Application of Machine Learning Methods for Epilepsy Risk Ranking in Patients with Hematopoietic Malignancies Using
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Datasets
- Age: 1–90 years old;
- Diagnosis: verified malignant neoplasms of lymphoid, hematopoietic, and related tissues;
- Case type: inpatient treatment.
- Absence of oncohematological or cardiac disease. Outpatient treatment was an exclusion criterion;
- A history of Acute symptomatic seizures (ASS) without a verified diagnosis of epilepsy (G40.0–G40.8);
- Outpatient treatment.
- Comorbidities: 14% of I60–I69, fibrillation—6%, epilepsy (G40.0–G40.8)—1.5%, hypertension—20%;
- Genetic sex: females—49%, males—51%;
- Age: mean age—52.5 (min—1, max—90, 25%—40, 50%—57, 75%—66).
- Age: 1–99 years old;
- Diagnosis: hypertension, acute coronary syndrome (ACS), strokes, coronary artery disease (CAD),congenital heart disease (CHD), verified malignant neoplasms of lymphoid, hematopoietic, and related tissues;
- Case type: inpatient treatment.
- Absence of cardiovascular disease and oncological disease;
- Outpatient treatment was an exclusion criterion;
- A history of Acute symptomatic seizures (ASS) without a verified diagnosis of epilepsy (G40.0–G40.8).
2.2. Correlation Analysis
2.3. Machine Learning Methods
2.4. Importance of Predictors
2.5. Cerebrovascular Disease
3. Results
3.1. Dataset I
3.2. Dataset II
4. Discussion
4.1. General
- vital signs (age, body mass index, patient weight);
- cardiovascular pathology, cerebrovascular pathology (arterial hypertension, stenosis or occlusion, occlusion and stenosis of precerebral arteries, cerebral sinus thrombosis, cerebral artery dissection without rupture, cerebral aneurysmatic disease, cerebral infarction);
- laboratory parameters (maximum absolute monocyte count, average hemoglobin content of red blood cells, neutrophil count, platelet count at hospital discharge, minimum hematocrit value, minimum and average blood sodium levels);
- hematopoietic stem cell transplantation parameters (donor blood group, number of transplanted cells).
4.2. Study Population
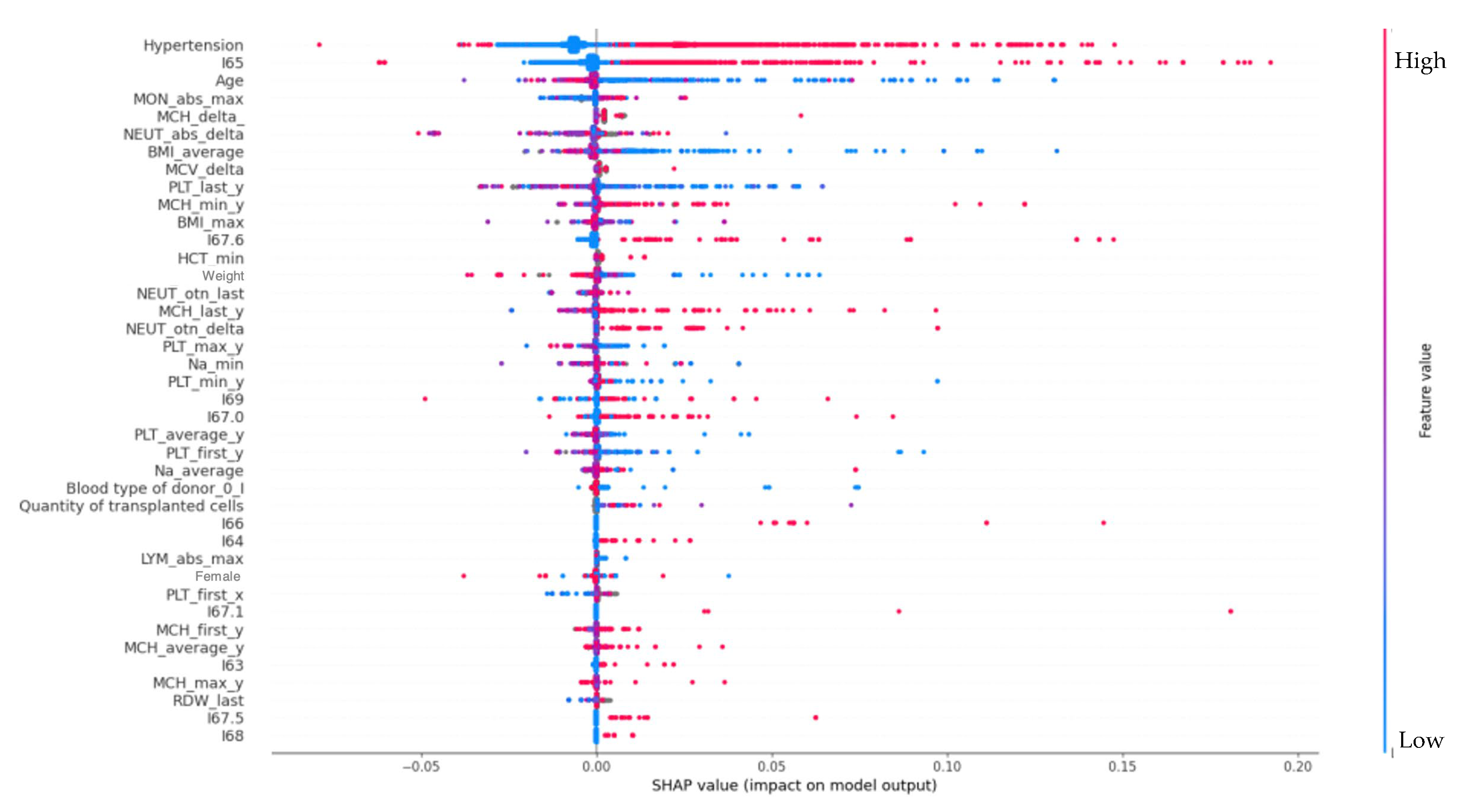
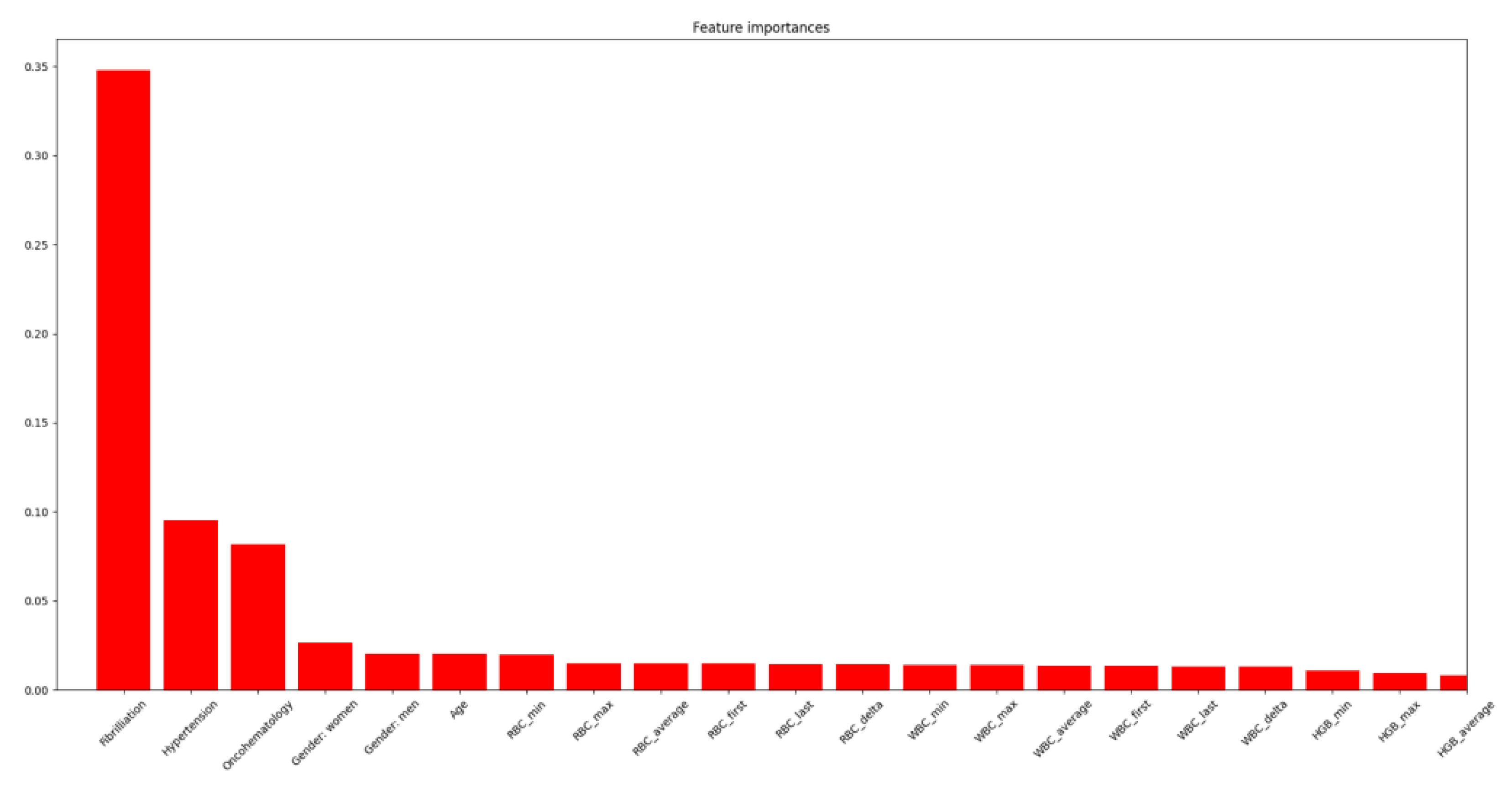
4.3. Risk Factors
4.4. Arterial Hypertension
4.5. Cerebral Sinus Thrombosis
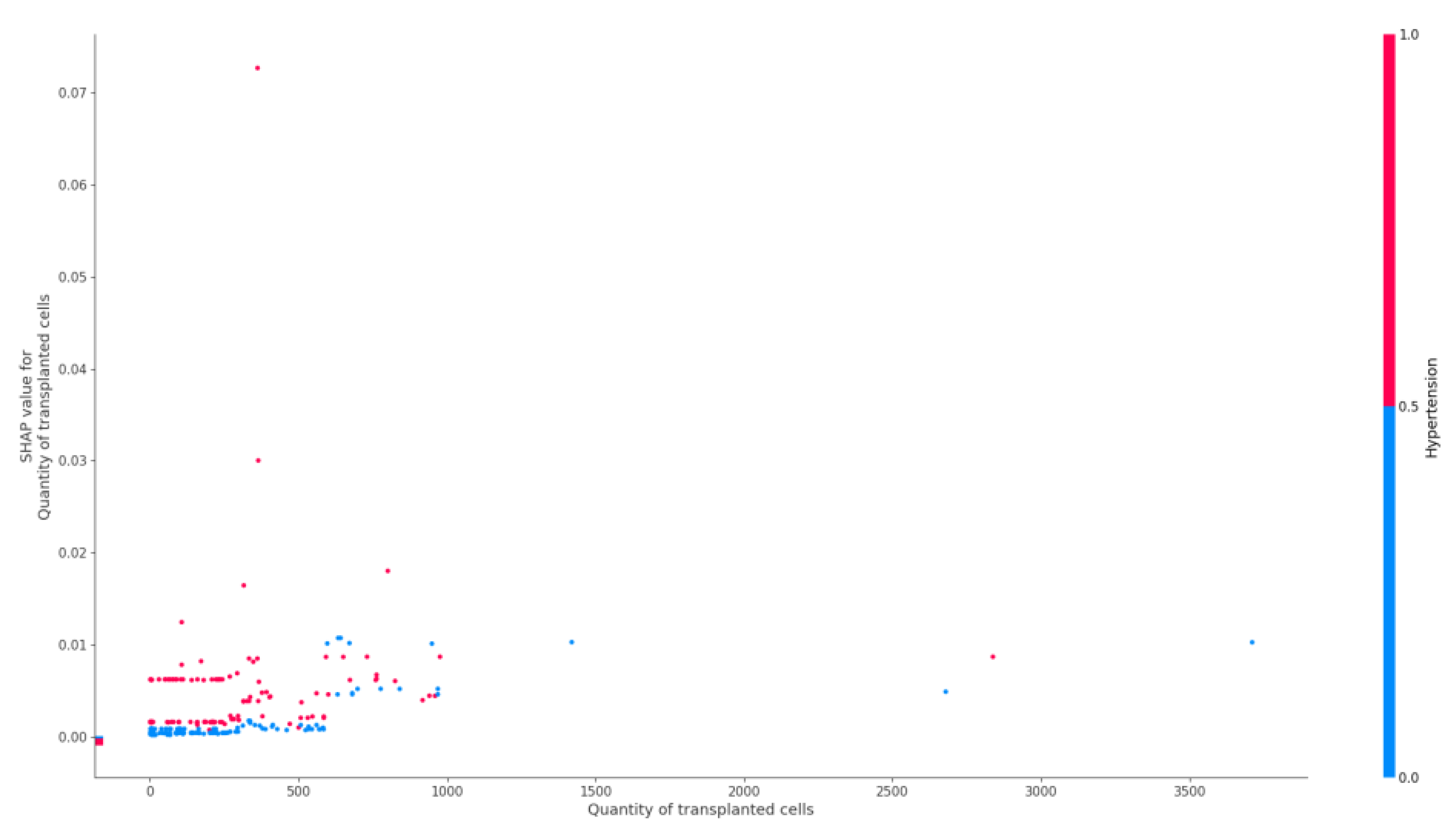
4.6. Transplanted Hematopoietic Stem Cells
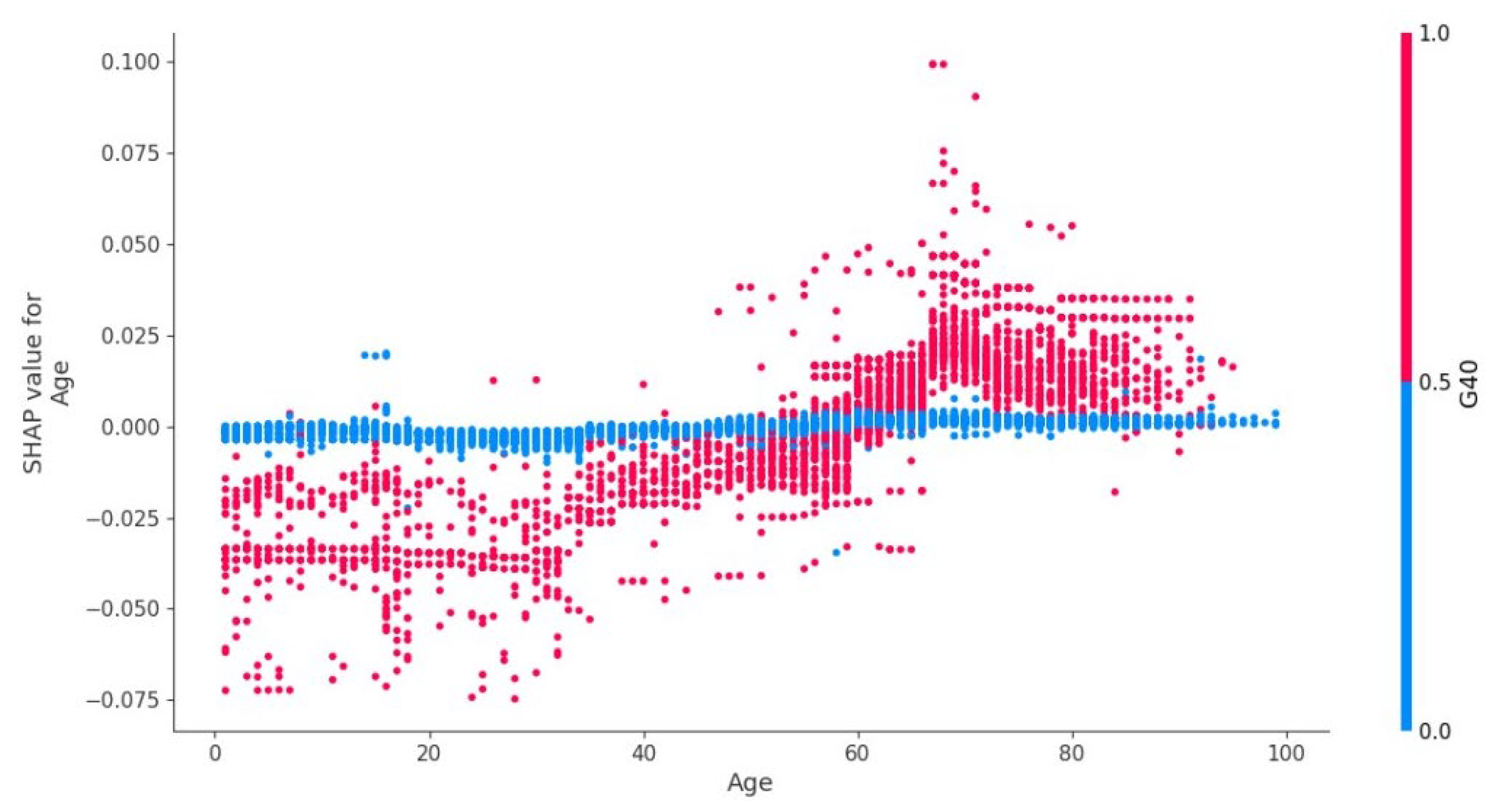
4.7. Age Factor
4.8. Dataset I vs. Dataset II Patients
4.9. Clinical Implications
4.10. Study Limitation
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ACS | Acute coronary seizure |
| ICD-10 | International Classification of Diseases, 10th revision |
| PRES | Posterior reversible encephalopathy syndrome |
| ASS | acute symphtomatic seizure |
| CVST | Cerebral venous sinus thrombosis |
| CI | confidence indicator |
| BMI | Body mass index |
| CAD | coronary artery disease |
| CHF | congestive heart failure |
| CHD | congenital heart disease |
| ANN | artificial neuron network |
| DT | decisions tree |
| AUC | Area under the Curve |
| ROC | receiver operating characteristic curve |
| PDW | platelet distribution width |
| SVM | support vector machine |
| HGB | Hemoglobin |
| LEU | Leukocytes |
| PLT | Platelets |
| MPW | Mean platelet volume |
| MCH | Mean cell hemoglobin |
| NEUT | Neutrophils |
| MCV | Mean corpuscular volume |
| PCT | Procalcitonin |
| RDW | Red blood cell distribution width |
| ALT | Alanine transaminase |
| PDW | Platelet distribution width |
| HDL | High-density lipoprotein |
| AST | Aspartate aminotransferase |
| WBC | White blood count |
| RBC | Red blood cell count |
| HCT | Hematocrit |
| LDL | Low-density lipoproteins |
| BLD | Blood in urine |
References
- Kang, J.M.; Kim, Y.J.; Kim, J.Y.; Cho, E.J.; Lee, J.H.; Lee, M.H.; Lee, S.H.; Sung, K.W.; Koo, H.H.; Yoo, K.H. Neurologic Complications after Allogeneic Hematopoietic Stem Cell Transplantation in Children: Analysis of Prognostic Factors. Biol. Blood Marrow Transplant. 2015, 21, 1091–1098. [Google Scholar] [CrossRef][Green Version]
- Khan, R.B.; Morris, E.B.; Pui, C.H.; Hudson, M.M.; Zhou, Y.; Ledet, D.S.; Howard, S.C. Long-Term Outcome and Risk Factors for Uncontrolled Seizures after a First Seizure in Children with Hematological Malignancies. J. Child Neurol. 2014, 29, 774–781. [Google Scholar] [CrossRef] [PubMed]
- Cordelli, D.M.; Masetti, R.; Zama, D.; Gueraldi, D.; Rondelli, R.; Cottone, C.; Prete, A.; Pession, A.; Franzoni, E. Etiology, Characteristics and Outcome of Seizures after Pediatric Hematopoietic Stem Cell Transplantation. Seizure 2014, 23, 140–145. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.H.; Xu, L.P.; Liu, D.H.; Chen, H.; Han, W.; Chen, Y.H.; Wang, F.R.; Wang, J.Z.; Wang, Y.; Zhao, T.; et al. Epileptic Seizures in Patients Following Allogeneic Hematopoietic Stem Cell Transplantation: A Retrospective Analysis of Incidence, Risk Factors, and Survival Rates. Clin. Transplant. 2013, 27, 80–89. [Google Scholar] [CrossRef]
- Yam, K.M.K.; Leung, W.K.A.; Zhu, X.L.; Fung, L.W.E. Drug Resistant Epilepsy with Mesial Temporal Sclerosis as Possible Late Neurological Complication in Two AML Survivors after Stem Cell Transplantation. Epilepsy Behav. Case Rep. 2018, 10, 71–77. [Google Scholar] [CrossRef]
- Leng, Y.; Yu, T.; Li, Y.; Chen, W. Surgical Treatment of Refractory Epilepsy after Chemotherapy in Two Children with Leukemia. Epilepsy Behav. Case Rep. 2013, 1, 32–34. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Steegmann, J.L.; Baccarani, M.; Breccia, M.; Casado, L.F.; García-Gutiérrez, V.; Hochhaus, A.; Kim, D.W.; Kim, T.D.; Khoury, H.J.; Le Coutre, P.; et al. European LeukemiaNet Recommendations for the Management and Avoidance of Adverse Events of Treatment in Chronic Myeloid Leukaemia. Leukemia 2016, 30, 1648–1671. [Google Scholar] [CrossRef]
- Armenian, S.H.; Chemaitilly, W.; Chen, M.; Chow, E.J.; Duncan, C.N.; Jones, L.W.; Pulsipher, M.A.; Remaley, A.T.; Rovo, A.; Salooja, N.; et al. National Institutes of Health Hematopoietic Cell Transplantation Late Effects Initiative: The Cardiovascular Disease and Associated Risk Factors Working Group Report. Biol. Blood Marrow Transplant. 2017, 23, 201–210. [Google Scholar] [CrossRef]
- Johnson, E.L.; Krauss, G.L.; Lee, A.K.; Schneider, A.L.C.; Dearborn, J.L.; Kucharska-Newton, A.M.; Huang, J.; Alonso, A.; Gottesman, R.F. Association between Midlife Risk Factors and Late-Onset Epilepsy: Results from the Atherosclerosis Risk in Communities Study. JAMA Neurol. 2018, 75, 1375–1382. [Google Scholar] [CrossRef]
- Gasparini, S.; Ferlazzo, E.; Sueri, C.; Cianci, V.; Ascoli, M.; Cavalli, S.M.; Beghi, E.; Belcastro, V.; Bianchi, A.; Benna, P.; et al. Hypertension, Seizures, and Epilepsy: A Review on Pathophysiology and Management. Neurol. Sci. 2019, 40, 1775–1783. [Google Scholar] [CrossRef]
- Sha, Z.; Moran, B.P.; McKinney, A.M.; Henry, T.R. Seizure Outcomes of Posterior Reversible Encephalopathy Syndrome and Correlations with Electroencephalographic Changes. Epilepsy Behav. 2015, 48, 70–74. [Google Scholar] [CrossRef]
- Chen, Q.; Zhao, X.; Fu, H.X.; Chen, Y.H.; Zhang, Y.Y.; Wang, J.Z.; Wang, Y.; Wang, F.R.; Mo, X.D.; Han, W.; et al. Posterior Reversible Encephalopathy Syndrome (PRES) after Haploidentical Haematopoietic Stem Cell Transplantation: Incidence, Risk Factors and Outcomes. Bone Marrow Transplant. 2020, 55, 2035–2042. [Google Scholar] [CrossRef]
- Datar, S.; Singh, T.; Rabinstein, A.A.; Fugate, J.E.; Hocker, S. Long-Term Risk of Seizures and Epilepsy in Patients with Posterior Reversible Encephalopathy Syndrome. Epilepsia 2015, 56, 564–568. [Google Scholar] [CrossRef] [PubMed]
- Heo, K.; Cho, K.H.; Lee, M.K.; Chung, S.J.; Cho, Y.J.; Lee, B.I. Development of Epilepsy after Posterior Reversible Encephalopathy Syndrome. Seizure 2016, 34, 90–94. [Google Scholar] [CrossRef] [PubMed]
- Gupta, V.; Braun, T.M.; Chowdhury, M.; Tewari, M.; Choi, S.W. A Systematic Review of Machine Learning Techniques in Hematopoietic Stem Cell Transplantation (HSCT). Sensors 2020, 20, 6100. [Google Scholar] [CrossRef] [PubMed]
- An, S.; Malhotra, K.; Dilley, C.; Han-Burgess, E.; Valdez, J.N.; Robertson, J.; Clark, C.; Westover, M.B.; Sun, J. Predicting Drug-Resistant Epilepsy—A Machine Learning Approach Based on Administrative Claims Data. Epilepsy Behav. 2018, 89, 118–125. [Google Scholar] [CrossRef] [PubMed]
- Park, S.C.; Chung, C.K. Postoperative Seizure Outcome-Guided Machine Learning for Interictal Electrocorticography in Neocortical Epilepsy. J. Neurophysiol. 2018, 119, 2265–2275. [Google Scholar] [CrossRef]
- Nissen, I.A.; Stam, C.J.; van Straaten, E.C.W.; Wottschel, V.; Reijneveld, J.C.; Baayen, J.C.; Hamer, P.C. de W.; Idema, S.; Velis, D.N.; Hillebrand, A. Localization of the Epileptogenic Zone Using Inter-ictal MEG and Machine Learning in a Large Cohort of Drug-Resistant Epilepsy Patients. Front. Neurol. 2018, 9, 647. [Google Scholar] [CrossRef]
- Bharath, R.D.; Panda, R.; Raj, J.; Bhardwaj, S.; Sinha, S.; Chaitanya, G.; Raghavendra, K.; Mundlamuri, R.C.; Arimappamagan, A.; Rao, M.B.; et al. Machine Learning Identifies “RsfMRI Epilepsy Networks” in Temporal Lobe Epilepsy. Eur. Radiol. 2019, 29, 3496–3505. [Google Scholar] [CrossRef]
- Rudie, J.D.; Colby, J.B.; Salamon, N. Machine Learning Classification of Mesial Temporal Sclerosis in Epilepsy Patients. Epilepsy Res. 2015, 117, 63–69. [Google Scholar] [CrossRef]
- Del Gaizo, J.; Mofrad, N.; Jensen, J.H.; Clark, D.; Glenn, R.; Helpern, J.; Bonilha, L. Using Machine Learning to Classify Temporal Lobe Epilepsy Based on Diffusion MRI. Brain Behav. 2017, 7, e00801. [Google Scholar] [CrossRef] [PubMed]
- Abbasi, B.; Goldenholz, D.M. Machine Learning Applications in Epilepsy. Epilepsia 2019, 60, 2037–2047. [Google Scholar] [CrossRef]
- Chiang, S.; Vannucci, M.; Goldenholz, D.M.; Moss, R.; Stern, J.M. Epilepsy as a Dynamic Disease: A Bayesian Model for Differentiating Seizure Risk from Natural Variability. Epilepsia Open 2018, 3, 236–246. [Google Scholar] [CrossRef] [PubMed]
- Chiang, S.; Goldenholz, D.M.; Moss, R.; Rao, V.R.; Haneef, Z.; Theodore, W.H.; Kleen, J.K.; Gavvala, J.; Vannucci, M.; Stern, J.M. Prospective Validation Study of an Epilepsy Seizure Risk System for Outpatient Evaluation. Epilepsia 2020, 61, 29–38. [Google Scholar] [CrossRef]
- Kalin, F.; Akinci, T.C.; Türkpence, D.; Seker, S.; Korkmaz, U. Detection of Epileptic Seizure Using STFT and Statistical Analysis. In Advances in Neural Signal Processing; Intech Open: London, UK, 2020. [Google Scholar] [CrossRef]
- Frolov, N.S.; Grubov, V.V.; Maksimenko, V.A.; Lüttjohann, A.; Makarov, V.V.; Pavlov, A.N.; Sitnikova, E.; Pisarchik, A.N.; Kurths, J.; Hramov, A.E. Statistical Properties and Predictability of Extreme Epileptic Events. Sci. Rep. 2019, 9, 7243. [Google Scholar] [CrossRef]
- Fiest, K.M.; Sauro, K.M.; Wiebe, S.; Patten, S.B.; Kwon, C.-S.; Dykeman, J.; Pringsheim, T.; Lorenzetti, D.L.; Jetté, N. Prevalence and Incidence of Epilepsy: A Systematic Review and Meta-Analysis of International Studies. Neurology 2017, 88, 296. [Google Scholar] [CrossRef]
- Schnabel, R.B.; Yin, X.; Gona, P.; Larson, M.G.; Beiser, A.; McManus, D.D.; Newton-Cheh, C.; Lubitz, S.A.; Magnani, J.W.; Ellinor, P.; et al. 50 Year Trends in Atrial Fibrillation Prevalence, Incidence, Risk Factors, and Mortality in the Fram-ingham Heart Study: A Cohort Study. Lancet 2015, 386, 154–162. [Google Scholar] [CrossRef]
- Shkolnikova, M.A.; Jdanov, D.A.; Ildarova, R.A.; Shcherbakova, N.V.; Polyakova, E.B.; Mikhaylov, E.N.; Shalnova, S.A.; Shkolnikov, V.M. Atrial Fibrillation among Russian Men and Women Aged 55 Years and Older: Prevalence, Mortality, and Associations with Biomarkers in a Population-Based Study. J. Geriatr. Cardiol. JGC 2020, 17, 74. [Google Scholar] [CrossRef] [PubMed]
- Börschel, C.S.; Schnabel, R.B. The Imminent Epidemic of Atrial Fibrillation and Its Concomitant Diseases—Myocardial Infarction and Heart Failure—A Cause for Concern. Int. J. Cardiol. 2019, 287, 162–173. [Google Scholar] [CrossRef]
- Chen, C.-C.; Chen, L.-S.; Yen, M.-F.; Chen, H.-H.; Liou, H.-H. Geographic Variation in the Age- and Gender-Specific Prevalence and Incidence of Epilepsy: Analysis of Taiwanese National Health Insurance-Based Data. Epilepsia 2012, 53, 283–290. [Google Scholar] [CrossRef]
- Tichy, E.; Lam, S.; Militano, U.; Bessmertny, O. A Case of Severe Thrombocytopenia and Antiepileptic Hypersensitivity Syndrome. J. Pediatric Pharmacol. Ther. 2003, 8, 29–33. [Google Scholar] [CrossRef] [PubMed]
- Basith, M.; Francis, A.; Bellon, A. Reversible Thrombocytopenia after Gabapentin in an HIV-Positive Patient. Case Rep. Psychiatry 2018, 2018, 5927065. [Google Scholar] [CrossRef] [PubMed]
- Nasreddine, W.; Beydoun, A. Valproate-Induced Thrombocytopenia: A Prospective Monotherapy Study. Epilepsia 2008, 49, 438–445. [Google Scholar] [CrossRef] [PubMed]
- Chi, N.F.; Kuan, Y.C.; Huang, Y.H.; Chan, L.; Hu, C.J.; Liu, H.Y.; Chiou, H.Y.; Chien, L.N. Development and Validation of Risk Score to Estimate 1-Year Late Poststroke Epilepsy Risk in Ischemic Stroke Patients. Clin. Epidemiol. 2018, 10, 1001–1011. [Google Scholar] [CrossRef] [PubMed]
- Spatola, M.; Dalmau, J. Seizures and Risk of Epilepsy in Autoimmune and Other Inflammatory Encephalitis. Curr. Opin. Neurol. 2017, 30, 345–353. [Google Scholar] [CrossRef]
- Burneo, J.G.; Antaya, T.C.; Allen, B.N.; Belisle, A.; Shariff, S.Z.; Saposnik, G. The Risk of New-Onset Epilepsy and Refractory Epilepsy in Older Adult Stroke Survivors. Neurology 2019, 93, E568–E577. [Google Scholar] [CrossRef]
- Kurien, M.; Ludvigsson, J.F.; Sanders, D.S.; Zylberberg, H.M.; Green, P.H.; Sundelin, H.E.K.; Lebwohl, B. Persistent Mucosal Damage and Risk of Epilepsy in People with Celiac Disease. European J. Neurol. 2018, 25, 592-e38. [Google Scholar] [CrossRef]
- Pinto, M.J.; Medeiros, P.B.; Príncipe, F.; Carvalho, M. Cerebral Venous Thrombosis in Hematological Malignancy: Balancing the Risks. J. Stroke Cerebrovasc. Dis. 2020, 29, 104683. [Google Scholar] [CrossRef]
- Silvis, S.M.; Hiltunen, S.; Lindgren, E.; Jood, K.; Zuurbier, S.M.; Middeldorp, S.; Putaala, J.; Cannegieter, S.C.; Tatlisumak, T.; Coutinho, J.M. Cancer and Risk of Cerebral Venous Thrombosis: A Case–Control Study. J. Thromb. Haemost. 2018, 16, 90–95. [Google Scholar] [CrossRef]
- Underwood, B.; Zhao, Q.; Walker, A.R.; Mims, A.S.; Vasu, S.; Long, M.; Haque, T.Z.; Blaser, B.W.; Grieselhuber, N.R.; Wall, S.A.; et al. Incidence of Venous Thrombosis after Peg-Asparaginase in Adolescent and Young Adults with Acute Lymphoblastic Leukemia. Int. J. Hematol. Oncol. 2020, 9, IJH28. [Google Scholar] [CrossRef]
- Bauwens, D.; Hantson, P.; Laterre, P.F.; Michaux, L.; Latinne, D.; De Tourtchaninoff, M.; Cosnard, G.; Hernalsteen, D. Recurrent Seizure and Sustained Encephalopathy Associated with Dimethylsulfoxide-Preserved Stem Cell Infusion. Leuk. Lymphoma 2005, 46, 1671–1674. [Google Scholar] [CrossRef] [PubMed]
- Maral, S.; Albayrak, M.; Pala, C.; Yildiz, A.; Sahin, O.; Ozturk, H.B. Dimethyl Sulfoxide-Induced Tonic-Clonic Seizure and Cardiac Arrest During Infusion of Autologous Peripheral Blood Stem Cells. Cell Tissue Bank. 2018, 19, 831–832. [Google Scholar] [CrossRef]
- Chen-Plotkin, A.S.; Vossel, K.A.; Samuels, M.A.; Chen, M.H. Encephalopathy, Stroke, and Myocardial Infarction with DMSO Use in Stem Cell Transplantation. Neurology 2007, 68, 859–861. [Google Scholar] [CrossRef] [PubMed]
- Argañaraz, G.A.; Konno, A.C.; Perosa, S.R.; Santiago, J.F.C.; Boim, M.A.; Vidotti, D.B.; Varella, P.P.V.; Costa, L.G.; Canzian, M.; Porcionatto, M.A.; et al. The Renin-Angiotensin System Is Upregulated in the Cortex and Hippocampus of Patients with Temporal Lobe Epilepsy Related to Mesial Temporal Sclerosis. Epilepsia 2008, 49, 1348–1357. [Google Scholar] [CrossRef] [PubMed]
- Berg, A.T.; Zelko, F.A.; Levy, S.R.; Testa, F.M. Age at Onset of Epilepsy, Pharmacoresistance, and Cognitive Outcomes: A Prospective Cohort Study. Neurology 2012, 79, 1384. [Google Scholar] [CrossRef] [PubMed]
- Mahale, R.; Mehta, A.; John, A.A.; Buddaraju, K.; Shankar, A.K.; Javali, M.; Srinivasa, R. Acute Seizures in Cerebral Venous Sinus Thrombosis: What Predicts It? Epilepsy Res. 2016, 123, 1–5. [Google Scholar] [CrossRef]
- Ferro, J.M.; Canhão, P.; Bousser, M.G.; Stam, J.; Barinagarrementeria, F. Early Seizures in Cerebral Vein and Dural Sinus Thrombosis: Risk Factors and Role of Antiepileptics. Stroke 2008, 39, 1152–1158. [Google Scholar] [CrossRef]
- Uluduz, D.; Midi, I.; Duman, T.; Yayla, V.; Karahan, A.Y.; Afsar, N.; Goksu, E.O.; Mengulluoglu, N.; Aytac, E.; Sungur, M.A.; et al. Epileptic Seizures in Cerebral Venous Sinus Thrombosis: Subgroup Analysis of VENOST Study. Seizure 2020, 78, 113–117. [Google Scholar] [CrossRef]
- Xian, Z.; Chen, Y.; Chen, L.; Lu, Q.; Huang, G.; Qin, Q.; Zeng, J.; Liang, Z. A Clinical Research on the Potential Pathogenesis of Somatic Cancer Related Cerebral Venous Sinus Thrombosis. Medicine 2019, 98, e15134. [Google Scholar] [CrossRef]
- Mineyko, A.; Kirton, A.; Billinghurst, L.; Tatishvili, N.N.; Wintermark, M.; deVeber, G.; Fox, C.; Abdalla, A.; Zafeiriou, D.; Friedman, N.; et al. Seizures and Outcome One Year After Neonatal and Childhood Cerebral Sinovenous Thrombosis. Pediatric Neurol. 2020, 105, 21–26. [Google Scholar] [CrossRef]
- Fitzgerald, K.C.; Williams, L.S.; Garg, B.P.; Carvalho, K.S.; Golomb, M.R. Cerebral Sinovenous Thrombosis in the Neonate. Arch. Neurol. 2006, 63, 405–409. [Google Scholar] [CrossRef] [PubMed]
- Ahlers, F.S.; Benros, M.E.; Dreier, J.W.; Christensen, J. Infections and Risk of Epilepsy in Children and Young Adults: A Nationwide Study. Epilepsia 2019, 60, 275–283. [Google Scholar] [CrossRef]
- Skiba, Y.B.; Polushin, A.Y.; Prokudin, M.Y.; Vladovskaya, M.D.; Kulagin, A.D. Acute Symptomatic Seizures during Haematopoietic Stem Cell Transplantation. Epilepsy Paroxysmal Cond. 2021, 13, 65–82. [Google Scholar] [CrossRef]
- Gesche, J.; Christensen, J.; Hjalgrim, H.; Rubboli, G.; Beier, C.P. Epidemiology and Outcome of Idiopathic Generalized Epilepsy in Adults. Eur. J. Neurol. 2020, 27, 676–684. [Google Scholar] [CrossRef] [PubMed]
- Schröder, H.; Kjeldahlk, M.; Boesen, A.M.; Nielsen, O.J.; Schmidt, K.; Johnsen, H.E.; Gregersen, H.; Heyman, M.; Gustafsson, G. Acute Lymphoblastic Leukemia in Adolescents Betweeen 10 and 19 Years of Age in Denmark—Secondary Publication. Dan. Med. Bull. 2006, 53, 76–79. [Google Scholar]
- Yang, H.; Rajah, G.; Guo, A.; Wang, Y.; Wang, Q. Pathogenesis of Epileptic Seizures and Epilepsy after Stroke. Neurol. Res. 2018, 40, 426–432. [Google Scholar] [CrossRef]
- Dammann, P.; Schaller, C.; Sure, U. Should We Resect Peri-Lesional Hemosiderin Deposits When Performing Lesionectomy in Patients with Cavernoma-Related Epilepsy (CRE)? Neurosurg. Rev. 2017, 40, 39–43. [Google Scholar] [CrossRef]



| Dataset | Males | Females | Mean Age | Age 25% | Age 50% | Age 75% | Comorbidities |
|---|---|---|---|---|---|---|---|
| Dataset I | 51% | 49% | 52.5 | 40 | 57 | 66 | 14% of I60–I69, Fibrillation—6%, epilepsy (G40.0–G40.8)—1.5%, hypertension—20% |
| Dataset II | 44% | 56% | 55 | 46 | 60 | 69 | presence of comorbid diseases (hypertension, cerebral vascular disease, infarcts, atrial fibrillation and congenital heart disease (CHD), blood pressure, fibrillation (13%), G40—8% |
| Method | Accuracy | Precision | Recall | F1-Score | AUC of ROC |
|---|---|---|---|---|---|
| Gradient Boosting | 0.96 | 0.93 | 0.96 | 0.98 | 0.94 |
| Random forest | 0.92 | 0.89 | 0.93 | 0.94 | 0.91 |
| Method | Cross-Validation Score | Precision | Recall | F1-Score | AUC of ROC |
|---|---|---|---|---|---|
| Gradient Boosting | 0.93 | 0.91 | 0.94 | 0.94 | 0.94 |
| Random forest | 0.89 | 0.82 | 0.91 | 0.90 | 0.90 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Skiba, I.; Kopanitsa, G.; Metsker, O.; Yanishevskiy, S.; Polushin, A. Application of Machine Learning Methods for Epilepsy Risk Ranking in Patients with Hematopoietic Malignancies Using. J. Pers. Med. 2022, 12, 1306. https://doi.org/10.3390/jpm12081306
Skiba I, Kopanitsa G, Metsker O, Yanishevskiy S, Polushin A. Application of Machine Learning Methods for Epilepsy Risk Ranking in Patients with Hematopoietic Malignancies Using. Journal of Personalized Medicine. 2022; 12(8):1306. https://doi.org/10.3390/jpm12081306
Chicago/Turabian StyleSkiba, Iaroslav, Georgy Kopanitsa, Oleg Metsker, Stanislav Yanishevskiy, and Alexey Polushin. 2022. "Application of Machine Learning Methods for Epilepsy Risk Ranking in Patients with Hematopoietic Malignancies Using" Journal of Personalized Medicine 12, no. 8: 1306. https://doi.org/10.3390/jpm12081306
APA StyleSkiba, I., Kopanitsa, G., Metsker, O., Yanishevskiy, S., & Polushin, A. (2022). Application of Machine Learning Methods for Epilepsy Risk Ranking in Patients with Hematopoietic Malignancies Using. Journal of Personalized Medicine, 12(8), 1306. https://doi.org/10.3390/jpm12081306






