Management with Santorini’s Plexus Should Be Personalized during Prostatectomy
Abstract
:1. Introduction
2. Methods
2.1. Patients and General Characterization
2.2. Functional Evaluation
2.2.1. Erectile Function
2.2.2. Continence
3. Statistical Methods
4. Results
5. Complications
6. Histology
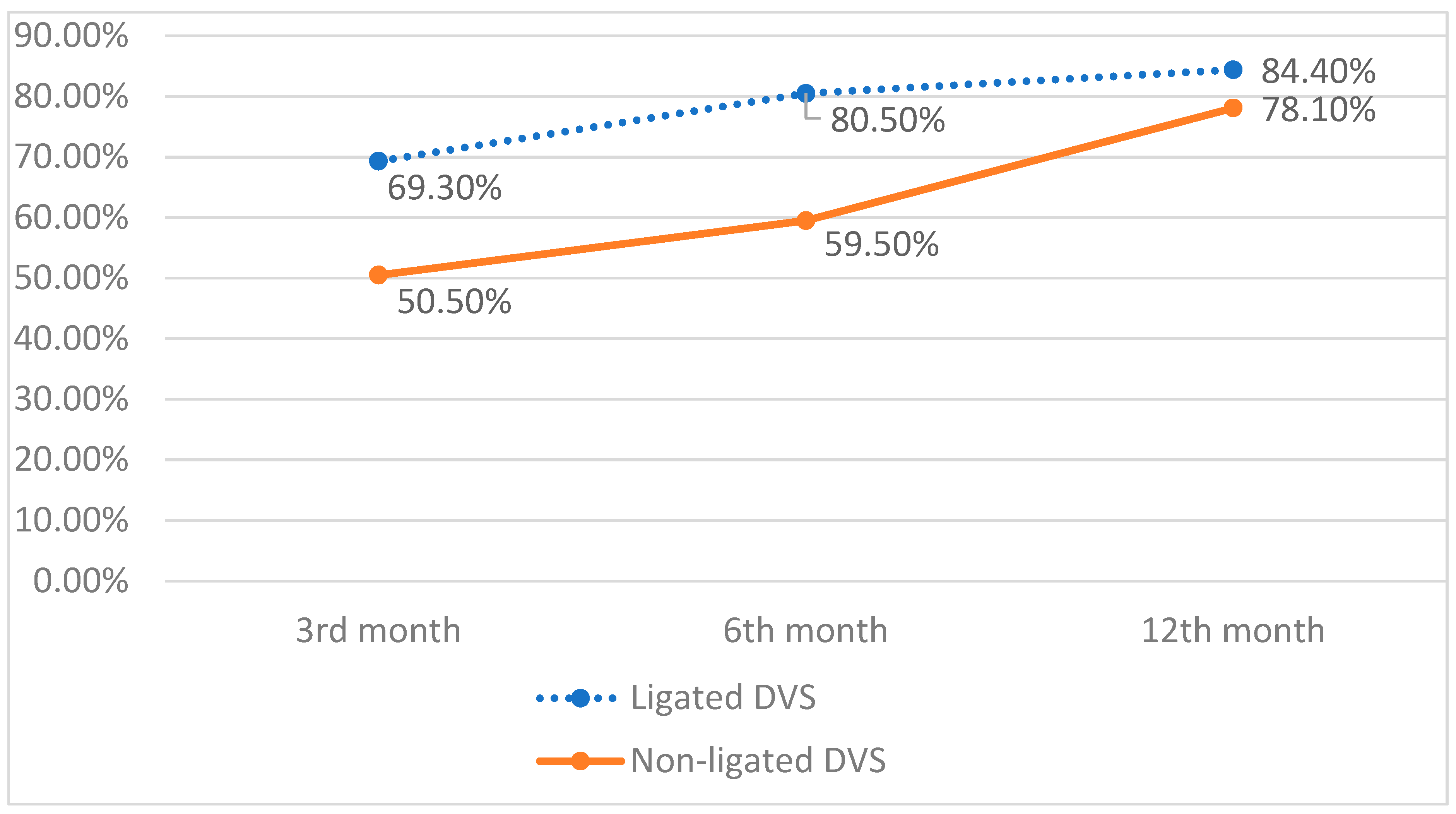
7. Continence
8. Erectile Function
9. Discussion
10. Limitation and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Rassweiler, J.J.; Teber, D. Advances in laparoscopic surgery in urology. Nat. Rev. Urol. 2016, 13, 387–399. [Google Scholar] [CrossRef]
- Wang, F.; Rudin, C.; McCormick, T.H.; Gore, J.L. Modeling recovery curves with application to prostatectomy. Biostatistics 2019, 20, 549–564. [Google Scholar] [CrossRef] [PubMed]
- Power, N.E.; Silberstein, J.L.; Kulkarni, G.S.; Laudone, V.P. The dorsal venous complex (DVC): Dorsal venous or dorsal vasculature complex? Santorini’s plexus revisited. BJU Int. 2011, 108, 930–932. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Curto, F.; Benijts, J.; Pansadoro, A.; Barmoshe, S.; Hoepffner, J.; Mugnier, C.; Piechaud, T.; Gaston, R. Nerve Sparing Laparoscopic Radical Prostatectomy: Our Technique. Eur. Urol. 2006, 49, 344–352. [Google Scholar] [CrossRef] [PubMed]
- Sharma, N.L.; Shah, N.C.; Neal, D.E. Robotic-assisted laparoscopic prostatectomy. Br. J. Cancer 2009, 101, 1491–1496. [Google Scholar] [CrossRef] [PubMed]
- Cristini, C.; di Pierro, G.B.; Leonardo, C.; de Nunzio, C.; Franco, G. Safe digital isolation of the santorini plexus during radical retropubic prostatectomy. BMC Urol. 2013, 13, 13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guru, K.A.; Perlmutter, A.E.; Sheldon, M.J.; Butt, Z.M.; Zhang, S.; Tan, W.; Wilding, G.; Kim, H.L.; Mohler, J.L. Apical Margins after Robot-Assisted Radical Prostatectomy: Does Technique Matter? J. Endourol. 2009, 23, 123–127. [Google Scholar] [CrossRef]
- Antonelli, A.; Palumbo, C.; Veccia, A.; Fisogni, S.; Zamboni, S.; Furlan, M.; Francavilla, S.; Lattarulo, M.; De Marzo, E.; Mirabella, G.; et al. Standard vs delayed ligature of the dorsal vascular complex during robot-assisted radical prostatectomy: Results from a randomized controlled trial. J. Robot. Surg. 2019, 13, 253–260. [Google Scholar] [CrossRef] [Green Version]
- Hruza, M.; Weiß, H.O.; Pini, G.; Goezen, A.S.; Schulze, M.; Teber, D.; Rassweiler, J.J. Complications in 2200 Consecutive Laparoscopic Radical Prostatectomies: Standardised Evaluation and Analysis of Learning Curves. Eur. Urol. 2010, 58, 733–741. [Google Scholar] [CrossRef]
- Jarzemski, P.; Listopadzki, S.; Słupski, P.; Jarzemski, M.; Brzoszczyk, B.; Sosnowski, R. Laparoscopic radical prostatectomy and extended pelvic lymph node dissection: A combined technique. Videosurg. Other Miniinvasive Tech. 2020, 15, 192–198. [Google Scholar] [CrossRef]
- Porpiglia, F.; Fiori, C.; Grande, S.; Morra, I.; Scarpa, R.M. Selective versus Standard Ligature of the Deep Venous Complex during Laparoscopic Radical Prostatectomy: Effects on Continence, Blood Loss, and Margin Status. Eur. Urol. 2009, 55, 1377–1385. [Google Scholar] [CrossRef] [PubMed]
- Kessler, T.M.; Burkhard, F.C.; Studer, U.E. Nerve-Sparing Open Radical Retropubic Prostatectomy. Eur. Urol. 2007, 51, 90–97. [Google Scholar] [CrossRef] [PubMed]
- Biso, G.M.N.R.; Munakomi, S. Neuroanatomy, Neurapraxia. StatPearls, 30 October 2021. Available online: https://www.ncbi.nlm.nih.gov/books/NBK557746/ (accessed on 18 March 2022).
- Karam, I.; Droupy, S.; Abd-Alsamad, I.; Korbage, A.; Uhl, J.; Benoit, G.; Delmas, V. The Precise Location and Nature of the Nerves to the Male Human Urethra: Histological and Immunohistochemical Studies with Three-Dimensional Reconstruction. Eur. Urol. 2005, 48, 858–864. [Google Scholar] [CrossRef] [PubMed]
- Hoshi, A.; Usui, Y.; Shimizu, Y.; Tomonaga, T.; Kawakami, M.; Nakajima, N.; Hanai, K.; Nomoto, T.; Terachi, T. Dorsal vein complex preserving technique for intrafascial nerve-sparing laparoscopic radical prostatectomy. Int. J. Urol. Off. J. Jpn. Urol. Assoc. 2013, 20, 493–500. [Google Scholar] [CrossRef]
- Feng, T.; Heulitt, G.; Lee, J.J.; Liao, M.; Li, H.F.; Porter, J.R. Randomised comparison of techniques for control of the dorsal venous complex during robot-assisted laparoscopic radical prostatectomy. BJU Int. 2020, 126, 586–594. [Google Scholar] [CrossRef]
- Dalela, D.; Jeong, W.; Prasad, M.A.; Sood, A.; Abdollah, F.; Diaz, M.; Karabon, P.; Sammon, J.; Jamil, M.; Baize, B.; et al. A Pragmatic Randomized Controlled Trial Examining the Impact of the Retzius-sparing Approach on Early Urinary Continence Recovery After Robot-assisted Radical Prostatectomy. Eur. Urol. 2017, 72, 677–685. [Google Scholar] [CrossRef]
- Poulakis, V.; Skriapas, K.; de Vries, R.; Dillenburg, W.; Witzsch, U.; Becht, E. Vesicourethral anastomosis during endoscopic extraperitoneal radical prostatectomy: A prospective comparison between the single-knot running and interrupted technique. Urology 2006, 68, 1284–1289. [Google Scholar] [CrossRef]
- Lima, T.F.N.; Bitran, J.; Frech, F.S.; Ramasamy, R. Prevalence of post-prostatectomy erectile dysfunction and a review of the recommended therapeutic modalities. Int. J. Impot. Res. 2020, 33, 401–409. [Google Scholar] [CrossRef]
- Tissot, S.J.; Costello, A.J. Potency outcomes after robot-assisted radical prostatectomy. Nat. Rev. Urol. 2021, 19, 195–196. [Google Scholar] [CrossRef]
- Mandhani, A. Prostatic fascia and recovery of sexual function after radical prostatectomy: Is it a “Veil of Aphrodite” or “Veil of mystery”! Indian J. Urol. IJU J. Urol. Soc. India 2009, 25, 146. [Google Scholar] [CrossRef]
- Lei, Y.; Alemozaffar, M.; Williams, S.B.; Hevelone, N.; Lipsitz, S.R.; Plaster, B.A.; Amarasekera, C.A.; Ulmer, W.D.; Huang, A.C.; Kowalczyk, K.J.; et al. Athermal Division and Selective Suture Ligation of the Dorsal Vein Complex During Robot-Assisted Laparoscopic Radical Prostatectomy: Description of Technique and Outcomes. Eur. Urol. 2011, 59, 235–243. [Google Scholar] [CrossRef] [PubMed]
- Basourakos, S.P.; Kowalczyk, K.J.; Moschovas, M.C.; Dudley, V.; Hung, A.J.; Shoag, J.E.; Patel, V.; Hu, J.C. Robot-Assisted Radical Prostatectomy Maneuvers to Attenuate Erectile Dysfunction: Technical Description and Video Compilation. J. Endourol. 2021, 35, 1601–1609. [Google Scholar] [CrossRef] [PubMed]
- Bader, P.; Hugonnet, C.L.; Burkhard, F.C.; Studer, U.E. Inefficient urethral milking secondary to urethral dysfunction as an additional risk factor for incontinence after radical prostatectomy. J. Urol. 2001, 166, 2247–2252. [Google Scholar] [CrossRef]
- Ferrara, V.; Giannubilo, W.; Azizi, B.; Scaldazza, C.V.; Garritano, A. Laparoscopic radical prostatectomy without ligation of the Santorini’s venous plexus. Urologia 2010, 77, 57–62. [Google Scholar] [CrossRef] [PubMed]
- ED Can Improve Years After Prostate Surgery. Available online: https://www.webmd.com/prostate-cancer/news/20100601/ed-can-improve-years-after-prostate-surgery (accessed on 18 March 2022).

| All Patients n = 415 (%) | Ligated DVC n = 205 (%) | Non-Ligated DVC n = 210 (%) | p-Value | |
|---|---|---|---|---|
| ISUP/Gleason grade group, | 0.439 | |||
| ISUP 1/Gleason 6 | 246 (59.3) | 120 (58.5) | 126 (60) | |
| ISUP 2/Gleason 7 (3 + 4) | 82 (19.8) | 42 (20.5) | 40 (19) | |
| ISUP 3/Gleason 7 (4 + 3) | 40 (9.6) | 19 (9.3) | 21 (10) | |
| ISUP 4/Gleason 8 | 40 (9.6) | 22 (10.7) | 18 (8.6) | |
| ISUP 5/Gleason 9 i 10 | 7 (1.7) | 2 (1) | 5 (2.4) | |
| PSA (ng) | 0.513 | |||
| min. max. | 1.5–94 | 1.65–94 | 1.5–90 | |
| median | 12.03 | 12.12 | 11.94 | |
| ≤10 | 254 (61.2) | 123 (60) | 131 (62.4) | |
| 10, 1–20 | 112 (27) | 54 (26.3) | 58 (27.6) | |
| >20 | 49 (11.8) | 28 (13.7) | 21 (10) | |
| Cancer stage | 0.439 | |||
| cT1a-b | 0 (0) | 0 (0) | 0 (0) | |
| cT1c-T2 | 377 (90.8) | 189 (92.2) | 188 (89.5) | |
| cT3 | 38 (9.2)) | 16 (7.8) | 22 (10.5) | |
| cT4 | 0 (0) | 0 (0) | 0 (0) | |
| Prostate volume (mL) | 0.568 | |||
| min. max. | 15–160 mL | 15–100 mL | 15–160 mL | |
| median | 43.3 mL | 41.95 mL | 44.68 ml | |
| ≤30 | 113 (27.2) | 60 (29.3) | 53 (25.2) | |
| 30, 1–50 | 208 (50.1) | 102 (49.8) | 106 (50.5) | |
| >50 | 94 (22.7) | 43 (21) | 51 (24.3) | |
| D’Amico Classification | 0.412 | |||
| low risk | 125 (30.1) | 48 (23.4) | 77 (36.7) | |
| intermediate risk | 165 (39.8) | 87 (42.4) | 78 (37.1) | |
| high risk | 125 (30.1) | 70 (34.1) | 55 (26.2) |
| All Patients n = 415 (%) | Ligated DVC n = 205 (%) | Non-Ligated DVC n = 210 (%) | p-Value | |
|---|---|---|---|---|
| Age | ||||
| min. max. | 45–84 | 45–76 | 45–84 | |
| median | 64.5 | 64.08 | 64.91 | 0.179 |
| BMI | ||||
| min. max. | 17.3–39.18 | 19.62–38.53 | 17.3–39.18 | |
| median | 27.86 | 27.75 | 27.97 | 0.530 |
| Arterial hypertension | 229 (55.2) | 108 (52.7) | 121 (57.6) | 0.362 |
| Ischemic heart disease | 72 (17.3) | 22 (10.7) | 48 (22.9) | 0.002 |
| Diabetes | 53 (12.8) | 22 (10.7) | 31 (14.8) | 0.279 |
| Asthma | 15 (3.6) | 4 (2) | 11 (5.2) | 0.126 |
| Atrial fibrillation | 14 (3.4) | 4 (2) | 10 (4.8) | 0.189 |
| ASA I | 39 (9.4) | 37 (18) | 3 (1.4) | <0.001 |
| ASA II | 279 (67.2) | 163 (79.5) | 116 (55.2) | |
| ASA III | 97 (23.4) | 6 (2.9) | 91 (43.3) | |
| ASA IV | 0 (0) | 0 (0) | 0 (0) |
| Parameter | All patients n = 415 (%) | Ligated DVC n = 205 (%) | Non-Ligated DVC n = 210 (%) | p-Value |
|---|---|---|---|---|
| Surgery duration (min.) | <0.001 | |||
| min.max. | 50–230 min | 80–230 min | 50–185 min | |
| median | 119 min | 140 min | 98 min | |
| Hospital stay (days): | >0.05 | |||
| min.max. | 1–15 | 1–7 | 1–15 | |
| median | 2.7 | 2.7 | 2.7 | |
| Intraoperative blood loss (mL): | <0.001 | |||
| min.max. | 0–1800 mL | 0–1000 mL | 10–1800 mL | |
| median | 266 mL | 223 mL | 30 | |
| ≤100 mL | 86 (20.7) | 47 (22.9) | 39 (18.6) | |
| 101–200 mL | 140 (33.7) | 82 (40.0) | 58 (27.6) | |
| 201–500 mL | 144 (34.7) | 64 (31.2) | 80 (38.1) | |
| >500 ml | 45 (10.9) | 12 (5.9) | 33 (15.7) | |
| NVB sparing: | >0.05 | |||
| Bilateral | 275 (66.2) | 133 (64.9) | 142 (67.6) | |
| Unilateral | 31 (7.5) | 14 (6.8) | 17 (8.1) | |
| Abandon | 109 (26.3) | 58 (28.3) | 51 (24.3) | |
| Hb decrease (g/dL): | 0.921 | |||
| min.max. | 0.2–7.9 g/dL | 0.6–6.4 g/dL | 0.2–7.9 g/dL | |
| median | 3.17 g/dL | 3.15 g/dL | 3.20 g/dL | |
| Drain leak (mL): | 0.155 | |||
| min.max. | 0–2400 mL | 0–2400 mL | 0–1360 | |
| median | 237 mL | 298 mL | 177 mL | |
| ≤100 mL | 164 (39.5) | 79 (38.5) | 85 (40.5) | |
| 101–200 mL | 108 (26.0) | 38 (18.5) | 65 (31.0) | |
| 201–500 mL | 101 (24.5) | 54 (26.3) | 44 (21.0) | |
| 501–1000 mL | 21 (5.0) | 11 (5.4) | 8 (3.8) | |
| >1000 ml | 21 (5.0) | 16 (7.8) | 2 (1.0) |
| Clavien–Dindo Grade | Ligated DVC | Non-Ligated DVC | p-Value |
|---|---|---|---|
| Grade I (67) | 38 | 29 | 0.340 |
| Lymphocele | 25 | 18 | 0.444 |
| Anastomosis Leakage | 5 | 3 | 0.499 |
| Wound infection | 0 | 6 | 0.030 |
| Limb lymphedema | 3 | 0 | 0.120 |
| Hematoma | 3 | 1 | 0.367 |
| Obturator nerve injury | 2 | 1 | 0.620 |
| Grade II (73) | 31 | 42 | 0.264 |
| UTI | 12 | 17 | 0.869 |
| Blood transfusion | 10 | 14 | 0.889 |
| Intraoperative rectal injury | 2 | 4 | |
| Hematuria | 5 | 5 | >0.999 |
| Thrombosis | 1 | 1 | 0.543 |
| Ileus | 1 | 1 | >0.999 |
| Grade IIIa (44) | 16 | 29 | 0.113 |
| Percutaneous drainage (lymphocele. abscess. hematoma) | 14 | 24 | 0.278 |
| Nephrostomy | 1 | 2 | 0.499 |
| Suprapubic cystostomy (Anastomosis Leakage) | 1 | 3 | 0.623 |
| Grade IIIb 17 | 13 | 4 | 0.042 |
| laparotomy (rectal injury) | 2 | 2 | >0.999 |
| Laparotomy | 4 | 0 | 0.059 |
| Laparotomy (anastomosis leak) | 1 | 0 | 0.494 |
| fenestration of lymphocele | 1 | 0 | 0.494 |
| Anastomosis stricture | 3 | 0 | 0.120 |
| Orchidectomy | 2 | 1 | 0.256 |
| Postoperative hernia surgery | 0 | 1 | >0.999 |
| Grade IV. V (6) | 2 | 4 | 0.685 |
| Urosepsis | 1 | 2 | >0.999 |
| Pulmonary embolism | 1 | 1 | >0.999 |
| Myocardial infarction | 0 | 1 | >0.999 |
| All Patients n = 415 (%) | Ligated DVC n = 205 (%) | Non-ligated DVC n = 210 (%) | p-Value | |
|---|---|---|---|---|
| Cancer stage | ||||
| pT2 | 276 (66.5) | 141 (68.8) | 135 (64.2) | 0.387 |
| pT3 | 139 (33.5) | 64 (31.2) | 75 (35.8) | 0.387 |
| pT3a | 79 (19.0) | 41 (20.0) | 38 (18.2) | 0.712 |
| pT3b | 60 (14.5) | 23 (11.2) | 37 (17.6) | 0.087 |
| pT4 | 0 (0) | 0 (0) | 0 (0) | |
| Positive surgical margin | ||||
| Overall | 139 (33.5) | 78 (38) | 61 (29) | 0.052 |
| Right | 35 (8.4) | 22 (10.7) | 13 (6.2) | 0.137 |
| Left | 34 (8.2) | 16 (7.8) | 18 (8.6) | 0.916 |
| Bilateral | 22 (5.3) | 11 (5.4) | 11 (5.2) | 1.000 |
| Apex | 58 (14.0) | 34 (16.6) | 24 (11.4) | 0.145 |
| Positive surgical margin | 139 (33.5) | 78 (38.0) | 61 (29) | 0.052 |
| Positive surgical margin—apex (all patients) | 58 (18.9) | 34 (26.2) | 24 (13.6) | 0.145 |
| Cancer identified in apex | 307 (74.0 | 130 (63.4) | 177(84.3) | <0.001 |
| Positive surgical margin—apex (cancer localized in apex) | 58 | 34 (26.2) | 24 (13.6) | 0.005 |
| ISUP/Gleason grade: | ||||
| ISUP 1/Gleason ≤ 6 | 134 (32.3) | 57 (27.8) | 77 (36.7) | 0.081 |
| ISUP 2/Gleason 7 (3 + 4) | 178 (42.9) | 94 (45.9) | 84 (40.0) | 0.259 |
| ISUP 3/Gleason 7 (4 + 3) | 52 (12.5) | 31 (15.1) | 21 (10.0) | 0.342 |
| ISUP 4/Gleason 8 | 36 (8.7) | 18 (8.8) | 18 (8.5) | 0.945 |
| ISUP 5/Gleason 9 I 10 | 15 (3.6) | 5 (2.4) | 10 (4.8) | 0.201 |
| Incontinence Grade ICS Scale | Ligated DVC | Non-Ligated DVC | |
|---|---|---|---|
| 3 months Number of patients | |||
| Mild | 32 | 29 | p > 0.05 |
| Average | 19 | 34 | |
| Severe | 12 | 41 | p = 0.004 |
| 6 months | |||
| Mild | 23 | 43 | p > 0.05 |
| Average | 11 | 28 | |
| Severe | 6 | 14 | |
| 12 months | |||
| Mild | 32 | 46 | p > 0.05 |
| Average | 21 | 26 | |
| Severe | 3 | 7 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wilamowski, J.; Wojtarowicz, M.; Adamowicz, J.; Golab, A.; Pozniak, M.; Leminski, A.; Kuffel, B.; Slojewski, M.; Drewa, T. Management with Santorini’s Plexus Should Be Personalized during Prostatectomy. J. Pers. Med. 2022, 12, 769. https://doi.org/10.3390/jpm12050769
Wilamowski J, Wojtarowicz M, Adamowicz J, Golab A, Pozniak M, Leminski A, Kuffel B, Slojewski M, Drewa T. Management with Santorini’s Plexus Should Be Personalized during Prostatectomy. Journal of Personalized Medicine. 2022; 12(5):769. https://doi.org/10.3390/jpm12050769
Chicago/Turabian StyleWilamowski, Jacek, Mateusz Wojtarowicz, Jan Adamowicz, Adam Golab, Michal Pozniak, Artur Leminski, Blazej Kuffel, Marcin Slojewski, and Tomasz Drewa. 2022. "Management with Santorini’s Plexus Should Be Personalized during Prostatectomy" Journal of Personalized Medicine 12, no. 5: 769. https://doi.org/10.3390/jpm12050769
APA StyleWilamowski, J., Wojtarowicz, M., Adamowicz, J., Golab, A., Pozniak, M., Leminski, A., Kuffel, B., Slojewski, M., & Drewa, T. (2022). Management with Santorini’s Plexus Should Be Personalized during Prostatectomy. Journal of Personalized Medicine, 12(5), 769. https://doi.org/10.3390/jpm12050769








