Assessment of Mastectomy Skin Flaps for Immediate Reconstruction with Implants via Thermal Imaging—A Suitable, Personalized Approach?
Abstract
:1. Introduction
2. Methods
2.1. Study Population
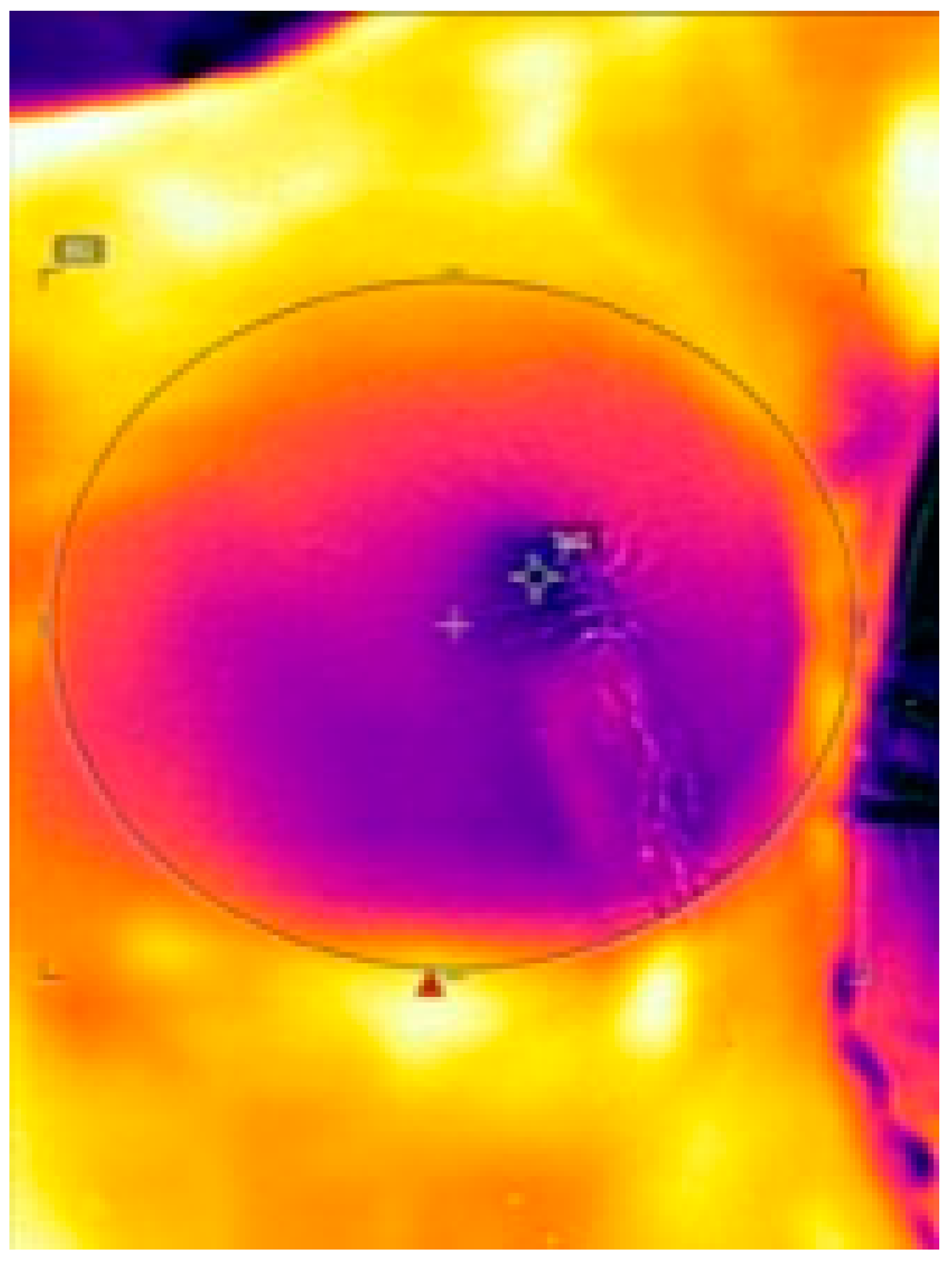
2.2. FLIR ONE
- -
- Preoperative: immediately after anesthesia induction before disinfection
- -
- Intraoperative 1: immediately after NSM/SSM
- -
- Intraoperative 2: immediately after implant placement and wound closure
- -
- Postoperative: 24 h postoperative
2.3. Statistical Analysis
3. Results
3.1. Surface Temperature of the Mastectomy Skin Flap
3.2. Wound-Healing Disorders and Necrosis
3.2.1. Surgery-Related Risk Factors
3.2.2. Patient-Related Risk Factors
4. Discussion
4.1. Examination of Skin-Flap Perfusion via Thermal Imaging
4.2. Detection of Hypoperfused Areas at Risk for WHD and MSFN
4.3. Detection of Patient- and Surgery-Related Risk Factors for WHD and MSFN
4.4. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jahkola, T.; Asko-Seljavaara, S.; von Smitten, K. Immediate Breast Reconstruction. Scand. J. Surg. 2003, 92, 249–256. [Google Scholar] [CrossRef] [PubMed]
- Albornoz, C.R.; Cordeiro, P.G.; Pusic, A.L.; McCarthy, C.M.; Mehrara, B.J.; Disa, J.J.; Matros, E. Diminishing relative contraindications for immediate breast reconstruction: A multicenter study. J. Am. Coll. Surg. 2014, 219, 788–795. [Google Scholar] [CrossRef] [PubMed]
- Panchal, H.; Matros, E. Current Trends in Postmastectomy Breast Reconstruction. Plast. Reconstr. Surg. 2017, 140, 7S–13S. [Google Scholar] [CrossRef] [PubMed]
- Zenn, M.; Venturi, M.; Pittman, T.; Spear, S.; Gurtner, G.; Robb, G.; Mesbahi, A.; Dayan, J. Optimizing Outcomes of Postmastectomy Breast Reconstruction With Acellular Dermal Matrix: A Review of Recent Clinical Data. Eplasty 2017, 17, e18. [Google Scholar] [PubMed]
- Chiappa, C.; Fachinetti, A.; Boeri, C.; Arlant, V.; Rausei, S.; Dionigi, G.; Rovera, F. Wound healing and postsurgical complications in breast cancer surgery: A comparison between PEAK PlasmaBlade and conventional electrosurgery—A preliminary report of a case series. Ann. Surg. Treat. Res. 2018, 95, 129. [Google Scholar] [CrossRef] [PubMed]
- Antony, A.K.; Mehrara, B.M.; McCarthy, C.M.; Zhong, T.; Kropf, N.; Disa, J.J.; Pusic, A.; Cordeiro, P.G. Salvage of tissue expander in the setting of mastectomy flap necrosis: A 13-year experience using timed excision with continued expansion. Plast. Reconstr. Surg. 2009, 124, 356–363. [Google Scholar] [CrossRef]
- Patel, K.M.; Hill, L.M.; Gatti, M.E.; Nahabedian, M.Y. Management of Massive Mastectomy Skin Flap Necrosis Following Autologous Breast Reconstruction. Ann. Plast. Surg. 2012, 69, 139–144. [Google Scholar] [CrossRef]
- Margulies, A.G.; Hochberg, J.; Kepple, J.; Henry-Tillman, R.S.; Westbrook, K.; Klimberg, V.S. Total skin-sparing mastectomy without preservation of the nipple-areola complex. Am. J. Surg. 2005, 190, 920–926. [Google Scholar] [CrossRef]
- Matsen, C.B.; Mehrara, B.; Eaton, A.; Capko, D.; Berg, A.; Stempel, M.; Van Zee, K.J.; Pusic, A.; King, T.A.; Cody, H.S.; et al. Skin Flap Necrosis After Mastectomy With Reconstruction: A Prospective Study. Ann. Surg. Oncol. 2016, 23, 257–264. [Google Scholar] [CrossRef] [Green Version]
- Lee, K.-T.; Mun, G.-H. Necrotic Complications in Nipple-Sparing Mastectomy Followed by Immediate Breast Reconstruction: Systematic Review with Pooled Analysis. J. Korean Soc. Microsurg. 2014, 23, 51–64. [Google Scholar] [CrossRef]
- Robertson, S.; Jeevaratnam, J.; Agrawal, A.; Cutress, R. Mastectomy skin flap necrosis: Challenges and solutions. Breast Cancer Targets Ther. 2017, 9, 141–152. [Google Scholar] [CrossRef] [Green Version]
- Jaspers, M.E.H.; Carrière, M.E.; Meij-de Vries, A.; Klaessens, J.H.G.M.; van Zuijlen, P.P.M. The FLIR ONE thermal imager for the assessment of burn wounds: Reliability and validity study. Burns 2017, 43, 1516–1523. [Google Scholar] [CrossRef] [PubMed]
- Hallock, G.G. Smartphone Thermal Imaging Can Enable the Safer Use of Propeller Flaps. Semin. Plast. Surg. 2020, 34, 161–164. [Google Scholar] [CrossRef] [PubMed]
- FLIR ONE. Available online: https://www.flir.com/products/flir-one-gen-3/ (accessed on 19 September 2021).
- Phillips, B.T.; Lanier, S.T.; Conkling, N.; Wang, E.; Dagum, A.B.; Ganz, J.C.; Khan, S.U.; Bui, D.T. Intraoperative Perfusion Techniques Can Accurately Predict Mastectomy Skin Flap Necrosis in Breast Reconstruction. Plast. Reconstr. Surg. 2012, 129, 778e–788e. [Google Scholar] [CrossRef]
- Xue, E.Y.; Chandler, L.K.; Viviano, S.L.; Keith, J.D. Use of FLIR ONE Smartphone Thermography in Burn Wound Assessment. Ann. Plast. Surg. 2018, 80, S236–S238. [Google Scholar] [CrossRef] [PubMed]
- Goel, J.; Nizamoglu, M.; Tan, A.; Gerrish, H.; Cranmer, K.; El-Muttardi, N.; Barnes, D.; Dziewulski, P. A prospective study comparing the FLIR ONE with laser Doppler imaging in the assessment of burn depth by a tertiary burns unit in the United Kingdom. Scars Burn. Heal. 2020, 6, 205951312097426. [Google Scholar] [CrossRef]
- Nischwitz, S.P.; Luze, H.; Kamolz, L.-P. Thermal imaging via FLIR One—A promising tool in clinical burn care and research. Burns 2020, 46, 988–989. [Google Scholar] [CrossRef]
- Dhatt, S.; Krauss, E.M.; Winston, P. The Role of FLIR ONE Thermography in Complex Regional Pain Syndrome. Am. J. Phys. Med. Rehabil. 2021, 100, e48–e51. [Google Scholar] [CrossRef]
- Rabbani, M.J.; Ilyas, A.; Rabbani, A.; Abidin, Z.U.; Tarar, M.N. Accuracy of Thermal Imaging Camera in Identification of Perforators. J. Coll. Physicians Surg. Pak. 2020, 30, 512–515. [Google Scholar] [CrossRef]
- Nischwitz, S.P.; Luze, H.; Schellnegger, M.; Gatterer, S.J.; Tuca, A.-C.; Winter, R.; Kamolz, L.-P. Thermal, Hyperspectral, and Laser Doppler Imaging: Non-Invasive Tools for Detection of The Deep Inferior Epigastric Artery Perforators—A Prospective Comparison Study. J. Pers. Med. 2021, 11, 1005. [Google Scholar] [CrossRef] [PubMed]
- Prabha, S. Thermal Imaging Techniques for Breast Screening—A Survey. Curr. Med. Imaging Rev. 2020, 16, 855–862. [Google Scholar] [CrossRef]
- Hakim, A.; Awale, R.N. Thermal Imaging—An Emerging Modality for Breast Cancer Detection: A Comprehensive Review. J. Med. Syst. 2020, 44, 136. [Google Scholar] [CrossRef] [PubMed]
- Whatley, J.A.; Kay, S. Using thermal imaging to measure changes in breast cancer-related lymphoedema during reflexology. Br. J. Community Nurs. 2020, 25 (Suppl. 10), S6–S11. [Google Scholar] [CrossRef]
- Khavanin, N.; Qiu, C.; Darrach, H.; Kraenzlin, F.; Kokosis, G.; Han, T.; Sacks, J.M. Intraoperative Perfusion Assessment in Mastectomy Skin Flaps: How Close are We to Preventing Complications? J. Reconstr. Microsurg. 2019, 35, 471–478. [Google Scholar] [CrossRef] [Green Version]
- Larson, D.L.; Basir, Z.; Bruce, T. Is Oncologic Safety Compatible with a Predictably Viable Mastectomy Skin Flap? Plast. Reconstr. Surg. 2011, 127, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Brigitte, W.F.Y.; Childs, C. A systematic review on the role of extremity skin temperature as a non-invasive marker for hypoperfusion in critically ill adults in the intensive care setting. JBI Libr. Syst. Rev. 2010, 8 (Suppl. 34), 1–26. Available online: http://journals.lww.com/01583928-201008341-00007 (accessed on 2 February 2022).
- Pereira, G.; Pereira, C. Skin edge debridement made easy. Injury 2003, 34, 954–956. [Google Scholar] [CrossRef]
- Woerdeman, L.A.E.; Hage, J.J.; Hofland, M.M.I.; Rutgers, E.J.T. A Prospective Assessment of Surgical Risk Factors in 400 Cases of Skin-Sparing Mastectomy and Immediate Breast Reconstruction with Implants to Establish Selection Criteria. Plast. Reconstr. Surg. 2007, 119, 455–463. [Google Scholar] [CrossRef]
- Davies, K.; Allan, L.; Roblin, P.; Ross, D.; Farhadi, J. Factors affecting post-operative complications following skin sparing mastectomy with immediate breast reconstruction. Breast 2011, 20, 21–25. [Google Scholar] [CrossRef]


| Highest T. [°C] | Lowest T. [°C] | Average T. [°C] | |
|---|---|---|---|
 | |||
| Highest T. [°C] | Lowest T. [°C] | Average T. [°C] | ||||
|---|---|---|---|---|---|---|
 | ||||||
| No Complication | Complication | ||
|---|---|---|---|
| Patients (n = 15) | 11 (73.3%) | 4 (26.7%) | |
| Age [years] | 47.6 (±10.6) | 46.3 (±1.5) | |
| BMI [kg/m2] | 25.3 (±3.0) | 28.1 (±0.6) | |
| Body temperature [°C] | ★ | 36.0 (±0.1) | 35.6 (±0.4) |
| Surgery duration [min] | ★ | 142 (±0.43) | 178 (±42) |
| Implant size [cm3] | ★ | 320.5 (±42.5) | 370 (±43.3) |
| Room temperature [°C] | ★ | 23.0 (±0.2) | 21.2 (±1.2) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luze, H.; Nischwitz, S.P.; Wurzer, P.; Winter, R.; Spendel, S.; Kamolz, L.-P.; Bjelic-Radisic, V. Assessment of Mastectomy Skin Flaps for Immediate Reconstruction with Implants via Thermal Imaging—A Suitable, Personalized Approach? J. Pers. Med. 2022, 12, 740. https://doi.org/10.3390/jpm12050740
Luze H, Nischwitz SP, Wurzer P, Winter R, Spendel S, Kamolz L-P, Bjelic-Radisic V. Assessment of Mastectomy Skin Flaps for Immediate Reconstruction with Implants via Thermal Imaging—A Suitable, Personalized Approach? Journal of Personalized Medicine. 2022; 12(5):740. https://doi.org/10.3390/jpm12050740
Chicago/Turabian StyleLuze, Hanna, Sebastian Philipp Nischwitz, Paul Wurzer, Raimund Winter, Stephan Spendel, Lars-Peter Kamolz, and Vesna Bjelic-Radisic. 2022. "Assessment of Mastectomy Skin Flaps for Immediate Reconstruction with Implants via Thermal Imaging—A Suitable, Personalized Approach?" Journal of Personalized Medicine 12, no. 5: 740. https://doi.org/10.3390/jpm12050740
APA StyleLuze, H., Nischwitz, S. P., Wurzer, P., Winter, R., Spendel, S., Kamolz, L.-P., & Bjelic-Radisic, V. (2022). Assessment of Mastectomy Skin Flaps for Immediate Reconstruction with Implants via Thermal Imaging—A Suitable, Personalized Approach? Journal of Personalized Medicine, 12(5), 740. https://doi.org/10.3390/jpm12050740








