Effects of Complex Rehabilitation Program on Reducing Pain and Disability in Patients with Lumbar Disc Protrusion—Is Early Intervention the Best Recommendation?
Abstract
:1. Introduction
2. Methods
2.1. Study Design
2.2. Setting
2.3. Participants
2.4. Variables
2.5. Data Sources
2.6. Bias
2.7. Study Size
2.8. Quantitative Variables
2.9. Statistical Methods
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dalstra, J.A.A.; Kunst, A.E.; Borrell, C.; Breeze, E.; Cambois, E.; Costa, G.; Geurts, J.J.M.; Lahelma, E.; Van Oyen, H.; Rasmussen, N.K.; et al. Socioeconomic differences in theprevalence of common chronic disease: An overview of eight European countries. Int. J. Epidemiol. 2005, 34, 316–326. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dora, C.; Wälchli, B.; Elfering, A.; Gal, I.; Weishaupt, D.; Boos, N. The significance of spinal canal dimensions in discriminating symptomatic from asymptomatic disc herniations. Eur. Spine J. 2002, 11, 575–581. [Google Scholar] [CrossRef]
- Feng, T.; Zhao, P.; Liang, G. Clinical significance on protruded nucleus pulposus: A comparative study of 44 patients with lumbar intervertebral disc protrusion and 73 asymptomatic control in tridimensional computed tomography. Zhongguo Zhong Xi Yi Jie He Za Zhi 2000, 20, 347–349. [Google Scholar]
- Matsumoto, M.; Okada, E.; Toyama, Y.; Fujiwara, H.; Momoshima, S.; Takahata, T. Tandem age-related lumbar and cervical intervertebral disc changes in asymptomatic subjects. Eur. Spine J. 2013, 22, 708–713. [Google Scholar] [CrossRef] [Green Version]
- Hoy, D.; Bain, C.; Williams, G.; March, L.; Brooks, P.; Blyth, F.; Woolf, A.; Vos, T.; Buchbinder, R. A systematicreview of the global prevalence of low back pain. Arthritis Rheum. 2012, 64, 2028–2037. [Google Scholar] [CrossRef]
- Busse, R.; Schreyogg, J.; Smith, P.C. Variability in healthcare treatment costs amongst nine EU countries-results from the Healthbasket project. Health Econ. 2008, 17, 51–58. [Google Scholar]
- Deyo, R.A.; Weinstein, J.N. Low back pain. N. Engl. J. Med. 2001, 344, 363–370. [Google Scholar] [CrossRef] [PubMed]
- Ala-Kokko, L. Genetic riskfactors for lumbar disc disease. Ann. Med. 2002, 34, 42–47. [Google Scholar] [CrossRef] [PubMed]
- Sirbu, E.; Buleu, F.; Tudor, A.; Dragan, S. Vitamin D and disease activity in rheumatoid arthritis patients: A retrospective study in a Romanian cohort. Acta Biochim. Pol. 2020, 67, 267–272. [Google Scholar] [CrossRef]
- Stoicănescu, L.D.; Cevei, M.L.; Sirbu, E.M.; Zdrîncă, M.M.; Muţiu, G. Unusual occurrence of avascular necrosis with bilateral involvement and ankylosing spondylitis, meningioma and Hodgkin lymphoma. Rom J. Morphol. Embryol. 2019, 60, 1003–1007. [Google Scholar] [PubMed]
- George, S.Z.; Fritz, J.M.; Silfies, S.P.; Schneider, M.J.; Beneciuk, J.M.; Lentz, T.A.; Gilliam, J.R.; Hendren, S.; Norman, K.S. Interventions for the Management of Acute and Chronic Low Back Pain: Revision 2021. J. Orthop. Sports Phys. Ther. 2021, 51, CPG1–CPG60. [Google Scholar] [CrossRef] [PubMed]
- Fritz, J.M.; Magel, J.S.; McFadden, M.; Asche, C.; Thackeray, A.; Meier, W.; Brennan, G. Early physical therapy vs usual care in patients with recent-onset low back pain: A randomized clinical trial. JAMA 2015, 314, 1459–1467. [Google Scholar] [CrossRef] [Green Version]
- Krekoukias, G.; Gelalis, I.D.; Xenakis, T.; Gioftsos, G.; Dimitriadis, Z.; Sakellari, V. Spinal mobilization vs conventional physiotherapy in the management of chronic low back pain due to spinal disk degeneration: A randomized controlled trial. J. Man. Manip. Ther. 2017, 25, 66–73. [Google Scholar] [CrossRef] [Green Version]
- Vaseghnia, A.; Shadmehr, A.; Moghadam, B.A.; Olyaei, G.; Reza Hadian, M.; Khazaeipour, Z. The therapeutic effects of manipulation technique on sacroiliac joint dysfunction in young women. Muscles Ligaments Tendons J. 2018, 8, 526–533. [Google Scholar] [CrossRef] [Green Version]
- Vieira-Pellenz, F.; Oliva-Pascual-Vaca, Á.; Rodriguez-Blanco, C.; Heredia-Rizo, A.M.; Ricard, F.; Almazán-Campos, G. Short-term effect of spinal manipulation on pain perception, spinal mobility, and full height recovery in male subjects with degenerative disk disease: A randomized controlled trial. Arch. Phys. Med. Rehabil. 2014, 95, 1613–1619. [Google Scholar] [CrossRef]
- Sarker, K.K.; Sethi, J.; Mohanty, U. Effect of spinal manipulation on pain sensitivity, postural sway, and health-related quality of life among patients with non-specific chronic low back pain: A randomised control trial. J. Clin. Diagn. Res. 2019, 13, YC01–YC05. [Google Scholar] [CrossRef]
- Tagliaferri, S.D.; Miller, C.T.; Ford, J.J.; Hahne, A.J.; Main, L.C.; Rantalainen, T.; Connell, D.A.; Simson, K.J.; Owen, P.J.; Belavy, D.L. Randomized trial of general strength and conditioning versus motor control and manual therapy for chronic low back pain on physical and self-report outcomes. J. Clin. Med. 2020, 9, 1726. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, A.L.S.; da Silva, N.F.; da Silva Filho, E.M. Evaluation of functional disability and pain in patients with chronic low back pain submitted to physiotherapy. Man. Ther. Posturology Rehabil. J. 2016, 14, 374. [Google Scholar] [CrossRef]
- Albornoz-Cabello, M.; Maya-Martín, J.; Domínguez-Maldonado, G.; Espejo-Antúnez, L.; Heredia-Rizo, A.M. Effect of interferential current therapy on pain perception and disability level in subjects with chronic low back pain: A randomized controlled trial. Clin. Rehabil. 2017, 31, 242–249. [Google Scholar] [CrossRef]
- Facci, L.M.; Nowotny, J.P.; Tormem, F.; Trevisani, V.F. Effects of transcutaneous electrical nerve stimulation (TENS) and interferential currents (IFC) in patients with nonspecific chronic low back pain: Randomized clinical trial. Sao Paulo Med. J. 2011, 129, 206–216. [Google Scholar] [CrossRef] [Green Version]
- Cheng, Z.X.; Zheng, Y.J.; Feng, Z.Y.; Fang, H.W.; Zhang, J.Y.; Wang, X.R. Chinese Association for the Study of Pain: Expert consensus on diagnosis and treatment for lumbar disc herniation. World J. Clin. Cases 2021, 9, 2058–2067. [Google Scholar] [CrossRef] [PubMed]
- Kose, G.; Tastan, S.; Temiz, N.C.; Sari, M.; Izci, Y. The Effect of Low Back Pain on Daily Activities and Sleep Quality in Patients With Lumbar Disc Herniation: A Pilot Study. J. Neurosci. Nurs. 2019, 51, 184–189. [Google Scholar] [CrossRef] [PubMed]
- Mooventhan, A.; Nivethitha, L. Scientificevidence-based effects of hydrotherapy on various systems of the body. N. Am. J. Med. Sci. 2014, 6, 199–209. [Google Scholar] [CrossRef] [Green Version]
- Radulović, N.; Pavlović, R.; Mihajlović, I.; Nikolić, S. Diagnostic of spinal column mobility using Schober’s test for lumbal syndrome by application of physical therapy and sport recreation. Eur. J. Phys. Educ. Sport Sci. 2017, 3, 51–66. [Google Scholar]
- Schober, P. The lumbar vertebral column in backache. Munch. Med. Wochenschr. 1937, 84, 336–338. [Google Scholar]
- Moll, J.; Wright, V. Measurement of spinal movement. In The Lumbar Spine and Back Pain, 3rd ed.; Jayson, M.I.V., Ed.; Churchill Livingstone: London, UK, 1987; Volume 11, pp. 215–234. [Google Scholar]
- Sinaki, M.; Mokri, B. Low back pain and disorders of the lumbar spine. In Physical Medicine & Rehabilitation; Braddom, R.L., Buschbacher, R.M., Dumitru, D., Johnson, E.W., Matthews, D., Sinaki, M., Eds.; WB Saunders Co.: Philadelphia, PA, USA, 1996; Volume 39, pp. 813–850. [Google Scholar]
- Tousignant, M.; Poulin, L.; Marchand, S.; Viau, A.; Place, C. TheModified–Modified Schober Test for range of motion assessment of lumbar flexion in patients with low back pain: A study of criterion validity, intra- and inter- rater reliability and minimum metrically detectable change. Disabil. Rehabil. 2005, 27, 553–559. [Google Scholar] [CrossRef]
- Strand, L.I.; Ljunggren, A.E.; Bogen, B.; Ask, T.; Johnsen, T.B. The Short-Form McGill Pain Questionnaire as an outcome measure: Test-retest reliability and responsiveness to change. Eur. J. Pain. 2008, 12, 917–925. [Google Scholar] [CrossRef]
- Lee, C.P.; Fu, T.S.; Liu, C.Y.; Hung, C.I. Psychometric evaluation of the Oswestry Disability Index in patients with chronic low back pain: Factor and Mokken analyses. Health Qual. Life Outcomes 2017, 15, 192. [Google Scholar] [CrossRef] [Green Version]
- Grafton, K.V. Test-retest reliability of the Short-Form McGill Pain Questionnaire: Rassessment of intraclass correlation coefficients and limits of agreement in patients with osteoarthritis. Clin. J. Pain 2005, 21, 73–82. [Google Scholar] [CrossRef] [Green Version]
- Trudeau, J.; Turk, D.; Dworkin, R.; Benson, C.; Biondi, D.; Kim, M.; Mou, J.; Katz, N. Validation of the revised short form McGill Pain Questionnaire (SF-MPQ-2) for self-report of pain qualities in patients with acute low back pain. J. Pain 2012, 13, S4. [Google Scholar] [CrossRef]
- Chauffe, A.; Yakuboff, M.; Tanabe, T. Responsiveness of the VAS and McGill pain questionnaire in measuring changes in musculoskeletal pain. J. Sport Rehabil. 2011, 20, 250–255. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Webography W1. Available online: http://www.chcr.brown.edu/pcoc/shortmcgillquest.pdf (accessed on 30 November 2021).
- Melzack, R. The short form McGill pain questionnaire. Pain 1987, 30, 191–197. [Google Scholar] [CrossRef]
- Byrne, M.; Troy, A.; Bradley, L.A.; Marchisello, P.J.; Geisinger, K.F.; Van der Heide, L.H.; Prieto, E.J. Cross-validation of the factor structure of the McGill Pain Questionnaire. Pain 1982, 13, 193–201. [Google Scholar] [CrossRef]
- Dworkin, R.H.; Turk, D.C.; Trudeau, J.J.; Benson, C.; Biondi, D.M.; Katz, N.P.; Kim, M. Validation of the Short-form McGill Pain Questionnaire-2 (SF-MPQ-2) in acute low back pain. J. Pain 2015, 16, 357–366. [Google Scholar] [CrossRef]
- Fairbank, J.C.; Pynsent, P.B. The Oswestry disability index. Spine (Phila Pa 1976) 2000, 25, 2940–2952. [Google Scholar] [CrossRef]
- Tomkins-Lane, C.C.; Battié, M.C. Validity and reproducibility of self-report measures of walking capacity in lumbar spinal stenosis. Spine (Phila Pa 1976) 2010, 35, 2097–2102. [Google Scholar] [CrossRef]
- Wu, A.; March, L.; Zheng, X.; Huang, J.; Wang, X.; Zhao, J.; Blyth, F.M.; Smith, E.; Buchbinder, R.; Hoy, D. Global low back pain prevalence and years lived with disability from 1990 to 2017: Estimates from the Global Burden of Disease Study 2017. Ann. Transl. Med. 2020, 8, 299. [Google Scholar] [CrossRef]
- Kolu, E.; Buyukavci, R.; Akturk, S.; Eren, F.; Ersoy, Y. Comparison of high-intensity laser therapy and combination of transcutaneous nerve stimulation and ultrasound treatment in patients with chronic lumbar radiculopathy: A randomized single-blind study. Pak. J. Med. Sci. 2018, 34, 530–534. [Google Scholar] [CrossRef]
- Zdrodowska, B.; Leszczyńska-Filus, M.; Leszczyński, R.; Błaszczyk, J. Comparison of the effect of laser and magnetic therapy for pain level and the range of motion of the spine of people with osteoarthritis lower back. Pol. Merkur. Lek. 2015, 38, 26–31. [Google Scholar]
- Gugliotta, M.; da Costa, B.R.; Dabis, E.; Theiler, R.; Jüni, P.; Reichenbach, S.; Landolt, H.; Hasler, P. Surgical versus conservative treatment for lumbar disc herniation: A prospective cohort study. BMJ Open 2016, 6, e012938. [Google Scholar] [CrossRef] [Green Version]
- Ariel, E.; Levkovitz, Y.; Goor-Aryeh, I.; Ratmansky, M. The effects of TENS, interferential stimulation, and combined interferential stimulation and pulsed ultrasound on patients with disc herniation-induced radicular pain. J. Back Musculoskelet. Rehabil. 2021, 35, 363–371. [Google Scholar] [CrossRef] [PubMed]
- Ozkaraoglu, D.K.; Tarakci, D.; Algun, Z.C. Comparison of two different electrotherapy methods in low back pain treatment. J. Back Musculoskelet. Rehabil. 2020, 33, 193–199. [Google Scholar] [CrossRef] [PubMed]
- Yurdakul, O.V.; Beydoğan, E.; YılmazYalçınkaya, E. Effects of physical therapy agents on pain, disability, quality of life, and lumbar paravertebral muscle stiffness via elastography in patients with chronic low back pain. Turk. J. Phys. Med. Rehabil. 2019, 65, 30–39. [Google Scholar] [CrossRef] [PubMed]
- Sawant, R.S.; Shinde, S.B. Effect of hydrotherapy based exercises for chronic nonspecific low back pain. Indian J. Physiother. Occup. Ther. 2019, 13, 133–138. [Google Scholar] [CrossRef]
- Dias, N.T.; Santos, P.R.; Cândido, T.A.; Pinto, R.M.C.; Resende, A.P.M.; Pereira-Baldon, V.S. Effects of the addition of transcutaneous electrical stimulation to non-pharmacological measures in labor pain: Study protocol for a randomized controlled trial. Trials 2022, 23, 44. [Google Scholar] [CrossRef] [PubMed]
- Rampazo, É.P.; Liebano, R.E. Analgesic Effects of Interferential Current Therapy: A Narrative Review. Medicina 2022, 58, 141. [Google Scholar] [CrossRef]
- Noble, G.J.; Lowe, A.S.; Walsh, D.M. Interferential Therapy Review. Part Mechanism of Analgesic Action and Clinical Usage. Phys. Ther. Rev. 2000, 5, 239–245. [Google Scholar] [CrossRef]
- Dedering, A.; Harms-Ringdahl, K.; Nèmeth, G. Back extensor muscle fatigue in patients with lumbar disc herniation. Pre-operative and post-operative analysis of electromyography, endurance time and subjective factors. Eur. Spine J. 2006, 15, 559–569. [Google Scholar] [CrossRef] [Green Version]
- Arbak, B.; Hendricks, O.; Horslev-Petersen, K.; Jurik, A.G.; Pedersen, S.J.; Ostergaard, M.; Hermansen, L.T.; Loft, A.G.; Jensen, T.S.; Manniche, C. The discriminative value of inflammatory back pain in patients with persistent low back pain. Scand. J. Rheumatol. 2016, 45, 321–328. [Google Scholar] [CrossRef]
- Fritz, J.M.; Hebert, J.; Koppenhaver, S.; Parent, E. Beyond minimally important change: Defining a successful outcome of physical therapy for patients with low back pain. Spine (Phila Pa 1976) 2009, 34, 2803–2809. [Google Scholar] [CrossRef] [PubMed]
- Schwind, J.; Learman, K.; O’Halloran, B.; Showalter, C.; Cook, C. Different minimally important clinical difference (MCID) scores lead to different clinical prediction rules for the Oswestry disability index for the same sample of patients. J. Man. Manip. Ther. 2013, 21, 71–78. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gatchel, R.J.; Polatin, P.B.; Noe, C.; Gardea, M.; Pulliam, C.; Thompson, J. Treatment- and cost-effectiveness of early intervention for acute low-back pain patients: A one-year prospective study. J. Occup. Rehabil. 2003, 13, 1–9. [Google Scholar] [CrossRef]
- Molde Hagen, E.; Grasdal, A.; Eriksen, H.R. Does early intervention with a light mobilization program reduce long-term sick leave for low back pain: A 3-year follow-up study. Spine (Phila Pa 1976) 2003, 28, 2309–2315. [Google Scholar] [CrossRef] [PubMed]
- Childs, J.D.; Fritz, J.M.; Wu, S.S.; Flynn, T.W.; Wainner, R.S.; Robertson, E.K.; Kim, F.S.; George, S.Z. Implications of early and guideline adherent physical therapy for low back pain on utilization and costs. BMC Health Serv. Res. 2015, 15, 150. [Google Scholar] [CrossRef] [Green Version]
- Chadha, S.; Behl, T.; Bungau, S.; Kumar, A.; Arora, R.; Gupta, A.; Uddin, M.S.; Zengin, G.; Aleya, L.; Setia, D.; et al. Mechanistic insights into the role of pyroptosis in rheumatoid arthritis. Curr. Res. Transl. Med. 2020, 68, 151–158. [Google Scholar] [CrossRef]
- Radu, A.F.; Bungau, S.G. Management of Rheumatoid Arthritis: An Overview. Cells 2021, 10, 2857. [Google Scholar] [CrossRef]
- Rhon, D.; Fritz, J. COMParative Early Treatment Effectiveness between physical therapy and usual care for low back pain (COMPETE): Study protocol for a randomized controlled trial. Trials 2015, 16, 423. [Google Scholar] [CrossRef] [Green Version]
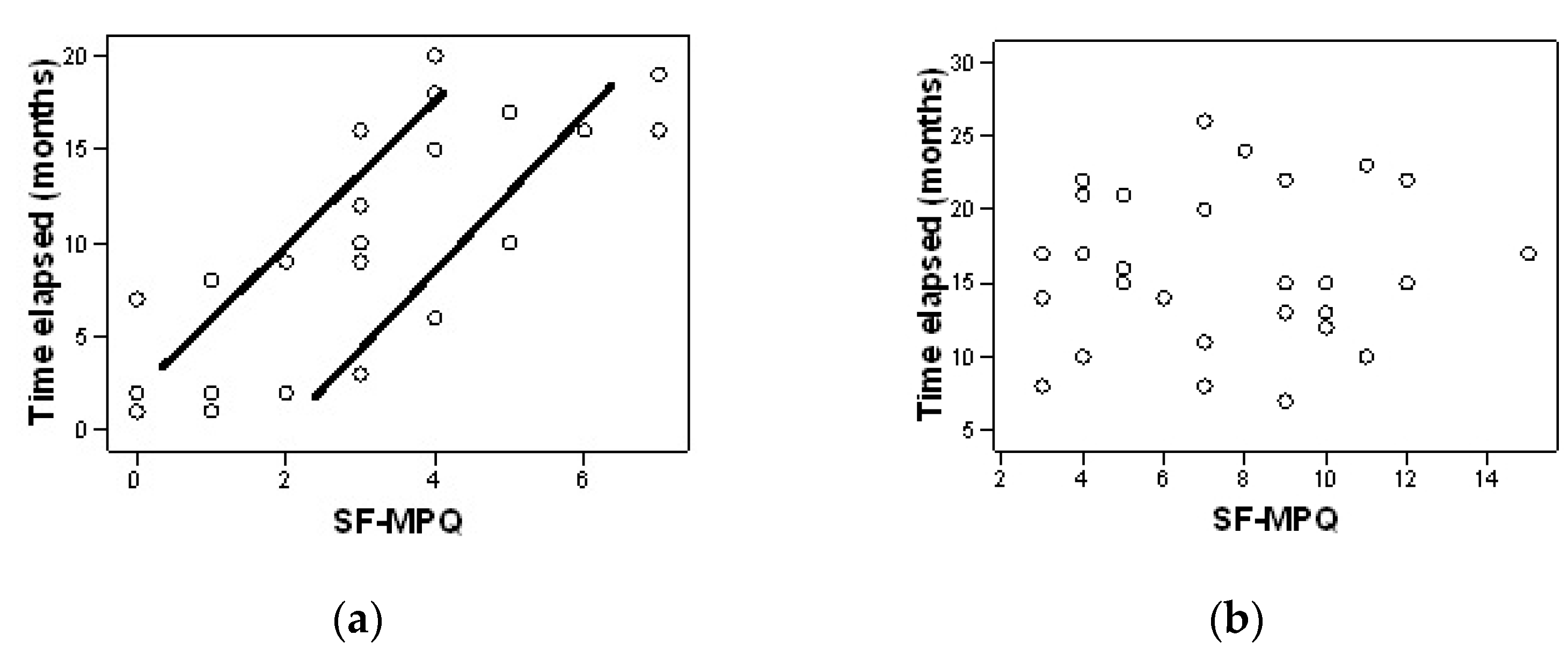
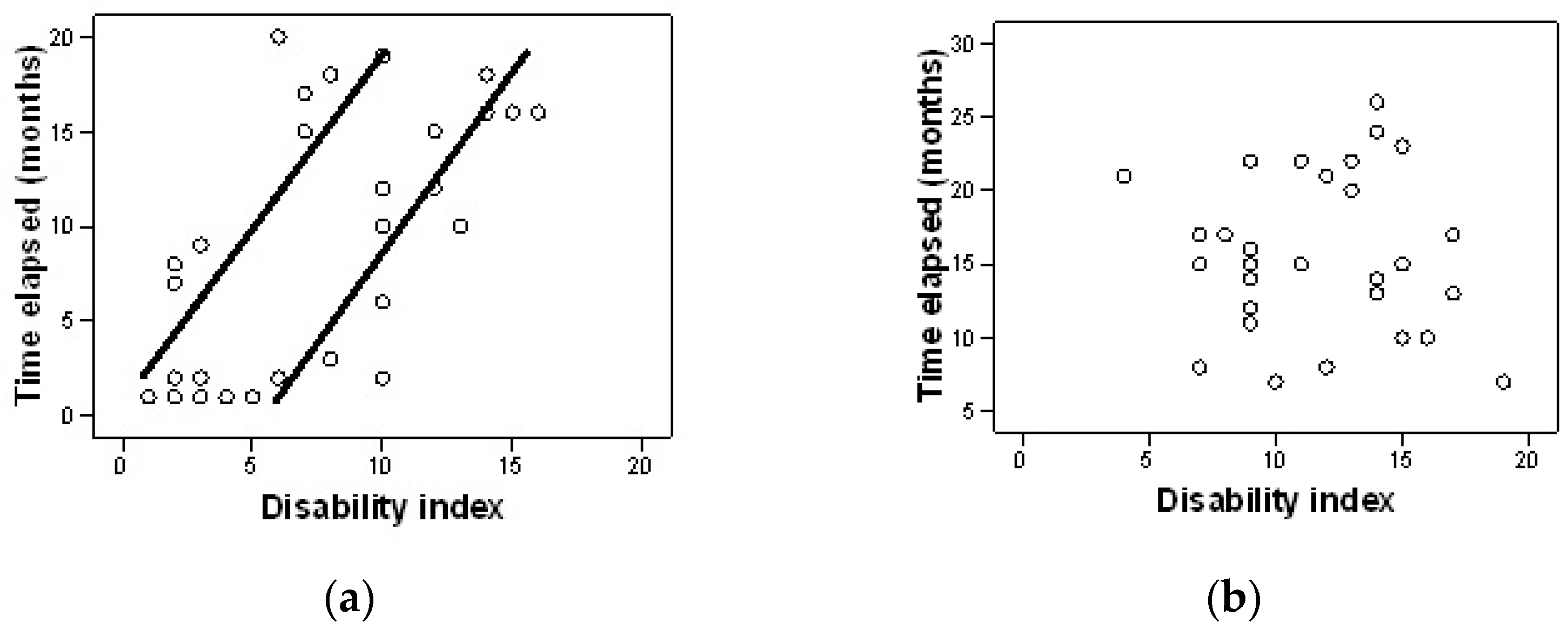
| Characteristics | Group A (n = 30) | Group B (n = 30) | p |
|---|---|---|---|
| Age (years) | 53.30 ± 11.20 | 52.97 ± 11.04 | 0.908 |
| BMI (kg/m2) | 29.28 ± 4.71 | 29.89 ± 4.41 | 0.685 |
| Time elapsed (months) | 9.0 ± 6.73 | 10.63 ± 5.32 | 0.920 |
| Gender (%) men women | 46.7 | 36.7 | 0.432 |
| 53.3 | 63.3 | ||
| Comorbidities (%) yes no | 53.3 | 60.0 | 0.602 |
| 46.7 | 40.0 | ||
| Radiculopathy (%) right left no | 26.7 | 33.3 | 0.717 |
| 30.0 | 33.3 | ||
| 43.3 | 33.3 |
| Parameters | Group A (n = 30) | Group B (n = 30) | p * | 95% CI [Lower/Upper] |
|---|---|---|---|---|
| VAS-M | 3.80 ± 1.972 | 4.07 ± 1.721 | 0.435 | −1.223/0.690 |
| VAS-E | 3.17 ± 1.724 | 3.60 ± 1.567 | 0.614 | −1.285/0.418 |
| SF-MPQ | 12.57 ± 6.027 | 12.77 ± 4.897 | 0.888 | −3.040/2.640 |
| FTF | 17.02 ± 10.841 | 17.57 ± 4.321 | 0.797 | −3.715/4.815 |
| LS | 11.20 ± 1.126 | 12.48 ± 1.334 | 0.736 | −0.692/0.492 |
| ILS | 8.38 ± 0.537 | 8.04 ± 0.666 | 0.058 | 0.575/1.185 |
| Right LLF | 12.90 ± 3.573 | 13.81 ± 3.008 | 0.289 | −2.620/0.794 |
| Left LLF | 12.62 ± 3.314 | 13.85 ± 2.948 | 0.134 | −2.852/0.391 |
| LF strength | 10.00 ± 5.206 | 12.63 ± 2.109 | 0.013 | −4.686/−0.581 |
| LE strength | 9.80 ± 6.641 | 13.00 ± 2.101 | 0.015 | −5.745/−0.655 |
| ODQ | 17.37 ± 5.605 | 17.53 ± 4.455 | 0.899 | −2.783/2.450 |
| ODQ (%) | 34.73 ± 11.209 | 35.07 ± 8.909 | 0.899 | −5.566/4.900 |
| Group A (n = 30) | Group B (n = 30) | Inter-Action | Effect Size | Group A Changes | Group B Changes | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Baseline (Mean ± SD) | Post (Mean ± SD) | Baseline (Mean ± SD) | Post (Mean ± SD) | p | p | p | 95% CI Lower/Upper | p | 95% CI Lower/Upper | |
| VAS-M | 3.80 ± 1.972 | 1.30 ± 1.343 | 4.07 ± 1.721 | 2.72 ± 1.907 | 0.002 * | 0.163 | 0.000 * | 1.882/3.118 | 0.001 * | 1.111/1.578 |
| VAS-E | 3.17 ± 1.724 | 0.60 ± 0.724 | 3.60 ± 1.567 | 2.72 ± 1.701 | 0.000 * | 0.296 | 0.000 * | 1.965/3.169 | 0.051 * | 1.087/1.580 |
| SF-MPQ | 12.57 ± 6.027 | 2.70 ± 2.120 | 12.77 ± 4.897 | 7.47 ± 3.137 | 0.000 * | 0.457 | 0.000 * | 7.996/11.671 | 0.000 * | 4.521/6.076 |
| FTF | 17.02 ± 10.841 | 11.20 ± 1.126 | 17.57 ± 4.321 | 18.12 ± 6.102 | 0.005 * | 0.126 | 0.000 * | −7.687/−3.940 | 0.001 * | −0.244/1.344 |
| LS | 11.20 ± 1.126 | 13.82 ± 0.932 | 12.48 ± 1.334 | 13.92 ± 1.325 | 0.000 * | 0.313 | 0.005 * | 2.277/2.963 | 0.005 * | 0.706/1.454 |
| ILS | 8.38 ± 0.537 | 8.93 ± 0.452 | 8.04 ± 0.666 | 8.05 ± 0.702 | 0.034 | 0.076 | 0.004 * | 0.366/0.734 | 0.841 | 0.091/0.111 |
| Right LLF | 12.90 ± 3.573 | 14.13 ± 3.386 | 13.81 ± 3.008 | 13.65 ± 3.094 | 0.571 | 0.001 | 0.001 * | −1.781/−0.685 | 0.249 | −0.157/−0.517 |
| Left LLF | 12.62 ± 3.314 | 13.48 ± 2.845 | 13.85 ± 2.948 | 13.67 ± 3.061 | 0.821 | 0.006 | 0.005 * | −1.901/−0.545 | 0.284 | −0.116/0.429 |
| LF strength | 10.00 ± 5.206 | 14.80 ± 5.517 | 12.63 ± 2.109 | 12.47 ± 2.501 | 0.093 | 0.071 | 0.001 * | −5.970/−3.630 | 0.538 | −0.380/0.713 |
| LE strength | 9.80 ± 6.641 | 14.30 ± 7.489 | 13.00 ± 2.101 | 13.13 ± 2.763 | 1.167 | 0.011 | 0.000 * | −5.676/−3.324 | 0.670 | −0.767/0.500 |
| ODQ | 17.37 ± 5.605 | 8.13 ± 3.785 | 17.53 ± 4.455 | 15.60 ± 3.616 | 0.001 * | 0.185 | 0.000 * | 8.026/10.441 | 0.000 * | 5.220/6.647 |
| ODI (%) | 34.73 ± 11.209 | 17.93 ± 6.269 | 35.07 ± 8.909 | 23.13 ± 7.099 | 0.004 * | 0.135 | 0.000 * | 13.927/19.673 | 0.000 * | 10.526/13.341 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tarcău, E.; Ianc, D.; Sirbu, E.; Ciobanu, D.; Boca, I.C.; Marcu, F. Effects of Complex Rehabilitation Program on Reducing Pain and Disability in Patients with Lumbar Disc Protrusion—Is Early Intervention the Best Recommendation? J. Pers. Med. 2022, 12, 741. https://doi.org/10.3390/jpm12050741
Tarcău E, Ianc D, Sirbu E, Ciobanu D, Boca IC, Marcu F. Effects of Complex Rehabilitation Program on Reducing Pain and Disability in Patients with Lumbar Disc Protrusion—Is Early Intervention the Best Recommendation? Journal of Personalized Medicine. 2022; 12(5):741. https://doi.org/10.3390/jpm12050741
Chicago/Turabian StyleTarcău, Emilian, Dorina Ianc, Elena Sirbu, Doriana Ciobanu, Ioan Cosmin Boca, and Florin Marcu. 2022. "Effects of Complex Rehabilitation Program on Reducing Pain and Disability in Patients with Lumbar Disc Protrusion—Is Early Intervention the Best Recommendation?" Journal of Personalized Medicine 12, no. 5: 741. https://doi.org/10.3390/jpm12050741
APA StyleTarcău, E., Ianc, D., Sirbu, E., Ciobanu, D., Boca, I. C., & Marcu, F. (2022). Effects of Complex Rehabilitation Program on Reducing Pain and Disability in Patients with Lumbar Disc Protrusion—Is Early Intervention the Best Recommendation? Journal of Personalized Medicine, 12(5), 741. https://doi.org/10.3390/jpm12050741






