol. Oncol. 2019, 53, 373–387. [Google Scholar] [CrossRef]
- Argalia, G.; Tarantino, G.; Ventura, C.; Campioni, D.; Tagliati, C.; Guardati, P.; Kostandini, A.; Marzioni, M.; Giuseppetti, G.M.; Giovagnoni, A. Shear wave elastography and transient elastography in HCV patients after direct-acting antivirals. Radiol. Med. 2021, 126, 894–899. [Google Scholar] [CrossRef]
- Polesel, J.; Talamini, R.; Montella, M.; Maso, L.D.; Crovatto, M.; Parpinel, M.; Izzo, F.; Tommasi, L.G.; Serraino, D.; La Vecchia, C.; et al. Nutrients intake and the risk of hepatocellular carcinoma in Italy. Eur. J. Cancer 2007, 43, 2381–2387. [Google Scholar] [CrossRef]
- Cicero, G.; Mazziotti, S.; Silipigni, S.; Blandino, A.; Cantisani, V.; Pergolizzi, S.; D’Angelo, T.; Stagno, A.; Maimone, S.; Squadrito, G.; et al. Dual-energy CT quantification of fractional extracellular space in cirrhotic patients: Comparison between early and delayed equilibrium phases and correlation with oesophageal varices. Radiol. Med. 2021, 126, 761–767. [Google Scholar] [CrossRef]
- Granata, V.; Fusco, R.; Castelguidone, E.D.L.D.; Avallone, A.; Palaia, R.; Delrio, P.; Tatangelo, F.; Botti, G.; Grassi, R.; Izzo, F.; et al. Diagnostic performance of gadoxetic acid–enhanced liver MRI versus multidetector CT in the assessment of colorectal liver metastases compared to hepatic resection. BMC Gastroenterol. 2019, 19, 129. [Google Scholar] [CrossRef]
- Stefanini, M.; Simonetti, G. Interventional Magnetic Resonance Imaging Suite (IMRIS): How to build and how to use. Radiol. Med. 2022, 127, 1063–1067. [Google Scholar] [CrossRef]
- Granata, V.; Fusco, R.; Avallone, A.; Catalano, O.; Filice, F.; Leongito, M.; Palaia, R.; Izzo, F.; Petrillo, A. Major and ancillary magnetic resonance features of LI-RADS to assess HCC: An overview and update. Infect. Agents Cancer 2017, 12, 23. [Google Scholar] [CrossRef] [PubMed]
- Petrillo, A.; Fusco, R.; Granata, V.; Filice, S.; Sansone, M.; Rega, D.; Delrio, P.; Bianco, F.; Romano, G.M.; Tatangelo, F.; et al. Assessing response to neo-adjuvant therapy in locally advanced rectal cancer using Intra-voxel Incoherent Motion modelling by DWI data and Standardized Index of Shape from DCE-MRI. Ther. Adv. Med. Oncol. 2018, 10, 1758835918809875. [Google Scholar] [CrossRef] [PubMed]
- Fusco, R.; Sansone, M.; Granata, V.; Grimm, R.; Pace, U.; Delrio, P.; Tatangelo, F.; Botti, G.; Avallone, A.; Pecori, B.; et al. Diffusion and perfusion MR parameters to assess preoperative short-course radiotherapy response in locally advanced rectal cancer: A comparative explorative study among Standardized Index of Shape by DCE-MRI, intravoxel incoherent motion- and diffusion kurtosis imaging-derived parameters. Abdom. Imaging 2019, 44, 3683–3700. [Google Scholar] [CrossRef]
- Granata, V.; Fusco, R.; Filice, S.; Catalano, O.; Piccirillo, M.; Palaia, R.; Izzo, F.; Petrillo, A. The current role and future prospectives of functional parameters by diffusion weighted imaging in the assessment of histologic grade of HCC. Infect. Agents Cancer 2018, 13, 23. [Google Scholar] [CrossRef] [PubMed]
- Rega, D.; Pace, U.; Scala, D.; Chiodini, P.; Granata, V.; Bucci, A.F.; Pecori, B.; Delrio, P. Treatment of splenic flexure colon cancer: A comparison of three different surgical procedures: Experience of a high volume cancer center. Sci. Rep. 2019, 9, 10953. [Google Scholar] [CrossRef]
- Granata, V.; Fusco, R.; Avallone, A.; Filice, F.; Tatangelo, F.; Piccirillo, M.; Grassi, R.; Izzo, F.; Petrillo, A. Critical analysis of the major and ancillary imaging features of LI-RADS on 127 proven HCCs evaluated with functional and morphological MRI: Lights and shadows. Oncotarget 2017, 8, 51224–51237. [Google Scholar] [CrossRef]
- Petrillo, A.; Fusco, R.; Petrillo, M.; Granata, V.; Delrio, P.; Bianco, F.; Pecori, B.; Botti, G.; Tatangelo, F.; Caracò, C.; et al. Standardized Index of Shape (DCE-MRI) and Standardized Uptake Value (PET/CT): Two quantitative approaches to dis-criminate chemo- radiotherapy locally advanced rectal cancer responders under a functional profile. Oncotarget 2017, 8, 8143–8153. [Google Scholar] [CrossRef]
- Granata, V.; Fusco, R.; Catalano, O.; Setola, S.V.; Castelguidone, E.D.L.D.; Piccirillo, M.; Palaia, R.; Grassi, R.; Granata, F.; Izzo, F.; et al. Multidetector computer tomography in the pancreatic adenocarcinoma assessment: An update. Infect. Agents Cancer 2016, 11, 57. [Google Scholar] [CrossRef]
- Ruffino, M.A.; Fronda, M.; Bergamasco, L.; Natrella, M.; Fanelli, G.; Bellosta, R.; Pegorer, M.; Attisani, L.; Ruggiero, M.; Malfa, P.; et al. Prognostic risk factors for loss of patency after femoropopliteal bailout stenting with dual-component stent: Results from the TIGRIS Italian Multicenter Registry. Radiol. Med. 2021, 126, 1129–1137. [Google Scholar] [CrossRef]
- Giurazza, F.; Contegiacomo, A.; Calandri, M.; Mosconi, C.; Modestino, F.; Corvino, F.; Scrofani, A.R.; Marra, P.; Coniglio, G.; Failla, G.; et al. IVC filter retrieval: A multicenter proposal of two score systems to predict application of complex technique and procedural outcome. Radiol. Med. 2021, 126, 1007–1016. [Google Scholar] [CrossRef]
- Fushimi, Y.; Yoshida, K.; Okawa, M.; Maki, T.; Nakajima, S.; Sakata, A.; Okuchi, S.; Hinoda, T.; Kanagaki, M.; Nakamoto, Y. Vessel wall MR imaging in neuroradiology. Radiol. Med. 2022, 127, 1032–1045. [Google Scholar] [CrossRef]
- Granata, V.; Simonetti, I.; Fusco, R.; Setola, S.V.; Izzo, F.; Scarpato, L.; Vanella, V.; Festino, L.; Simeone, E.; Ascierto, P.A.; et al. Management of cutaneous melanoma: Radiologists challenging and risk assessment. Radiol. Med. 2022, 127, 899–911. [Google Scholar] [CrossRef] [PubMed]
- Cirillo, L.; Rustici, A.; Toni, F.; Zoli, M.; Bartiromo, F.; Gramegna, L.L.; Cicala, D.; Tonon, C.; Caranci, F.; Lodi, R. Vessel Wall MRI: Clinical implementation in cerebrovascular disorders—Technical aspects. Radiol. Med. 2022, 127, 645–651. [Google Scholar] [CrossRef]
- Renzulli, M.; Brandi, N.; Argalia, G.; Brocchi, S.; Farolfi, A.; Fanti, S.; Golfieri, R. Morphological, dynamic and functional characteristics of liver pseudolesions and benign lesions. Radiol. Med. 2022, 127, 129–144. [Google Scholar] [CrossRef]
- Li, N.; Wakim, J.; Koethe, Y.; Huber, T.; Schenning, R.; Gade, T.P.; Hunt, S.J.; Park, B.J. Multicenter assessment of augmented reality registration methods for image-guided interventions. Radiol. Med. 2022, 127, 857–865. [Google Scholar] [CrossRef] [PubMed]
- Ledda, R.E.; Silva, M.; McMichael, N.; Sartorio, C.; Branchi, C.; Milanese, G.; Nayak, S.M.; Sverzellati, N. The diagnostic value of grey-scale inversion technique in chest radiography. Radiol. Med. 2022, 127, 294–304. [Google Scholar] [CrossRef]
- Bianchi, A.; Mazzoni, L.N.; Busoni, S.; Pinna, N.; Albanesi, M.; Cavigli, E.; Cozzi, D.; Poggesi, A.; Miele, V.; Fainardi, E.; et al. Assessment of cerebrovascular disease with computed tomography in COVID-19 patients: Correlation of a novel specific visual score with increased mortality risk. Radiol. Med. 2021, 126, 570–576. [Google Scholar] [CrossRef] [PubMed]
- Cartocci, G.; Colaiacomo, M.C.; Lanciotti, S.; Andreoli, C.; De Cicco, M.L.; Brachetti, G.; Pugliese, S.; Capoccia, L.; Tortora, A.; Scala, A.; et al. Correction to: Chest CT for early detection and management of coronavirus disease (COVID-19): A report of 314 patients admitted to Emergency Department with suspected pneumonia. Radiol. Med. 2021, 126, 642. [Google Scholar] [CrossRef]
- Masci, G.M.; Iafrate, F.; Ciccarelli, F.; Pambianchi, G.; Panebianco, V.; Pasculli, P.; Ciardi, M.R.; Mastroianni, C.M.; Ricci, P.; Catalano, C.; et al. Tocilizumab effects in COVID-19 pneumonia: Role of CT texture analysis in quantitative assessment of response to therapy. Radiol. Med. 2021, 126, 1170–1180. [Google Scholar] [CrossRef]
- Francolini, G.; Desideri, I.; Stocchi, G.; Ciccone, L.P.; Salvestrini, V.; Garlatti, P.; Aquilano, M.; Greto, D.; Bonomo, P.; Meattini, I.; et al. Impact of COVID-19 on workload burden of a complex radiotherapy facility. Radiol. Med. 2021, 126, 717–721. [Google Scholar] [CrossRef]
- Pignata, S.; Gallo, C.; Daniele, B.; Elba, S.; Giorgio, A.; Capuano, G.; Adinolfi, L.E.; De Sio, I.; Izzo, F.; Farinati, F.; et al. Characteristics at presentation and outcome of hepatocellular carcinoma (HCC) in the elderly. A study of the Cancer of the Liver Italian Program (CLIP). Crit. Rev. Oncol. 2006, 59, 243–249. [Google Scholar] [CrossRef]
- Perillo, T.; Paolella, C.; Perrotta, G.; Serino, A.; Caranci, F.; Manto, A. Reversible cerebral vasoconstriction syndrome: Review of neuroimaging findings. Radiol. Med. 2022, 127, 981–990. [Google Scholar] [CrossRef]
- Caruso, D.; Polici, M.; Rinzivillo, M.; Zerunian, M.; Nacci, I.; Marasco, M.; Magi, L.; Tarallo, M.; Gargiulo, S.; Iannicelli, E.; et al. CT-based radiomics for prediction of therapeutic response to Everolimus in metastatic neuroendocrine tumors. Radiol. Med. 2022, 127, 691–701. [Google Scholar] [CrossRef] [PubMed]
- Han, D.; Yu, N.; Yu, Y.; He, T.; Duan, X. Performance of CT radiomics in predicting the overall survival of patients with stage III clear cell renal carcinoma after radical nephrectomy. Radiol. Med. 2022, 127, 837–847. [Google Scholar] [CrossRef] [PubMed]
- Granata, V.; Fusco, R.; Setola, S.; Galdiero, R.; Picone, C.; Izzo, F.; D’Aniello, R.; Miele, V.; Grassi, R.; Grassi, R.; et al. Lymphadenopathy after BNT162b2 COVID-19 Vaccine: Preliminary Ultrasound Findings. Biology 2021, 10, 214. [Google Scholar] [CrossRef]
- Masci, G.M.; Ciccarelli, F.; Mattei, F.I.; Grasso, D.; Accarpio, F.; Catalano, C.; Laghi, A.; Sammartino, P.; Iafrate, F. Role of CT texture analysis for predicting peritoneal metastases in patients with gastric cancer. Radiol. Med. 2022, 127, 251–258. [Google Scholar] [CrossRef] [PubMed]
- Izzo, F.; Piccirillo, M.; Albino, V.; Palaia, R.; Belli, A.; Granata, V.; Setola, S.; Fusco, R.; Petrillo, A.; Orlando, R.; et al. Prospective screening increases the detection of potentially curable hepatocellular carcinoma: Results in 8900 high-risk patients. HPB 2013, 15, 985–990. [Google Scholar] [CrossRef]
- Granata, V.; Castelguidone, E.D.L.D.; Fusco, R.; Catalano, O.; Piccirillo, M.; Palaia, R.; Izzo, F.; Gallipoli, A.D.; Petrillo, A. Irreversible electroporation of hepatocellular carcinoma: Preliminary report on the diagnostic accuracy of magnetic resonance, computer tomography, and contrast-enhanced ultrasound in evaluation of the ablated area. Radiol. Med. 2016, 121, 122–131. [Google Scholar] [CrossRef] [PubMed]
- Fanelli, F.; Cannavale, A.; Chisci, E.; Citone, M.; Falcone, G.M.; Michelagnoli, S.; Miele, V. Direct percutaneous embolization of aneurysm sac: A safe and effective procedure to treat post-EVAR type II endoleaks. Radiol. Med. 2021, 126, 258–263. [Google Scholar] [CrossRef]
- Battaglia, V.; Cervelli, R. Liver investigations: Updating on US technique and contrast-enhanced ultrasound (CEUS). Eur. J. Radiol. 2017, 96, 65–73. [Google Scholar] [CrossRef]
- Faccia, M.; Garcovich, M.; Ainora, M.E.; Riccardi, L.; Pompili, M.; Gasbarrini, A.; Zocco, M.A. Contrast-Enhanced Ultrasound for Monitoring Treatment Response in Different Stages of Hepatocellular Carcinoma. Cancers 2022, 14, 481. [Google Scholar] [CrossRef]
- Ossola, C.; Curti, M.; Calvi, M.; Tack, S.; Mazzoni, S.; Genesio, L.; Venturini, M.; Genovese, E.A. Role of ultrasound and magnetic resonance imaging in the prognosis and classification of muscle injuries in professional football players: Correlation between imaging and return to sport time. Radiol. Med. 2021, 126, 1460–1467. [Google Scholar] [CrossRef] [PubMed]
- Güldoğan, E.S.; Ergun, O.; Türkmenoğlu, T.T.; Yılmaz, K.B.; Akdağ, T.; Güneş, S.Ö.; Durmaz, H.A.; Hekimoğlu, B. The impact of TI-RADS in detecting thyroid malignancies: A prospective study. Radiol. Med. 2021, 126, 1335–1344. [Google Scholar] [CrossRef] [PubMed]
- Celletti, I.; Fresilli, D.; De Vito, C.; Bononi, M.; Cardaccio, S.; Cozzolino, A.; Durante, C.; Grani, G.; Grimaldi, G.; Isidori, A.M.; et al. TIRADS, SRE and SWE in INDETERMINATE thyroid nodule characterization: Which has better diagnostic performance? Radiol. Med. 2021, 126, 1189–1200. [Google Scholar] [CrossRef] [PubMed]
- Rosa, F.; Martinetti, C.; Veirana, M.A.; Attieh, A.; Trisoglio, A.; Sabattini, R.; Gandolfo, N.; Gastaldo, A. How embryology knowledge can help radiologists in the differential diagnosis of canal of Nuck pathologies. Radiol. Med. 2021, 126, 910–924. [Google Scholar] [CrossRef] [PubMed]
- Granata, V.; Fusco, R.; Sansone, M.; Grassi, R.; Maio, F.; Palaia, R.; Tatangelo, F.; Botti, G.; Grimm, R.; Curley, S.; et al. Magnetic resonance imaging in the assessment of pancreatic cancer with quantitative parameter extraction by means of dynamic contrast-enhanced magnetic resonance imaging, diffusion kurtosis imaging and intravoxel incoherent motion diffusion-weighted imaging. Ther. Adv. Gastroenterol. 2020, 13, 1756284819885052. [Google Scholar] [CrossRef]
- Masciocchi, C.; Sparvoli, L.; Barile, A. Diagnostic imaging of malignant cartilage tumors. Eur. J. Radiol. 1998, 27 (Suppl. 1), S86–S90. [Google Scholar] [CrossRef]
- Barile, A.; Bruno, F.; Mariani, S.; Arrigoni, F.; Reginelli, A.; De Filippo, M.; Zappia, M.; Splendiani, A.; Di Cesare, E.; Masciocchi, C. What can be seen after rotator cuff repair: A brief review of diagnostic imaging findings. Musculoskelet. Surg. 2017, 101 (Suppl. 1), 3–14. [Google Scholar] [CrossRef]
- Barile, A.; Quarchioni, S.; Bruno, F.; Ierardi, A.M.; Arrigoni, F.; Giordano, A.V.; Carducci, S.; Varrassi, M.; Carrafiello, G.; Caranci, F.; et al. Interventional radiology of the thyroid gland: Critical review and state of the art. Gland. Surg. 2018, 7, 132–146. [Google Scholar] [CrossRef]
- Available online: https://www.nccn.org (accessed on 17 September 2022).
- Available online: https://www.aiom.it (accessed on 17 September 2022).
- Available online: http://www.sirm.org (accessed on 17 September 2022).
- Perrone, F.; Gallo, C.; Daniele, B.; Gaeta, G.; Izzo, F.; Capuano, G.; Adinolfi, L.; Mazzanti, R.; Farinati, F.; Elba, S.; et al. Tamoxifen in the Treatment of Hepatocellular Carcinoma: 5-Year Results of the CLIP-1 Multicentre Randomised Controlled Trial. Curr. Pharm. Des. 2002, 8, 1013–1019. [Google Scholar] [CrossRef]
- Riva, F.; Garanzini, E.M.; Cascella, T.; Marchianò, A.V.; Spreafico, C. A “blood theft” after liver transplantation: The role of interventional radiology in the management and treatment of splenic artery steal syndrome. J. Radiol. Case Rep. 2022, 16, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Arrigoni, F.; Bruno, F.; Zugaro, L.; Natella, R.; Cappabianca, S.; Russo, U.; Papapietro, V.R.; Splendiani, A.; Di Cesare, E.; Masciocchi, C.; et al. Developments in the management of bone metastases with interventional radiology. Acta Biomed. 2018, 89, 166–174. [Google Scholar] [CrossRef]
- Serai, S.D.; Elsingergy, M.M.; Hartung, E.A.; Otero, H.J. Liver and spleen volume and stiffness in patients post-Fontan procedure and patients with ARPKD compared to normal controls. Clin. Imaging 2022, 89, 147–154. [Google Scholar] [CrossRef] [PubMed]
- Arrigoni, F.; Barile, A.; Zugaro, L.; Fascetti, E.; Zappia, M.; Brunese, L.; Masciocchi, C. CT-guided radiofrequency ablation of spinal osteoblastoma: Treatment and long-term follow-up. Int. J. Hyperth. 2018, 34, 321–327. [Google Scholar] [CrossRef]
- De Filippo, M.; Puglisi, S.; D’Amuri, F.; Gentili, F.; Paladini, I.; Carrafiello, G.; Maestroni, U.; Del Rio, P.; Ziglioli, F.; Pagnini, F. CT-guided percutaneous drainage of abdominopelvic collections: A pictorial essay. Radiol. Med. 2021, 126, 1561–1570. [Google Scholar] [CrossRef]
- Cannataci, C.; Cimo, B.; Mamone, G.; Tuzzolino, F.; D’Amico, M.; Cortis, K.; Maruzzelli, L.; Miraglia, R. Portal vein puncture-related complications during transjugular intrahepatic portosystemic shunt creation: Colapinto needle set vs. Rösch-Uchida needle set. Radiol. Med. 2021, 126, 1487–1495. [Google Scholar] [CrossRef] [PubMed]
- Mahnken, A.H.; Boullosa Seoane, E.; Cannavale, A.; de Haan, M.W.; Dezman, R.; Kloeckner, R.; O’Sullivan, G.; Ryan, A.; Tsoumakidou, G. CIRSE Clinical Practice Manual. Cardiovasc. Intervent. Radiol. 2021, 44, 1323–1353. [Google Scholar]
- Laurelli, G.; Falcone, F.; Gallo, M.S.; Scala, F.; Losito, S.; Granata, V.; Cascella, M.; Greggi, S. Long-Term Oncologic and Reproductive Outcomes in Young Women with Early Endometrial Cancer Conservatively Treated: A Prospective Study and Literature Update. Int. J. Gynecol. Cancer 2016, 26, 1650–1657. [Google Scholar] [CrossRef]
- De Cecco, C.N.; Buffa, V.; Fedeli, S.; Luzietti, M.; Vallone, A.; Ruopoli, R.; Miele, V.; Rengo, M.; MauriziEnrici, M.; Fina, P.; et al. Prelim-inary experience with abdominal dual-energy CT (DECT): True versus virtual nonenhanced images of the liver. Radiol. Med. 2010, 115, 1258–1266. [Google Scholar] [CrossRef]
- di Giacomo, V.; Trinci, M.; van der Byl, G.; Catania, V.D.; Calisti, A.; Miele, V. Ultrasoundinnewbornsandchildrensufferingfrom non-traumatic acute abdominal pain: Imaging with clinical and surgical correlation. J. Ultrasound 2014, 18, 385–393. [Google Scholar] [CrossRef]
- Izzo, F.; Palaia, R.; Albino, V.; Amore, A.; di Giacomo, R.; Piccirillo, M.; Leongito, M.; Nasto, A.; Granata, V.; Petrillo, A.; et al. Hepato-cellular carcinoma and liver metastases: Clinical data on a new dual-lumen catheter kit for surgical sealant infusion to prevent perihepatic bleeding and dissemination of cancer cells following biopsy and loco-regional treatments. Infect. Agent Cancer 2015, 10, 11. [Google Scholar] [CrossRef] [PubMed]
- Rahbari, N.N.; Garden, O.J.; Padbury, R.; Maddern, G.; Koch, M.; Hugh, T.J.; Fan, S.T.; Nimura, Y.; Figueras, J.; Vauthey, J.-N.; et al. Post-hepatectomy haemorrhage: A definition and grading by the International Study Group of Liver Surgery (ISGLS). HPB 2011, 13, 528–535. [Google Scholar] [CrossRef] [PubMed]
- Lubner, M.; Menias, C.; Rucker, C.; Bhalla, S.; Peterson, C.M.; Wang, L.; Gratz, B. Blood in the Belly: CT Findings of Hemoperitoneum. Radiographics 2007, 27, 109–125. [Google Scholar] [CrossRef]
- Ilyas, M.; Bashir, M.; Robbani, I.; Rasool, S.R.; Shera, F.A.; Hamid, I. Sentinel clot sign in hemoperitoneum. Abdom. Imaging 2019, 44, 1955–1956. [Google Scholar] [CrossRef]
- Fusco, R.; Sansone, M.; Filice, S.; Granata, V.; Catalano, O.; Amato, D.M.; Di Bonito, M.; D’Aiuto, M.; Capasso, I.; Rinaldo, M.; et al. Integration of DCE-MRI and DW-MRI Quantitative Parameters for Breast Lesion Classification. BioMed Res. Int. 2015, 2015, 237863. [Google Scholar] [CrossRef]
- Byun, J.; Kim, K.W.; Lee, J.; Kwon, H.-J.; Kwon, J.H.; Song, G.-W.; Lee, S.-G. The role of multiphase CT in patients with acute postoperative bleeding after liver transplantation. Abdom. Imaging 2019, 45, 141–152. [Google Scholar] [CrossRef] [PubMed]
- Di Domenico, S.; Rossini, A.; Petrocelli, F.; Valente, U.; Ferro, C. Recurrent acute Budd–Chiari syndrome after right hepatectomy: US color-Doppler vascular pattern and left hepatic vein stenting for treatment. Abdom. Imaging 2013, 38, 320–323. [Google Scholar] [CrossRef]
- Yoshiya, S.; Shirabe, K.; Nakagawara, H.; Soejima, Y.; Yoshizumi, T.; Ikegami, T.; Yamashita, Y.-I.; Harimoto, N.; Nishie, A.; Yamanaka, T.; et al. Portal Vein Thrombosis After Hepatectomy. World J. Surg. 2014, 38, 1491–1497. [Google Scholar] [CrossRef] [PubMed]
- Cohen, J.; Edelman, R.R.; Chopra, S. Portal vein thrombosis: A review. Am. J. Med. 1992, 92, 173–182. [Google Scholar] [CrossRef]
- Witte, C.L.; Brewer, M.L.; Witte, M.H.; Pond, G.B. Protean Manifestations of Pylethrombosis. A review of thirty-four patients. Ann. Surg. 1985, 202, 191–202. [Google Scholar] [CrossRef]
- Sheen, C.; Lamparelli, H.; Milne, A.; Green, I.; Ramage, J. Clinical features, diagnosis and outcome of acute portal vein thrombosis. QJM 2000, 93, 531–534. [Google Scholar] [CrossRef] [PubMed]
- Sakuraba, M.; Miyamoto, S.; Nagamatsu, S.; Kayano, S.; Taji, M.; Kinoshita, T.; Kosuge, T.; Kimata, Y. Hepatic artery reconstruction following ablative surgery for hepatobiliary and pancreatic malignancies. Eur. J. Surg. Oncol. 2012, 38, 580–585. [Google Scholar] [CrossRef] [PubMed]
- Silva, M.A.; Jambulingam, P.S.; Gunson, B.K.; Mayer, D.; Buckels, J.A.; Mirza, D.F.; Bramhall, S.R. Hepatic artery thrombosis following orthotopic liver transplantation: A 10-year experience from a single centre in the United Kingdom. Liver Transplant. 2006, 12, 146–151. [Google Scholar] [CrossRef]
- Bhattacharjya, S.; Gunson, B.K.; Mirza, D.F.; Mayer, D.A.; Buckels, J.A.; McMaster, P.; Neuberger, J.M. Delayed Hepatic Artery Thrombosis in Adult Orthotopic Liver Transplantation—A 12-Year Experience. Transplantation 2001, 71, 1592–1596. [Google Scholar] [CrossRef]
- Nagano, Y.; Togo, S.; Tanaka, K.; Masui, H.; Endo, I.; Sekido, H.; Nagahori, K.; Shimada, H. Risk Factors and Management of Bile Leakage after Hepatic Resection. World J. Surg. 2003, 27, 695–698. [Google Scholar] [CrossRef] [PubMed]
- Hoeffel, C.; Azizi, L.; Lewin, M.; Laurent, V.; Aubé, C.; Arrivé, L.; Tubiana, J.-M. Normal and Pathologic Features of the Postoperative Biliary Tract at 3D MR Cholangiopancreatography and MR Imaging. Radiographics 2006, 26, 1603–1620. [Google Scholar] [CrossRef] [PubMed]
- Castellanos, A.A.; Granados, J.F.M.; Fernandez, J.E.; Muñoz, I.G.; Tarradas, F.D.A.T. Early phase detection of bile leak after hepatobiliary surgery: Value of Gd-EOB-DTPA-enhanced MR cholangiography. Abdom. Imaging 2012, 37, 795–802. [Google Scholar] [CrossRef] [PubMed]
- Melamud, K.; LeBedis, C.A.; Anderson, S.W.; Soto, J.A. Biliary Imaging: Multimodality Approach to Imaging of Biliary Injuries and Their Complications. Radiographics 2014, 34, 613–623. [Google Scholar] [CrossRef]
- Thompson, C.M.; Saad, N.E.; Quazi, R.R.; Darcy, M.D.; Picus, D.D.; Menias, C.O. Management of Iatrogenic Bile Duct Injuries: Role of the Interventional Radiologist. Radiographics 2013, 33, 117–134. [Google Scholar] [CrossRef]
- Biller, L.H.; Schrag, D. Diagnosis and Treatment of Metastatic Colorectal Cancer: A Review. JAMA 2021, 325, 669–685. [Google Scholar] [CrossRef]
- Kopetz, S.; Chang, G.J.; Overman, M.J.; Eng, C.; Sargent, D.; Larson, D.W.; Grothey, A.; Vauthey, J.-N.; Nagorney, D.M.; McWilliams, R.R. Improved Survival in Metastatic Colorectal Cancer Is Associated With Adoption of Hepatic Resection and Improved Chemotherapy. J. Clin. Oncol. 2009, 27, 3677–3683. [Google Scholar] [CrossRef] [PubMed]
- Tagliafico, A.S.; Campi, C.; Bianca, B.; Bortolotto, C.; Buccicardi, D.; Francesca, C.; Prost, R.; Rengo, M.; Faggioni, L. Blockchain in radiology research and clinical practice: Current trends and future directions. Radiol. Med. 2022, 127, 391–397. [Google Scholar] [CrossRef]
- Rahbari, N.N.; Garden, O.J.; Padbury, R.; Brooke-Smith, M.; Crawford, M.; Adam, R.; Koch, M.; Makuuchi, M.; Dematteo, R.P.; Christophi, C.; et al. Posthepatectomy liver failure: A defi-nition and grading by the International Study Group of Liver Surgery (ISGLS). Surgery 2011, 149, 713–724. [Google Scholar] [CrossRef] [PubMed]
- Chiti, G.; Grazzini, G.; Flammia, F.; Matteuzzi, B.; Tortoli, P.; Bettarini, S.; Pasqualini, E.; Granata, V.; Busoni, S.; Messserini, L.; et al. Gastroenteropancreatic neuroendocrine neoplasms (GEP-NENs): A radiomic model to predict tumor grade. Radiol. Med. 2022, 127, 928–938. [Google Scholar] [CrossRef] [PubMed]
- Pawlik, T.M.; Scoggins, C.R.; Zorzi, D.; Abdalla, E.K.; Andres, A.; Eng, C.; Curley, S.A.; Loyer, E.M.; Muratore, A.; Mentha, G.; et al. Effect of Surgical Margin Status on Survival and Site of Recurrence After Hepatic Resection for Colorectal Metastases. Ann. Surg. 2005, 241, 715–724. [Google Scholar] [CrossRef]
- Borghetti, P.; Branz, J.; Volpi, G.; Pancera, S.; Buraschi, R.; Bianchi, L.N.C.; Bonù, M.L.; Greco, D.; Facheris, G.; Tomasi, C.; et al. Home-based pulmonary rehabilitation in patients undergoing (chemo)radiation therapy for unresectable lung cancer: A prospective explorative study. Radiol. Med. 2022, 127, 1322–1332. [Google Scholar] [CrossRef]
- Pawlik, T.M.; Schulick, R.D.; Choti, M.A. Expanding Criteria for Resectability of Colorectal Liver Metastases. Oncologist 2008, 13, 51–64. [Google Scholar] [CrossRef] [PubMed]
- Aquina, C.T.; Brown, Z.J.; Beane, J.D.; Ejaz, A.; Cloyd, J.M.; Tsung, A.; Adam, M.O.; Pawlik, T.M.; Kim, A.C. Disparities in Care Access to Liver-Directed Therapy Among Medicare Beneficiaries with Colorectal Cancer Liver Metastasis. Ann. Surg. Oncol. 2022, 30, 335–344. [Google Scholar] [CrossRef] [PubMed]
- Granata, V.; Fusco, R.; De Muzio, F.; Cutolo, C.; Setola, S.V.; Dell’Aversana, F.; Grassi, F.; Belli, A.; Silvestro, L.; Ottaiano, A.; et al. Radiomics and machine learning analysis based on magnetic resonance imaging in the assessment of liver mucinous colorectal metastases. Radiol. Med. 2022, 127, 763–772. [Google Scholar] [CrossRef]
- Ierardi, A.M.; Stellato, E.; Pellegrino, G.; Bonelli, C.; Cellina, M.; Renzulli, M.; Biondetti, P.; Carrafiello, G. Fluid-dynamic control microcatheter used with glue: Preliminary experience on its feasibility and safety. Radiol. Med. 2022, 127, 272–276. [Google Scholar] [CrossRef]
- Granata, V.; Fusco, R.; Catalano, O.; Filice, S.; Amato, D.M.; Nasti, G.; Avallone, A.; Izzo, F.; Petrillo, A. Early Assessment of Colorectal Cancer Patients with Liver Metastases Treated with Antiangiogenic Drugs: The Role of Intravoxel Incoherent Motion in Diffusion-Weighted Imaging. PLoS ONE 2015, 10, e0142876. [Google Scholar] [CrossRef] [PubMed]
- Granata, V.; Fusco, R.; Setola, S.V.; Raso, M.M.; Avallone, A.; De Stefano, A.; Nasti, G.; Palaia, R.; Delrio, P.; Petrillo, A.; et al. Liver radiologic findings of chemotherapy-induced toxicity in liver colorectal metastases patients. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 9697–9706. [Google Scholar] [PubMed]
- Intagliata, N.M.; Caldwell, S.H.; Tripodi, A. Diagnosis, Development, and Treatment of Portal Vein Thrombosis in Patients with and without Cirrhosis. Gastroenterology 2019, 156, 1582–1599.e1. [Google Scholar] [CrossRef] [PubMed]
- Barretta, M.L.; Catalano, O.; Setola, S.V.; Granata, V.; Marone, U.; Gallipoli, A.D. Gallbladder metastasis: Spectrum of imaging findings. Abdom. Imaging 2011, 36, 729–734. [Google Scholar] [CrossRef]
- Lopera, J.E.; Yamaguchi, S. Invited Commentary: Minimally Invasive Endovascular Management of Portal Vein Thrombosis. Radiographics 2022, 42, E169–E170. [Google Scholar] [CrossRef]
- Ak, C.; Adalı, G.; Sayar, S.; Ağaçkıran, A.; Kulalı, F.; Kahraman, R.; Öztürk, O.; Özdil, K. Portal Vein Thrombosis Risk Factors in Liver Transplant Candidates. Hepatol. Forum 2022, 3, 88–92. [Google Scholar] [CrossRef]
- Song, W.; Chen, Q.; Guo, D.; Jiang, C. Preoperative estimation of the survival of patients with unresectable hepatocellular carcinoma achieving complete response after conventional transcatheter arterial chemoembolization: Assessments of clinical and LI-RADS MR features. Radiol. Med. 2022, 127, 939–949. [Google Scholar] [CrossRef]
- Iacobellis, F.; Brillantino, A.; Di Serafino, M.; Orabona, G.D.; Grassi, R.; Cappabianca, S.; Scaglione, M.; Romano, L. Economic and clinical benefits of immediate total-body CT in the diagnostic approach to polytraumatized patients: A descriptive analysis through a literature review. Radiol. Med. 2022, 127, 637–644. [Google Scholar] [CrossRef]
- Paolucci, A.; Ierardi, A.M.; Hohenstatt, S.; Grassi, V.; Romagnoli, S.; Pignataro, L.; Trimarchi, S.; Carrafiello, G. Pre-surgical embolization of carotid body paragangliomas: Advantages of direct percutaneous approach and transitory balloon-occlusion at the origin of the external carotid artery. Radiol. Med. 2022, 127, 433–439. [Google Scholar] [CrossRef]
- Xiong, X.; Lou, Y.; Zhou, T.; Zheng, Z.; Liu, Y.; Liu, R.; Zhang, K.; Gong, Y.; Tang, C.; Jin, Z. Case report: A case of giant accessory hepatic lobe torsion combined with left hepatic vein branch thrombosis in a child. Front. Pediatr. 2022, 10, 970876. [Google Scholar] [CrossRef]
- Kato, H.; Asano, Y.; Ito, M.; Arakawa, S.; Shimura, M.; Koike, D.; Ochi, T.; Yasuoka, H.; Kawai, T.; Higashiguchi, T.; et al. A case of Vp4 hepatocellular carcinoma with tumor thrombosis extending into the confluence of the splenic/portal vein achieved a good prognosis with emergent hepatectomy and postoperative adjuvant therapy with lenvatinib. World J. Surg. Oncol. 2022, 20, 278. [Google Scholar] [CrossRef] [PubMed]
- Cortellini, A.; Bozzetti, F.; Palumbo, P.; Brocco, D.; Di Marino, P.; Tinari, N.; De Tursi, M.; Agostinelli, V.; Patruno, L.; Valdesi, C.; et al. Weighing the role of skeletal muscle mass and muscle density in cancer patients receiving PD-1/PD-L1 checkpoint inhibitors: A multicenter real-life study. Sci. Rep. 2020, 10, 1456. [Google Scholar] [CrossRef]
- Piccirillo, M.; Rinaldi, L.; Leongito, M.; Amore, A.; Crispo, A.; Granata, V.; Aprea, P.; Izzo, F. Percutaneous implant of Denver peri-toneo-venous shunt for treatment of refractory ascites: A single center retrospective study. Eur. Rev. Med. Pharmacol. Sci. 2017, 21, 3668–3673. [Google Scholar] [PubMed]
- Alam, H.; Kim, D.; Provido, H.; Kirkpatrick, J. Portal vein thrombosis in the adult: Surgical implications in an era of dynamic imaging. Am. Surg. 1997, 63, 681–685. [Google Scholar] [PubMed]
- Hanafy, A.S.; Abd-Elsalam, S.; Dawoud, M.M. Randomized controlled trial of rivaroxaban versus warfarin in the management of acute non-neoplastic portal vein thrombosis. Vasc. Pharmacol. 2019, 113, 86–91. [Google Scholar] [CrossRef] [PubMed]
- Xue, Z.; Chen, M.; Zhang, X.; Wang, G.; He, X.; Wu, L.; Ma, Y. Analysis of early hepatic artery thrombosis after liver transplantation. ANZ J. Surg. 2018, 88, 172–176. [Google Scholar] [CrossRef]
- McNaughton, D.A.; Abu-Yousef, M.M. Doppler US of the liver made simple. Radiographics 2011, 31, 161–188, Erratum in Radiographics 2011, 31, 904. [Google Scholar] [CrossRef]
- Yoon, J.H.; Choi, S.K.; Cho, S.B.; Kim, H.J.; Ko, Y.S.; Jun, C.H. Early extrahepatic recurrence as a pivotal factor for survival after hepatocellular carcinoma resection: A 15-year observational study. World J. Gastroenterol. 2022, 28, 5351–5363. [Google Scholar] [CrossRef]
- Xiong, Y.; Cao, P.; Lei, X.; Tang, W.; Ding, C.; Qi, S.; Chen, G. Accurate prediction of microvascular invasion occurrence and effective prognostic estimation for patients with hepatocellular carcinoma after radical surgical treatment. World J. Surg. Oncol. 2022, 20, 328. [Google Scholar] [CrossRef]
- Granata, V.; Fusco, R.; Setola, S.V.; Picone, C.; Vallone, P.; Belli, A.; Incollingo, P.; Albino, V.; Tatangelo, F.; Izzo, F.; et al. Microvascular invasion and grading in hepatocellular carcinoma: Correlation with major and ancillary features according to LIRADS. Abdom. Imaging 2019, 44, 2788–2800. [Google Scholar] [CrossRef]
- Masciocchi, C.; Arrigoni, F.; La Marra, A.; Mariani, S.; Zugaro, L.; Barile, A. Treatment of focal benign lesions of the bone: MRgFUS and RFA. Br. J. Radiol. 2016, 89, 20150356. [Google Scholar] [CrossRef]
- Granata, V.; Fusco, R.; D’Alessio, V.; Giannini, A.; Venanzio Setola, S.; Belli, A.; Palaia, R.; Petrillo, A.; Izzo, F. Electroporation-based treatments in minimally invasive percutaneous, laparoscopy and endoscopy procedures for treatment of deep-seated tumors. Eur. Rev. Med. Pharmacol. Sci. 2021, 25, 3536–3545. [Google Scholar] [CrossRef]
- Granata, V.; Fusco, R.; Risi, C.; Ottaiano, A.; Avallone, A.; De Stefano, A.; Grimm, R.; Grassi, R.; Brunese, L.; Izzo, F.; et al. Diffusion-Weighted MRI and Diffusion Kurtosis Imaging to Detect RAS Mutation in Colorectal Liver Metastasis. Cancers 2020, 12, 2420. [Google Scholar] [CrossRef]
- Vicini, S.; Bortolotto, C.; Rengo, M.; Ballerini, D.; Bellini, D.; Carbone, I.; Preda, L.; Laghi, A.; Coppola, F.; Faggioni, L. A narrative review on current imaging applications of artificial intelligence and radiomics in oncology: Focus on the three most common cancers. Radiol. Med. 2022, 127, 819–836. [Google Scholar] [CrossRef] [PubMed]
- Xue, K.; Liu, L.; Liu, Y.; Guo, Y.; Zhu, Y.; Zhang, M. Radiomics model based on multi-sequence MR images for predicting preoperative immunoscore in rectal cancer. Radiol. Med. 2022, 127, 702–713. [Google Scholar] [CrossRef] [PubMed]
- Gao, W.; Wang, W.; Song, D.; Yang, C.; Zhu, K.; Zeng, M.; Rao, S.-X.; Wang, M. A predictive model integrating deep and radiomics features based on gadobenate dimeglumine-enhanced MRI for postoperative early recurrence of hepatocellular carcinoma. Radiol. Med. 2022, 127, 259–271. [Google Scholar] [CrossRef] [PubMed]
- Scialpi, M.; Moschini, T.O.; De Filippis, G. PET/contrast-enhanced CT in oncology: “To do, or not to do, that is the question”. Radiol. Med. 2022, 127, 925–927. [Google Scholar] [CrossRef]
- Granata, V.; Catalano, O.; Fusco, R.; Tatangelo, F.; Rega, D.; Nasti, G.; Avallone, A.; Piccirillo, M.; Izzo, F.; Petrillo, A. The target sign in colorectal liver metastases: An atypical Gd-EOB-DTPA “uptake” on the hepatobiliary phase of MR imaging. Abdom. Imaging 2015, 40, 2364–2371. [Google Scholar] [CrossRef]
- Piccirillo, M.; Granata, V.; Albino, V.; Palaia, R.; Setola, S.V.; Petrillo, A.; Tatangelo, F.; Botti, G.; Foggia, M.; Izzo, F. Can Hepatocellular Carcinoma (HCC) Produce Unconventional Metastases? Four Cases of Extrahepatic HCC. Tumori J. 2013, 99, e19–e23. [Google Scholar] [CrossRef]
- De Muzio, F.; Grassi, F.; Dell’Aversana, F.; Fusco, R.; Danti, G.; Flammia, F.; Chiti, G.; Valeri, T.; Agostini, A.; Palumbo, P.; et al. A Narrative Review on LI-RADS Algorithm in Liver Tumors: Prospects and Pitfalls. Diagnostics 2022, 12, 1655. [Google Scholar] [CrossRef] [PubMed]
- Fusco, R.; Petrillo, M.; Granata, V.; Filice, S.; Sansone, M.; Catalano, O.; Petrillo, A. Magnetic resonance imaging evaluation in neoadjuvant therapy of locally advanced rectal cancer: A systematic review. Radiol. Oncol. 2017, 51, 252–262. [Google Scholar] [CrossRef] [PubMed]
- Takenaga, T.; Hanaoka, S.; Nomura, Y.; Nakao, T.; Shibata, H.; Miki, S.; Yoshikawa, T.; Hayashi, N.; Abe, O. Multichannel three-dimensional fully convolutional residual network-based focal liver lesion detection and classification in Gd-EOB-DTPA-enhanced MRI. Int. J. Comput. Assist. Radiol. Surg. 2021, 16, 1527–1536. [Google Scholar] [CrossRef] [PubMed]
- Fusco, R.; Sansone, M.; Granata, V.; Setola, S.V.; Petrillo, A.; Fusco, R.; Sansone, M.; Granata, V.; Setola, S.V.; Petrillo, A. A systematic review on multiparametric MR imaging in prostate cancer detection. Infect. Agents Cancer 2017, 12, 57. [Google Scholar] [CrossRef] [PubMed]
- Donato, H.; França, M.; Candelária, I.; Caseiro-Alves, F. Liver MRI: From basic protocol to advanced techniques. Eur. J. Radiol. 2017, 93, 30–39. [Google Scholar] [CrossRef]
- Culverwell, A.; Sheridan, M.; Guthrie, J.; Scarsbrook, A. Diffusion-weighted MRI of the liver—Interpretative pearls and pitfalls. Clin. Radiol. 2013, 68, 406–414. [Google Scholar] [CrossRef]
- Budjan, J.; Schoenberg, S.O.; Attenberger, U.I. CT und MRT der Leber: Wann, was, warum? CT and MRI of the liver: When, what, why? Der Radiol. 2017, 57, 366–372. [Google Scholar] [CrossRef]
- Miao, T.L.; Kielar, A.Z.; Hibbert, R.M.; Schieda, N. Utility of T1-weighted MRI as a predictor of liver lesion visibility on ultrasound: A clinical tool to determine feasibility of ultrasound-guided percutaneous interventions. Eur. J. Radiol. 2017, 90, 256–261. [Google Scholar] [CrossRef]
- Tanaka, O.; Ito, H.; Yamada, K.; Kubota, T.; Kizu, O.; Kato, T.; Yamagami, T.; Nishimura, T. Higher lesion conspicuity for SENSE dynamic MRI in detecting hypervascular hepatocellular carcinoma: Analysis through the measurements of liver SNR and lesion–liver CNR comparison with conventional dynamic MRI. Eur. Radiol. 2005, 15, 2427–2434. [Google Scholar] [CrossRef]
- Li, Z.; Mao, Y.; Huang, W.; Li, H.; Zhu, J.; Li, W.; Li, B. Texture-based classification of different single liver lesion based on SPAIR T2W MRI images. BMC Med. Imaging 2017, 17, 42. [Google Scholar] [CrossRef]
- Hori, M.; Murakami, T.; Kim, T.; Tomoda, K.; Nakamura, H. CT Scan and MRI in the Differentiation of Liver Tumors. Dig. Dis. 2004, 22, 39–55. [Google Scholar] [CrossRef]
- De Robertis, R.; Geraci, L.; Tomaiuolo, L.; Bortoli, L.; Beleù, A.; Malleo, G.; D’onofrio, M. Liver metastases in pancreatic ductal adenocarcinoma: A predictive model based on CT texture analysis. Radiol. Med. 2022, 127, 1079–1084. [Google Scholar] [CrossRef] [PubMed]
- Argalia, G.; Ventura, C.; Tosi, N.; Campioni, D.; Tagliati, C.; Tufillaro, M.; Cucco, M.; Baroni, G.S.; Giovagnoni, A. Comparison of point shear wave elastography and transient elastography in the evaluation of patients with NAFLD. Radiol. Med. 2022, 127, 571–576. [Google Scholar] [CrossRef] [PubMed]
- Zerunian, M.; Pucciarelli, F.; Caruso, D.; Polici, M.; Masci, B.; Guido, G.; De Santis, D.; Polverari, D.; Principessa, D.; Benvenga, A.; et al. Artificial intelligence based image quality enhancement in liver MRI: A quantitative and qualitative evaluation. Radiol. Med. 2022, 127, 1098–1105. [Google Scholar] [CrossRef]
- Grassi, R.; Cappabianca, S.; Urraro, F.; Feragalli, B.; Montanelli, A.; Patelli, G.; Granata, V.; Giacobbe, G.; Russo, G.; Grillo, A.; et al. Chest CT Computerized Aided Quantification of PNEUMONIA Lesions in COVID-19 Infection: A Comparison among Three Commercial Software. Int. J. Environ. Res. Public Health 2020, 17, 6914. [Google Scholar] [CrossRef] [PubMed]
- Inoue, Y.; Fujii, K.; Ishii, M.; Kagota, S.; Tomioka, A.; Hamamoto, H.; Osumi, W.; Tsuchimoto, Y.; Masubuchi, S.; Yamamoto, M.; et al. Volumetric and Functional Regeneration of Remnant Liver after Hepatectomy. J. Gastrointest. Surg. 2019, 23, 914–921. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Xu, M.; Lin, N.; Pan, C.; Zhou, B.; Zhong, Y.; Xu, R. Associating liver partition and portal vein ligation for staged hepatectomy versus conventional two-stage hepatectomy: A systematic review and meta-analysis. World J. Surg. Oncol. 2017, 15, 227. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cutolo, C.; Fusco, R.; Simonetti, I.; De Muzio, F.; Grassi, F.; Trovato, P.; Palumbo, P.; Bruno, F.; Maggialetti, N.; Borgheresi, A.; et al. Imaging Features of Main Hepatic Resections: The Radiologist Challenging. J. Pers. Med. 2023, 13, 134. https://doi.org/10.3390/jpm13010134
Cutolo C, Fusco R, Simonetti I, De Muzio F, Grassi F, Trovato P, Palumbo P, Bruno F, Maggialetti N, Borgheresi A, et al. Imaging Features of Main Hepatic Resections: The Radiologist Challenging. Journal of Personalized Medicine. 2023; 13(1):134. https://doi.org/10.3390/jpm13010134
Chicago/Turabian StyleCutolo, Carmen, Roberta Fusco, Igino Simonetti, Federica De Muzio, Francesca Grassi, Piero Trovato, Pierpaolo Palumbo, Federico Bruno, Nicola Maggialetti, Alessandra Borgheresi, and et al. 2023. "Imaging Features of Main Hepatic Resections: The Radiologist Challenging" Journal of Personalized Medicine 13, no. 1: 134. https://doi.org/10.3390/jpm13010134
APA StyleCutolo, C., Fusco, R., Simonetti, I., De Muzio, F., Grassi, F., Trovato, P., Palumbo, P., Bruno, F., Maggialetti, N., Borgheresi, A., Bruno, A., Chiti, G., Bicci, E., Brunese, M. C., Giovagnoni, A., Miele, V., Barile, A., Izzo, F., & Granata, V. (2023). Imaging Features of Main Hepatic Resections: The Radiologist Challenging. Journal of Personalized Medicine, 13(1), 134. https://doi.org/10.3390/jpm13010134