Contribution of Hypoalbuminemia and Anemia to the Prognostic Value of Plasma p-Cresyl Sulfate and p-Cresyl Glucuronide for Cardiovascular Outcome in Chronic Kidney Disease
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Laboratory Analysis
2.3. Statistical Analysis
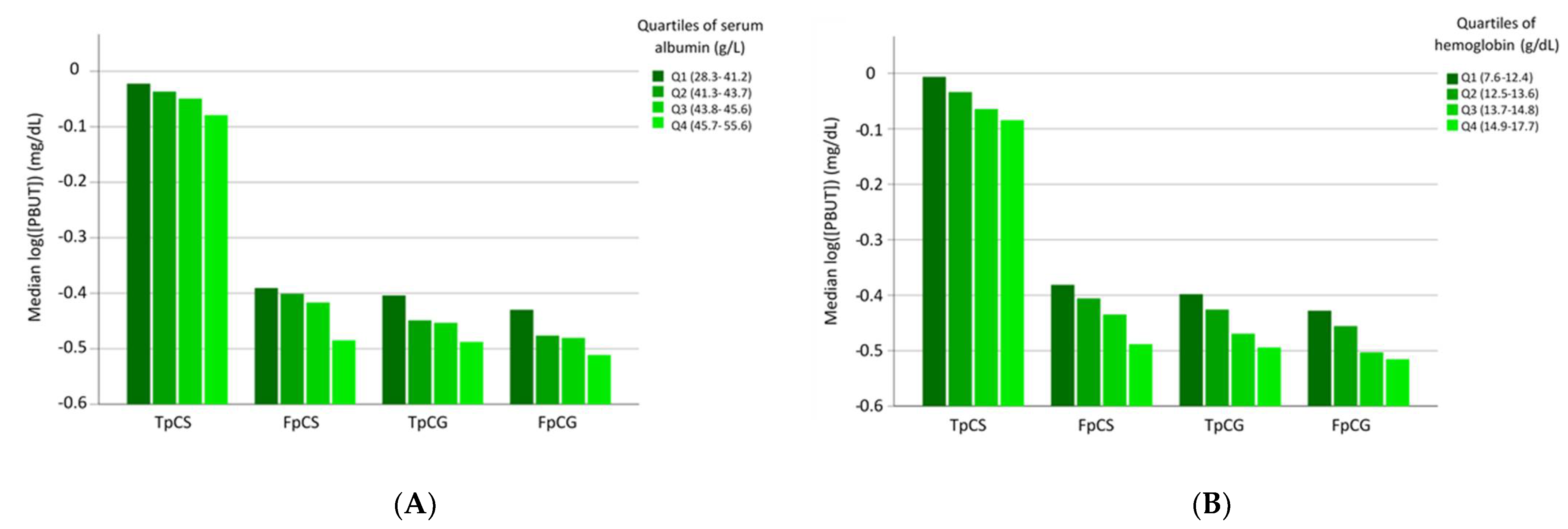
3. Results
3.1. Correlation Studies
3.2. Outcome
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vanholder, R.; Pletinck, A.; Schepers, E.; Glorieux, G. Biochemical and Clinical Impact of Organic Uremic Retention Solutes: A Comprehensive Update. Toxins 2018, 10, 33. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gryp, T.; Vanholder, R.; Vaneechoutte, M.; Glorieux, G. p-Cresyl Sulfate. Toxins 2017, 9, 52. [Google Scholar] [CrossRef] [Green Version]
- Glorieux, G.; Vanholder, R.; Van Biesen, W.; Pletinck, A.; Schepers, E.; Neirynck, N.; Speeckaert, M.; De Bacquer, D.; Verbeke, F. Free p-cresyl sulfate shows the highest association with cardiovascular outcome in chronic kidney disease. Nephrol. Dial. Transpl. 2021, 36, 998–1005. [Google Scholar] [CrossRef] [PubMed]
- Deltombe, O.; Glorieux, G.; Marzouki, S.; Masereeuw, R.; Schneditz, D.; Eloot, S. Selective Transport of Protein-Bound Uremic Toxins in Erythrocytes. Toxins 2019, 11, 385. [Google Scholar] [CrossRef] [Green Version]
- Dati, F.; Schumann, G.; Thomas, L.; Aguzzi, F.; Baudner, S.; Bienvenu, J.; Blaabjerg, O.; Blirup-Jensen, S.; Carlstrom, A.; Petersen, P.H.; et al. Consensus of a group of professional societies and diagnostic companies on guidelines for interim reference ranges for 14 proteins in serum based on the standardization against the IFCC/BCR/CAP Reference Material (CRM 470). International Federation of Clinical Chemistry. Community Bureau of Reference of the Commission of the European Communities. College of American Pathologists. Eur. J. Clin. Chem. Clin. Biochem. 1996, 34, 517–520. [Google Scholar]
- Barreto, F.C.; Barreto, D.V.; Liabeuf, S.; Meert, N.; Glorieux, G.; Temmar, M.; Choukroun, G.; Vanholder, R.; Massy, Z.A.; European Uremic Toxin Work, G. Serum indoxyl sulfate is associated with vascular disease and mortality in chronic kidney disease patients. Clin. J. Am. Soc. Nephrol. 2009, 4, 1551–1558. [Google Scholar] [CrossRef] [Green Version]
- Bammens, B.; Evenepoel, P.; Keuleers, H.; Verbeke, K.; Vanrenterghem, Y. Free serum concentrations of the protein-bound retention solute p-cresol predict mortality in hemodialysis patients. Kidney Int. 2006, 69, 1081–1087. [Google Scholar] [CrossRef]
- De Smet, R.; Van Kaer, J.; Liebich, H.; Lesaffer, G.; Verstraete, A.; Dhondt, A.; Duym, P.; Lameire, N.; Vanholder, R. Heparin-induced release of protein-bound solutes during hemodialysis is an in vitro artifact. Clin. Chem. 2001, 47, 901–909. [Google Scholar]
- Evenepoel, P.; Glorieux, G.; Meijers, B. p-cresol sulfate and indoxyl sulfate: Some clouds are gathering in the uremic toxin sky. Kidney Int. 2017, 92, 1323–1324. [Google Scholar] [CrossRef]
- Shafi, T.; Sirich, T.L.; Meyer, T.W.; Hostetter, T.H.; Plummer, N.S.; Hwang, S.; Melamed, M.L.; Banerjee, T.; Coresh, J.; Powe, N.R. Results of the HEMO Study suggest that p-cresol sulfate and indoxyl sulfate are not associated with cardiovascular outcomes. Kidney Int. 2017, 92, 1484–1492. [Google Scholar] [CrossRef]
- Liabeuf, S.; Barreto, D.V.; Barreto, F.C.; Meert, N.; Glorieux, G.; Schepers, E.; Temmar, M.; Choukroun, G.; Vanholder, R.; Massy, Z.A.; et al. Free p-cresylsulphate is a predictor of mortality in patients at different stages of chronic kidney disease. Nephrol. Dial. Transpl. 2010, 25, 1183–1191. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grossman, Y.; Barbash, I.M.; Fefer, P.; Goldenberg, I.; Berkovitch, A.; Regev, E.; Fink, N.; Ben-Zekry, S.; Brodov, Y.; Kogan, A.; et al. Addition of albumin to Traditional Risk Score Improved Prediction of Mortality in Individuals Undergoing Transcatheter Aortic Valve Replacement. J. Am. Geriatr. Soc. 2017, 65, 2413–2417. [Google Scholar] [CrossRef] [PubMed]
- Phillips, A.; Shaper, A.G.; Whincup, P.H. Association between serum albumin and mortality from cardiovascular disease, cancer, and other causes. Lancet 1989, 2, 1434–1436. [Google Scholar] [CrossRef]
- Shannon, C.M.; Ballew, S.H.; Daya, N.; Zhou, L.; Chang, A.R.; Sang, Y.; Coresh, J.; Selvin, E.; Grams, M.E. Serum albumin and risks of hospitalization and death: Findings from the Atherosclerosis Risk in Communities study. J. Am. Geriatr. Soc. 2021, 69, 2865–2876. [Google Scholar] [CrossRef]
- Foley, R.N.; Parfrey, P.S.; Harnett, J.D.; Kent, G.M.; Murray, D.C.; Barre, P.E. Hypoalbuminemia, cardiac morbidity, and mortality in end-stage renal disease. J. Am. Soc. Nephrol. 1996, 7, 728–736. [Google Scholar] [CrossRef] [PubMed]
- Leavey, S.F.; Strawderman, R.L.; Jones, C.A.; Port, F.K.; Held, P.J. Simple nutritional indicators as independent predictors of mortality in hemodialysis patients. Am. J. Kidney Dis. 1998, 31, 997–1006. [Google Scholar] [CrossRef]
- Lowrie, E.G.; Huang, W.H.; Lew, N.L. Death risk predictors among peritoneal dialysis and hemodialysis patients: A preliminary comparison. Am. J. Kidney. Dis. 1995, 26, 220–228. [Google Scholar] [CrossRef]
- Owen, W.F., Jr.; Lew, N.L.; Liu, Y.; Lowrie, E.G.; Lazarus, J.M. The urea reduction ratio and serum albumin concentration as predictors of mortality in patients undergoing hemodialysis. N. Engl. J. Med. 1993, 329, 1001–1006. [Google Scholar] [CrossRef]
- Soucie, J.M.; McClellan, W.M. Early death in dialysis patients: Risk factors and impact on incidence and mortality rates. J. Am. Soc. Nephrol. 1996, 7, 2169–2175. [Google Scholar] [CrossRef]
- Delliere, S.; Cynober, L. Is transthyretin a good marker of nutritional status? Clin. Nutr. 2017, 36, 364–370. [Google Scholar] [CrossRef]
- Oreopoulos, A.; Ezekowitz, J.A.; McAlister, F.A.; Kalantar-Zadeh, K.; Fonarow, G.C.; Norris, C.M.; Johnson, J.A.; Padwal, R.S. Association between direct measures of body composition and prognostic factors in chronic heart failure. Mayo Clin. Proc. 2010, 85, 609–617. [Google Scholar] [CrossRef] [Green Version]
- Joorgensen, K.A.; Stoffersen, E. Heparin like activity of albumin. Thromb. Res. 1979, 16, 569–574. [Google Scholar] [CrossRef]
- Jorgensen, K.A.; Stoffersen, E. On the inhibitory effect of albumin on platelet aggregation. Thromb. Res. 1980, 17, 13–18. [Google Scholar] [CrossRef]
- Joles, J.A.; Willekes-Koolschijn, N.; Koomans, H.A. Hypoalbuminemia causes high blood viscosity by increasing red cell lysophosphatidylcholine. Kidney Int. 1997, 52, 761–770. [Google Scholar] [CrossRef] [Green Version]
- Zhang, W.J.; Frei, B. Albumin selectively inhibits TNF alpha-induced expression of vascular cell adhesion molecule-1 in human aortic endothelial cells. Cardiovasc. Res. 2002, 55, 820–829. [Google Scholar] [CrossRef]
- Chien, S.C.; Chen, C.Y.; Lin, C.F.; Yeh, H.I. Critical appraisal of the role of serum albumin in cardiovascular disease. Biomark. Res. 2017, 5, 31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| PBUT | Correlation Coefficients | |
|---|---|---|
| Albumin | Hemoglobin | |
| TpCS | −0.157 | −0.284 |
| FpCS | −0.250 | −0.319 |
| TpCG | −0.163 | −0.271 |
| FpCG | −0.192 | −0.270 |
| Factor | Beta Coefficient | 95% CI | p |
|---|---|---|---|
| (Constant) | −2.297; −0.488 | 0.003 | |
| Hemoglobin (g/dL) | −0.149 | −1.133; −0.040 | <0.001 |
| Albumin (g/L) | −0.059 | −0.039; 0.005 | 0.14 |
| eGFR (mL/min/1.73 m2) | −0.501 | −0.021; −0.016 | <0.001 |
| Variable | HR | 95% CI | p |
|---|---|---|---|
| Age (1 SD) | 1.70 | 1.34; 2.18 | <0.001 |
| Sex (male = 1) | 1.81 | 1.27; 2.57 | 0.001 |
| Diabetes (yes = 1) | 2.65 | 1.89; 3.71 | <0.001 |
| SBP (1 SD) | 1.36 | 1.16; 1.60 | <0.001 |
| Log(FpCS) (1 SD) | 1.37 | 1.14; 1.65 | 0.001 |
| Albumin (1 SD) | 0.63 | 0.53; 0.75 | <0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Verbeke, F.; Vanholder, R.; Van Biesen, W.; Glorieux, G. Contribution of Hypoalbuminemia and Anemia to the Prognostic Value of Plasma p-Cresyl Sulfate and p-Cresyl Glucuronide for Cardiovascular Outcome in Chronic Kidney Disease. J. Pers. Med. 2022, 12, 1239. https://doi.org/10.3390/jpm12081239
Verbeke F, Vanholder R, Van Biesen W, Glorieux G. Contribution of Hypoalbuminemia and Anemia to the Prognostic Value of Plasma p-Cresyl Sulfate and p-Cresyl Glucuronide for Cardiovascular Outcome in Chronic Kidney Disease. Journal of Personalized Medicine. 2022; 12(8):1239. https://doi.org/10.3390/jpm12081239
Chicago/Turabian StyleVerbeke, Francis, Raymond Vanholder, Wim Van Biesen, and Griet Glorieux. 2022. "Contribution of Hypoalbuminemia and Anemia to the Prognostic Value of Plasma p-Cresyl Sulfate and p-Cresyl Glucuronide for Cardiovascular Outcome in Chronic Kidney Disease" Journal of Personalized Medicine 12, no. 8: 1239. https://doi.org/10.3390/jpm12081239
APA StyleVerbeke, F., Vanholder, R., Van Biesen, W., & Glorieux, G. (2022). Contribution of Hypoalbuminemia and Anemia to the Prognostic Value of Plasma p-Cresyl Sulfate and p-Cresyl Glucuronide for Cardiovascular Outcome in Chronic Kidney Disease. Journal of Personalized Medicine, 12(8), 1239. https://doi.org/10.3390/jpm12081239








