Early Diagnosis of Kidney Damage Associated with Tobacco Use: Preventive Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Aspects
2.2. Patients and Study Design
2.3. Inclusion and Exclusion Criteria
2.4. Sample Collection
2.5. Quantification of Kidney Damage Biomarkers in Urine Samples
2.6. Tobacco Use Biomarker: Urinary Cotinine
2.7. Oxidative Stress Determination
2.8. Temporal Evolution of Biomarkers Calculation
2.9. Statistical Analysis
3. Results
3.1. Patients’ Characteristics
3.2. Objective 1: To Evaluate the Association between Tobacco Use and Kidney Damage
3.3. Objective 2: To Evaluate the Progression of Subclinical Kidney Damage Associated with Tobacco after Two Years of Consumption
3.4. Objective 3: To Evaluate If Smoking Cessation Reduces Subclinical Renal Damage
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- WHO World Health Organization. Fact Sheets. Available online: https://www.who.int/es/news-room/fact-sheets/detail/tobacco (accessed on 21 January 2022).
- Hieshima, K.; Suzuki, T.; Sugiyama, S.; Kurinami, N.; Yoshida, A.; Miyamoto, F.; Kajiwara, K.; Jinnouchi, T.; Jinnouchi, H. Smoking Cessation Ameliorates Microalbuminuria with Reduction of Blood Pressure and Pulse Rate in Patients with Already Diagnosed Diabetes Mellitus. J. Clin. Med. Res. 2018, 10, 478–485. [Google Scholar] [CrossRef]
- Rose, K.; Flanagan, J.G.; Patel, S.R.; Cheng, R.; Hudson, C. Retinal Blood Flow and Vascular Reactivity in Chronic Smokers. Investig. Opthalmology Vis. Sci. 2014, 55, 4266. [Google Scholar] [CrossRef][Green Version]
- Xiao, Z.; Li, C.W.; Shan, J.; Luo, L.; Feng, L.; Lu, J.; Li, S.F. Interventions to Improve Chronic Cyclosporine a Nephrotoxicity through Inhibiting Renal Cell Apoptosis: A Systematic Review. Chin. Med. J. 2013, 126, 3767–3774. [Google Scholar] [CrossRef]
- Coresh, J.; Selvin, E.; Stevens, L.A.; Manzi, J.; Kusek, J.W.; Eggers, P.; Van Lente, F.; Levey, A.S. Prevalence of Chronic Kidney Disease in the United States. JAMA 2007, 298, 2038. [Google Scholar] [CrossRef]
- Hammer, Y.; Cohen, E.; Levi, A.; Krause, I. The Relationship between Cigarette Smoking and Renal Function: A Large Cohort Study. Isr. Med. Assoc. J. IMAJ 2016, 18, 4. [Google Scholar]
- Hallan, S.I.; Orth, S.R. Smoking Is a Risk Factor in the Progression to Kidney Failure. Kidney Int. 2011, 80, 516–523. [Google Scholar] [CrossRef]
- Orth, S.R. Effects of Smoking on Systemic and Intrarenal Hemodynamics: Influence on Renal Function. J. Am. Soc. Nephrol. 2004, 15, S58–S63. [Google Scholar] [CrossRef]
- Orth, S.R.; Viedt, C.; Ritz, E. Adverse Effects of Smoking in the Renal Patient. Tohoku J. Exp. Med. 2001, 194, 1–15. [Google Scholar] [CrossRef]
- Arany, I.; Grifoni, S.; Clark, J.S.; Csongradi, E.; Maric, C.; Juncos, L.A. Chronic Nicotine Exposure Exacerbates Acute Renal Ischemic Injury. Am. J. Physiol. Ren. Physiol. 2011, 301, F125–F133. [Google Scholar] [CrossRef]
- Bleyer, A.J.; Shemanski, L.R.; Burke, G.L.; Hansen, K.J.; Appel, R.G. Tobacco, Hypertension, and Vascular Disease: Risk Factors for Renal Functional Decline in an Older Population. Kidney Int. 2000, 57, 2072–2079. [Google Scholar] [CrossRef]
- Prieto, M.; Vicente-Vicente, L.; Casanova, A.G.; Hernández-Sánchez, M.T.; Gomez-Marcos, M.A.; Garcia-Ortiz, L.; Morales, A.I. Designing New Diagnostic Systems for the Early Detection of Tobacco-Associated Chronic Renal Damage in Patients of a Primary Care Centre in Salamanca, Spain: An Observational, Prospective Study Protocol. BMJ Open 2020, 10, e032918. [Google Scholar] [CrossRef]
- World Medical Association. WMA—The World Medical Association-Declaración de Helsinki de la AMM—Principios éticos Para las Investigaciones Médicas en Seres Humanos. 2017. Available online: https://www.wma.net/es/policies-post/declaracion-de-helsinki-de-la-amm-principios-eticos-para-las-investigaciones-medicas-en-seres-humanos/ (accessed on 21 January 2022).
- BOE. Ley 14/2007, de 3 de Julio, de Investigación Biomédica. 2007. Available online: https://www.boe.es/eli/es/l/2007/07/03/14 (accessed on 20 June 2022).
- Bradford, M.M. A Rapid and Sensitive Method for the Quantitation of Microgram Quantities of Protein Utilizing the Principle of Protein-Dye Binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Kidney Disease Improving Global Outcomes (KDIGO). Chapter 2: Definition, Identification, and Prediction of CKD Progression. Kidney Int. Suppl. 2013, 3, 63–72. [Google Scholar] [CrossRef]
- Klag, M.J.; Whelton, P.K.; Randall, B.L.; Neaton, J.D.; Brancati, F.L.; Ford, C.E.; Shulman, N.B.; Stamler, J. Blood Pressure and End-Stage Renal Disease in Men. N. Engl. J. Med. 1996, 334, 13–18. [Google Scholar] [CrossRef]
- Markowitz, G.S.; Lin, J.; Valeri, A.M.; Avila, C.; Nasr, S.H.; D’Agati, V.D. Idiopathic Nodular Glomerulosclerosis Is a Distinct Clinicopathologic Entity Linked to Hypertension and Smoking. Hum. Pathol. 2002, 33, 826–835. [Google Scholar] [CrossRef]
- Choi, Y.; Park, J.-H.; Kim, D.-H.; Kim, H.J.; Suh, E.; Kim, K.-H.; Ahn, J.J.; Lee, G.-N.; Jung, J.-H.; Han, K.; et al. Association between Cotinine-Verified Smoking Status and Moderately Increased Albuminuria in the Middle-Aged and Older Population in Korea: A Nationwide Population-Based Study. PLoS ONE 2021, 16, e0246017. [Google Scholar] [CrossRef]
- Maeda, I.; Hayashi, T.; Sato, K.K.; Koh, H.; Harita, N.; Nakamura, Y.; Endo, G.; Kambe, H.; Fukuda, K. Cigarette Smoking and the Association with Glomerular Hyperfiltration and Proteinuria in Healthy Middle-Aged Men. Clin. J. Am. Soc. Nephrol. 2011, 6, 2462–2469. [Google Scholar] [CrossRef]
- Podzolkov, V.I.; Bragina, A.E.; Druzhinina, N.A.; Vasil’eva, L.V.; Osadchiy, K.K.; Dubchak, A.E.; Khvalin, E.I. Relation between Tobacco Smoking/Electronic Smoking and Albuminuria/Vascular Stiffness in Young People without Cardiovascular Diseases. Kidney Blood Press. Res. 2020, 45, 467–476. [Google Scholar] [CrossRef]
- Halimi, J.-M.; Giraudeau, B.; Vol, S.; Cacès, E.; Nivet, H.; Lebranchu, Y.; Tichet, J. Effects of Current Smoking and Smoking Discontinuation on Renal Function and Proteinuria in the General Population. Kidney Int. 2000, 58, 1285–1292. [Google Scholar] [CrossRef]
- Pinto-Sietsma, S.-J. Smoking Is Related to Albuminuria and Abnormal Renal Function in Nondiabetic Persons. Ann. Intern. Med. 2000, 133, 585. [Google Scholar] [CrossRef]
- Han, W.K.; Bailly, V.; Abichandani, R.; Thadhani, R.; Bonventre, J.V. Kidney Injury Molecule-1 (KIM-1): A Novel Biomarker for Human Renal Proximal Tubule Injury. Kidney Int. 2002, 62, 237–244. [Google Scholar] [CrossRef]
- Soni, S.S.; Cruz, D.; Bobek, I.; Chionh, C.Y.; Nalesso, F.; Lentini, P.; de Cal, M.; Corradi, V.; Virzi, G.; Ronco, C. NGAL: A Biomarker of Acute Kidney Injury and Other Systemic Conditions. Int. Urol. Nephrol. 2010, 42, 141–150. [Google Scholar] [CrossRef]
- Supavekin, S.; Zhang, W.; Kucherlapati, R.; Kaskel, F.J.; Moore, L.C.; Devarajan, P. Differential Gene Expression Following Early Renal Ischemia/Reperfusion. Kidney Int. 2003, 63, 1714–1724. [Google Scholar] [CrossRef]
- Abd El Dayem, S.; El magd El Bohy, A.; El Shehaby, A. Value of the Intrarenal Arterial Resistivity Indices and Different Renal Biomarkers for Early Identification of Diabetic Nephropathy in Type 1 Diabetic Patients. J. Pediatric Endocrinol. Metab. 2016, 29, 273–279. [Google Scholar] [CrossRef]
- de Carvalho, J.A.M.; Tatsch, E.; Hausen, B.S.; Bollick, Y.S.; Moretto, M.B.; Duarte, T.; Duarte, M.M.M.F.; Londero, S.W.K.; Premaor, M.O.; Comim, F.V.; et al. Urinary Kidney Injury Molecule-1 and Neutrophil Gelatinase-Associated Lipocalin as Indicators of Tubular Damage in Normoalbuminuric Patients with Type 2 Diabetes. Clin. Biochem. 2016, 49, 232–236. [Google Scholar] [CrossRef]
- Lacquaniti, A.; Fenga, C.; Venuti, V.A.; Pernice, L.; Catanzariti, S.; Sirna, G.; Pernice, F.; Arena, A.; Lupica, R.; Abbate, C.; et al. Hydrocarbons and Kidney Damage: Potential Use of Neutrophil Gelatinase-Associated Lipocalin and Sister Chromatide Exchange. Am. J. Nephrol. 2012, 35, 271–278. [Google Scholar] [CrossRef]
- Casanova, A.G.; Vicente-Vicente, L.; Hernández-Sánchez, M.T.; Prieto, M.; Rihuete, M.I.; Ramis, L.M.; del Barco, E.; Cruz, J.J.; Ortiz, A.; Cruz-González, I.; et al. Urinary Transferrin Pre-Emptively Identifies the Risk of Renal Damage Posed by Subclinical Tubular Alterations. Biomed. Pharmacother. 2020, 121, 109684. [Google Scholar] [CrossRef]
- Quiros, Y.; Ferreira, L.; Sancho-Martínez, S.M.; González-Buitrago, J.M.; López-Novoa, J.M.; López-Hernández, F.J. Sub-Nephrotoxic Doses of Gentamicin Predispose Animals to Developing Acute Kidney Injury and to Excrete Ganglioside M2 Activator Protein. Kidney Int. 2010, 78, 1006–1015. [Google Scholar] [CrossRef]
- Sancho-Martínez, S.M.; Blanco-Gozalo, V.; Quiros, Y.; Prieto-García, L.; Montero-Gómez, M.J.; Docherty, N.G.; Martínez-Salgado, C.; Morales, A.I.; López-Novoa, J.M.; López-Hernández, F.J. Impaired Tubular Reabsorption Is the Main Mechanism Explaining Increases in Urinary NGAL Excretion Following Acute Kidney Injury in Rats. Toxicol. Sci. 2020, 175, 75–86. [Google Scholar] [CrossRef]
- Vicente-Vicente, L.; Casanova, A.G.; Hernández-Sánchez, M.T.; Prieto, M.; Martínez-Salgado, C.; López-Hernández, F.J.; Cruz-González, I.; Morales, A.I. Albuminuria Pre-Emptively Identifies Cardiac Patients at Risk of Contrast-Induced Nephropathy. J. Clin. Med. 2021, 10, 4942. [Google Scholar] [CrossRef]
- Vicente-Vicente, L.; Sánchez-Juanes, F.; García-Sánchez, O.; Blanco-Gozalo, V.; Pescador, M.; Sevilla, M.A.; González-Buitrago, J.M.; López-Hernández, F.J.; López-Novoa, J.M.; Morales, A.I. Sub-Nephrotoxic Cisplatin Sensitizes Rats to Acute Renal Failure and Increases Urinary Excretion of Fumarylacetoacetase. Toxicol. Lett. 2015, 234, 99–109. [Google Scholar] [CrossRef] [PubMed]
- Vicente-Vicente, L.; Ferreira, L.; González-Buitrago, J.M.; López-Hernández, F.J.; López-Novoa, J.M.; Morales, A.I. Increased Urinary Excretion of Albumin, Hemopexin, Transferrin and VDBP Correlates with Chronic Sensitization to Gentamicin Nephrotoxicity in Rats. Toxicology 2013, 304, 83–91. [Google Scholar] [CrossRef] [PubMed]
- Domrongkitchaiporn, S.; Sritara, P.; Kitiyakara, C.; Stitchantrakul, W.; Krittaphol, V.; Lolekha, P.; Cheepudomwit, S.; Yipintsoi, T. Risk Factors for Development of Decreased Kidney Function in a Southeast Asian Population: A 12-Year Cohort Study. J. Am. Soc. Nephrol. 2005, 16, 791–799. [Google Scholar] [CrossRef]
- Funk, J.A.; Schnellmann, R.G. Persistent Disruption of Mitochondrial Homeostasis after Acute Kidney Injury. Am. J. Physiol. Ren. Physiol. 2012, 302, F853–F864. [Google Scholar] [CrossRef]
- Haroun, M.K.; Jaar, B.G.; Hoffman, S.C.; Comstock, G.W.; Klag, M.J.; Coresh, J. Risk Factors for Chronic Kidney Disease: A Prospective Study of 23,534 Men and Women in Washington County, Maryland. J. Am. Soc. Nephrol. 2003, 14, 2934–2941. [Google Scholar] [CrossRef]
- Noronha-Dutra, A.A.; Epperlein, M.M.; Woolf, N. Effect of Cigarette Smoking on Cultured Human Endothelial Cells. Cardiovasc. Res. 1993, 27, 774–778. [Google Scholar] [CrossRef]
- Mimić-Oka, J.; Simić, T.; Djukanović, L.; Reljić, Z.; Davicević, Z. Alteration in Plasma Antioxidant Capacity in Various Degrees of Chronic Renal Failure. Clin. Nephrol. 1999, 51, 233–241. [Google Scholar]
- Lee, T.H.; Chen, J.-J.; Wu, C.-Y.; Yang, C.-W.; Yang, H.-Y. Hyperuricemia and Progression of Chronic Kidney Disease: A Review from Physiology and Pathogenesis to the Role of Urate-Lowering Therapy. Diagnostics 2021, 11, 1674. [Google Scholar] [CrossRef]
- Sawicki, P.T.; Didjurgeit, U.; Mühlhauser, I.; Bender, R.; Heinemann, L.; Berger, M. Smoking Is Associated with Progression of Diabetic Nephropathy. Diabetes Care 1994, 17, 126–131. [Google Scholar] [CrossRef]
- Pinto-Sietsma, S.J.; Janssen, W.M.; Hillege, H.L.; Navis, G.; De Zeeuw, D.; De Jong, P.E. Urinary Albumin Excretion Is Associated with Renal Functional Abnormalities in a Nondiabetic Population. J. Am. Soc. Nephrol. 2000, 11, 1882–1888. [Google Scholar] [CrossRef]

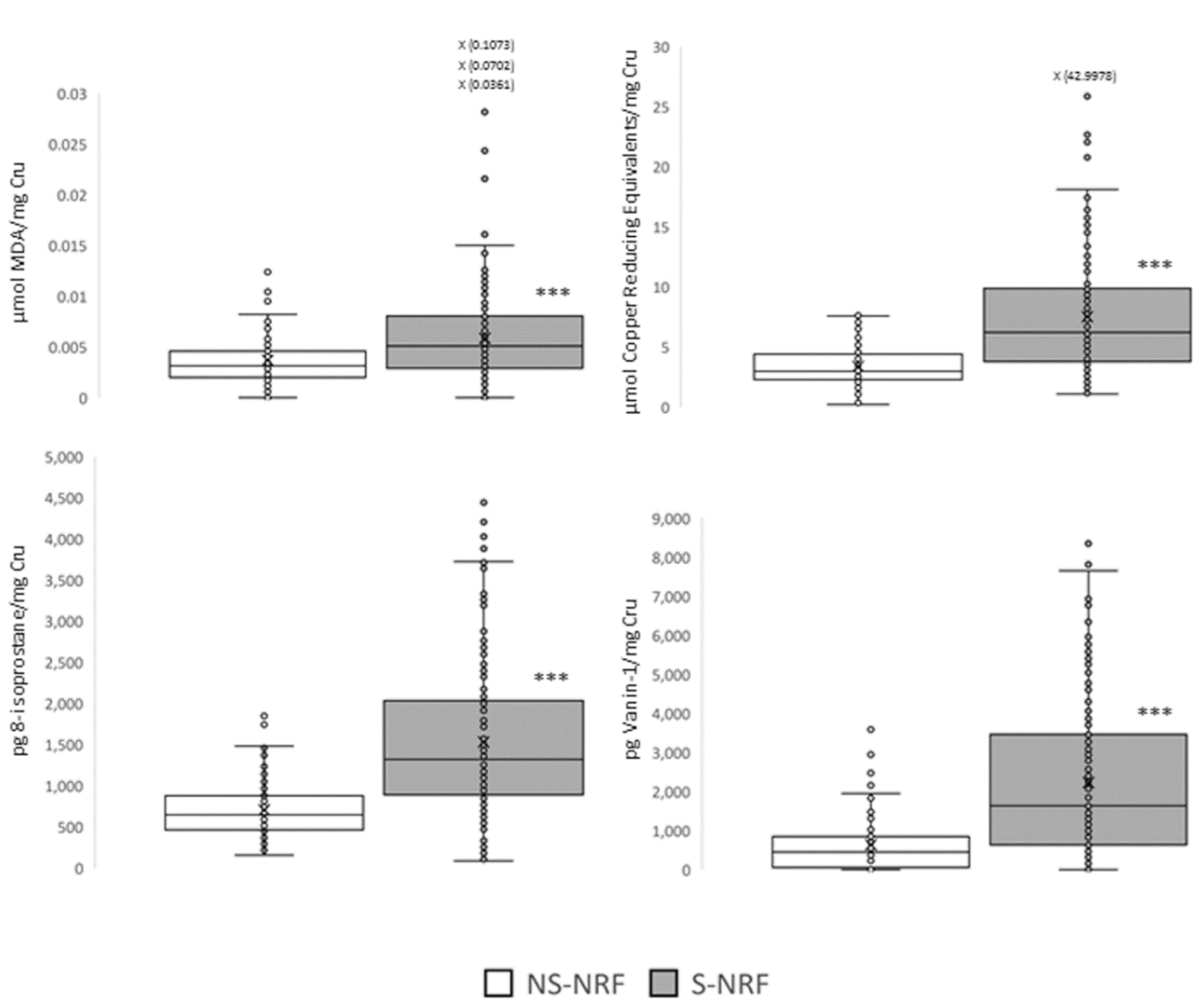
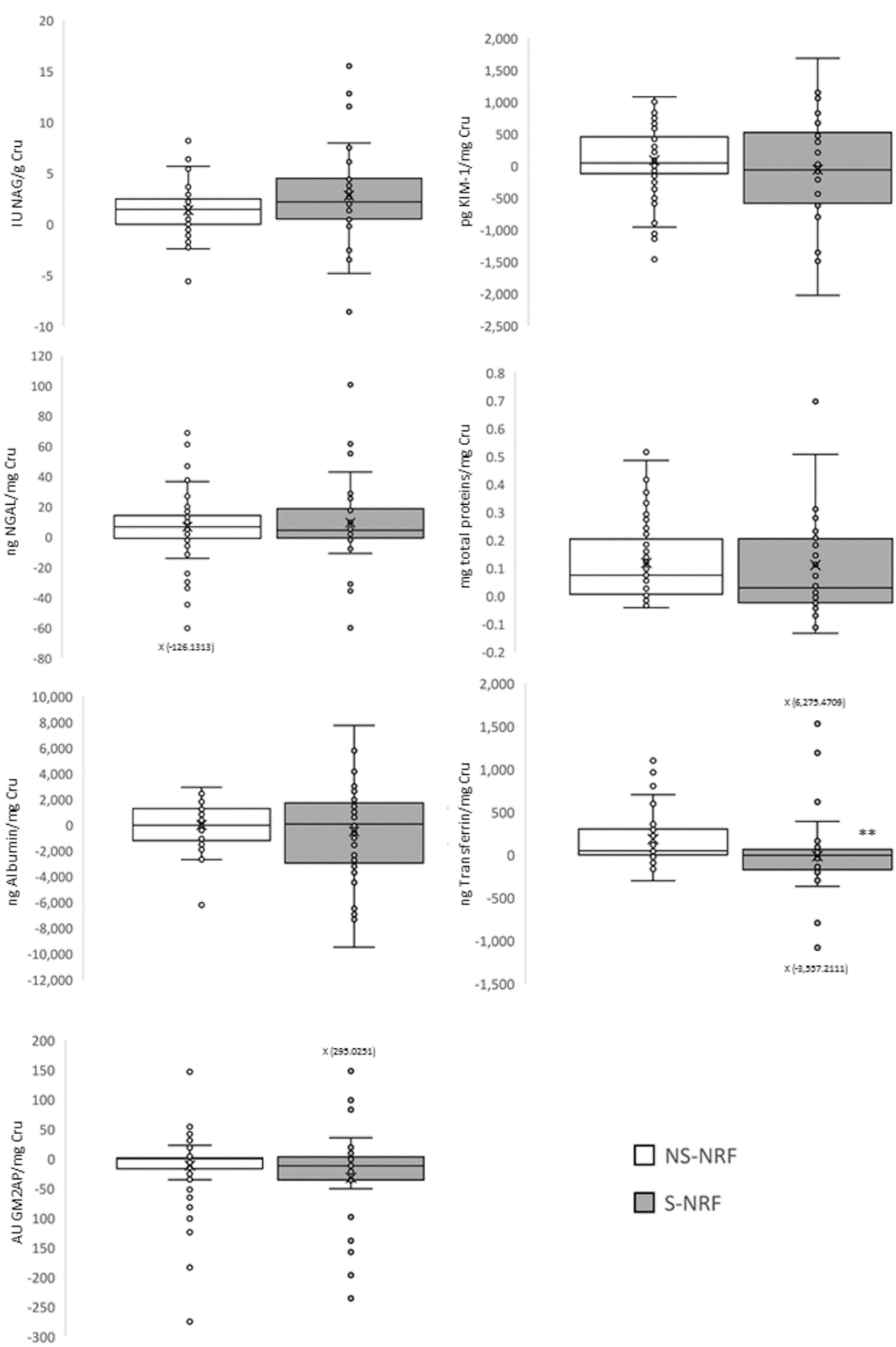
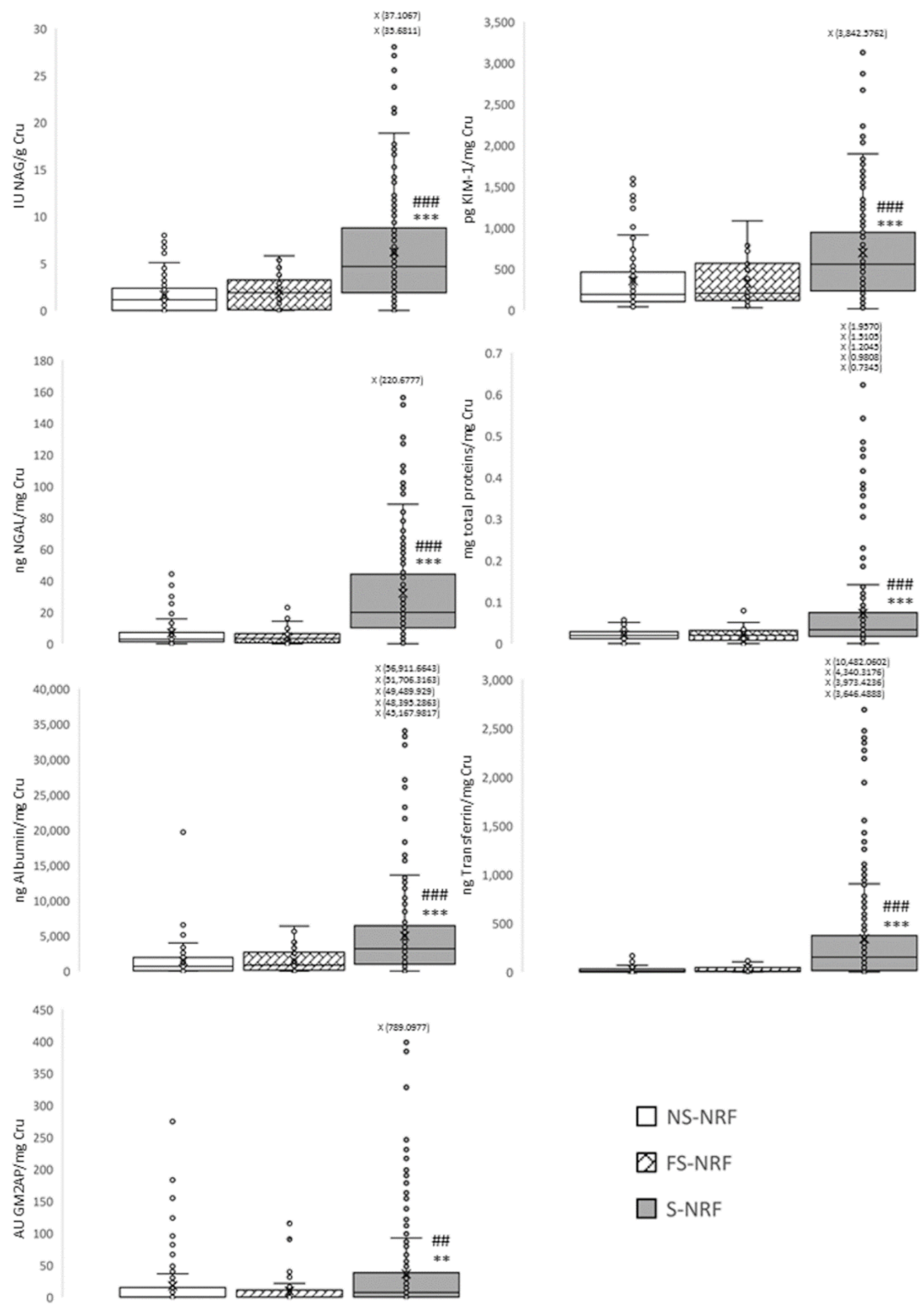
| Group | NS-NRF | NS-RF | S-NRF | S-RF | FS-NRF |
|---|---|---|---|---|---|
| Smoker | X | X | |||
| Former Smoker | X | ||||
| Risk factor | X | X |
| Non-Smokers | Smokers | Former Smokers | |||
|---|---|---|---|---|---|
| No Risk Factors (n = 105) | Risk Factors (n = 80) | No Risk Factors (n = 194) | Risk Factors (n = 114) | No Risk Factors (n = 46) | |
| Group Name | NS-NRF | NS-RF | S-NRF | S-RF | FS-NRF |
| Gender (female/male %) | 43.8/56.2 a | 51.2/48.8 a | 53.6/46.4 a | 39.5/60.5 a | 63.0/37.0 a |
| Age (years, mean ± SEM) | 53.28 ± 1.40 | 67.44 ± 1.18 *** | 49.76 ± 0.77 ### | 57.35 ± 0.96 ###&&& | 54.83 ± 1.57 ### |
| Weight (kg, mean ± SEM) | 72.35 ± 1.23 | 71.41 ± 1.28 | 72.62 ± 1.18 | 77.69 ± 1.42 *# | 71.97 ± 2.05 |
| Height (cm, mean ± SEM) | 166.55 ± 0.92 | 160.88 ± 1.13 ** | 166.28 ± 0.64 ## | 164.88 ± 0.85 | 166.02 ± 1.04 |
| BMI (kg/m2, mean ± SEM) | 26.05 ± 0.39 | 27.55 ± 0.37 | 26.11 ± 0.36 # | 28.41 ± 0.45 **&&& | 26.00 ± 0.60 $$$ |
| Plasma creatinine (mg/dL) | 0.83 ± 0.02 | 0.80 ± 0.02 | 0.79 ± 0.01 | 0.82 ± 0.02 | 0.81 ± 0.02 |
| eGFR CKD-EPI (mL/min/1.73 m2, mean ± SEM) | 94.03 ± 1.37 | 86.11 ± 1.49 ** | 97.14 ± 1.06 | 91.90 ± 1.44 # | 91.11 ± 1.76 |
| Previous kidney disease (%) | 0 a | 0 a | 0 a | 9.8 b | 0 a |
| Diabetes mellitus (%) | 0 a | 17.5 b | 0 a | 36.8 c | 0 a |
| Arterial hypertension (%) | 0 a | 87.5 b | 0 a | 64.9 c | 0 a |
| NSAIDs (%) | 0 a | 6.2 b | 0 a | 30.7 c | 0 a |
| Number of cigarettes per day (n, mean ± SEM) | n.a. | n.a. | 16.81 ± 0.66 | 14.78 ± 0.84 & | n.a. |
| Urinary cotinine (mean ± SEM) | 5.65 ± 2.81 | 3.42 ± 0.68 ** | 5289.83 ± 350.40 ### | 2413.73 ± 276.75 ***###&&& | 9.36 ± 7.82 #&&&$$$ |
| Urinary Biomarker | NAG | KIM-1 | NGAL | Total Proteins | Albumin | Transferrin | GM2AP |
|---|---|---|---|---|---|---|---|
| Cotinine | 0.488 *** | 0.366 *** | 0.473 *** | 0.360 *** | 0.447 *** | 0.411 *** | 0.218 *** |
| Urinary Biomarker | 8-Isoprostane | Vanin-1 | MDA | TAC |
|---|---|---|---|---|
| Cotinine | 0.592 *** | 0.524 *** | 0.472 *** | 0.569 *** |
| NAG | 0.142 * | 0.227 *** | 0.163 ** | 0.240 *** |
| KIM-1 | 0.243 *** | 0.301 *** | 0.192 *** | 0.238 *** |
| NGAL | 0.108 | 0.303 *** | 0.200 *** | 0.349 *** |
| Total proteins | 0.162 ** | 0.271 *** | 0.196 *** | 0.208 *** |
| Albumin | 0.142 * | 0.208 *** | 0.196 *** | 0.213 *** |
| Transferrin | 0.250 *** | 0.262 *** | 0.181 ** | 0.194 *** |
| GM2AP | 0.159 ** | −0.009 | 0.054 | 0.159 ** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tascón, J.; Prieto, M.; Casanova, A.G.; Sanz, F.J.; Hernández Mezquita, M.A.; Barrueco Ferrero, M.; Gomez-Marcos, M.A.; Garcia-Ortiz, L.; Vicente-Vicente, L.; Morales, A.I.; et al. Early Diagnosis of Kidney Damage Associated with Tobacco Use: Preventive Application. J. Pers. Med. 2022, 12, 1032. https://doi.org/10.3390/jpm12071032
Tascón J, Prieto M, Casanova AG, Sanz FJ, Hernández Mezquita MA, Barrueco Ferrero M, Gomez-Marcos MA, Garcia-Ortiz L, Vicente-Vicente L, Morales AI, et al. Early Diagnosis of Kidney Damage Associated with Tobacco Use: Preventive Application. Journal of Personalized Medicine. 2022; 12(7):1032. https://doi.org/10.3390/jpm12071032
Chicago/Turabian StyleTascón, Javier, Marta Prieto, Alfredo G. Casanova, Francisco J. Sanz, Miguel A. Hernández Mezquita, Miguel Barrueco Ferrero, Manuel A. Gomez-Marcos, Luis Garcia-Ortiz, Laura Vicente-Vicente, Ana I. Morales, and et al. 2022. "Early Diagnosis of Kidney Damage Associated with Tobacco Use: Preventive Application" Journal of Personalized Medicine 12, no. 7: 1032. https://doi.org/10.3390/jpm12071032
APA StyleTascón, J., Prieto, M., Casanova, A. G., Sanz, F. J., Hernández Mezquita, M. A., Barrueco Ferrero, M., Gomez-Marcos, M. A., Garcia-Ortiz, L., Vicente-Vicente, L., Morales, A. I., & on behalf of BIOTAB Team. (2022). Early Diagnosis of Kidney Damage Associated with Tobacco Use: Preventive Application. Journal of Personalized Medicine, 12(7), 1032. https://doi.org/10.3390/jpm12071032








