Cognitive Impairment after Post-Acute COVID-19 Infection: A Systematic Review of the Literature
Abstract
1. Introduction
2. Materials and Methods
3. Results
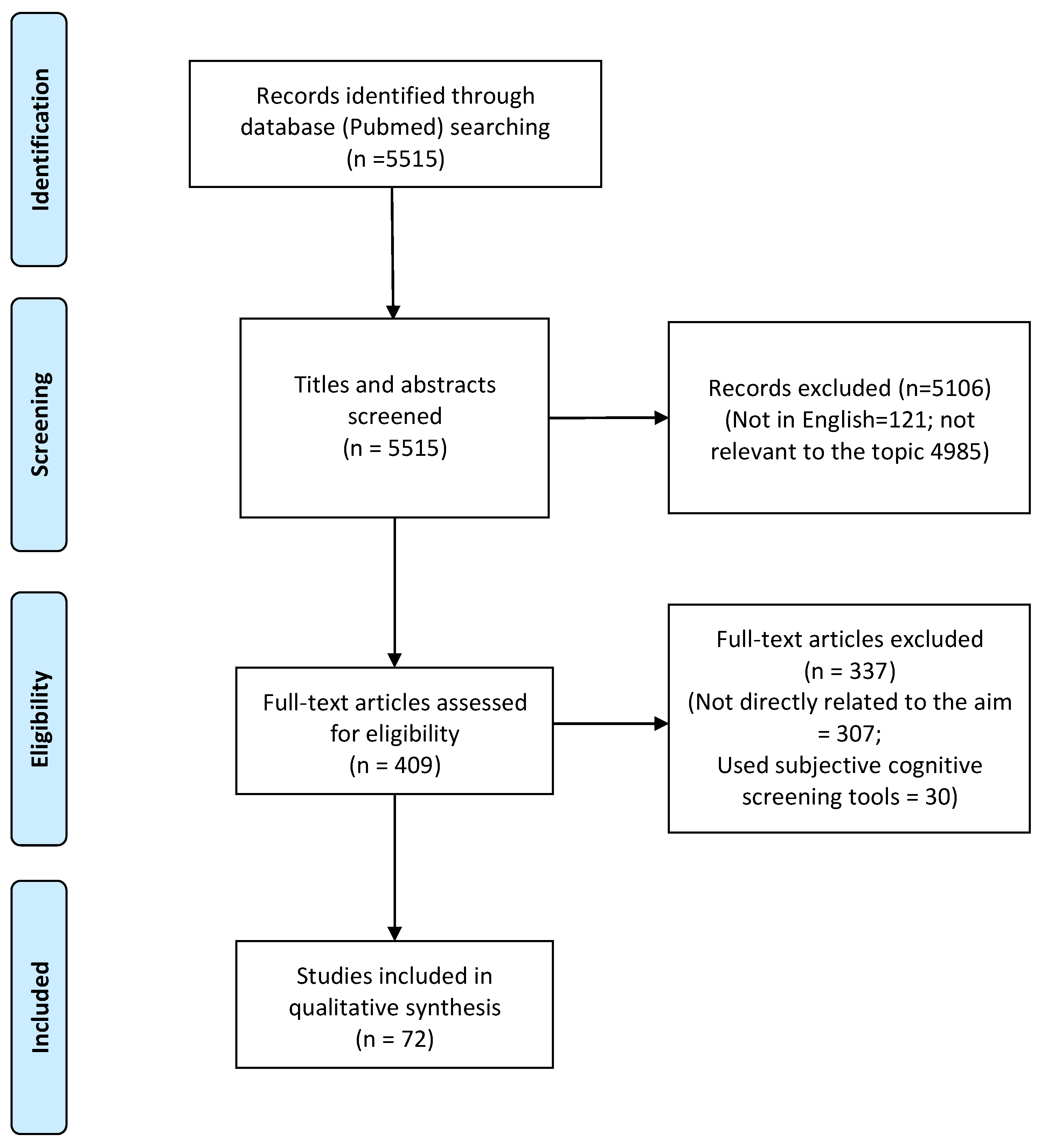
3.1. Search Results and Characteristics of Studies
3.2. Sample Characteristics
3.2.1. Size, Inclusion Criteria and General Design Characteristics of the Studies
3.2.2. Age and Gender
3.2.3. Hospitalization Rates and Severity of the Respiratory Pathology
3.3. Evaluation of Cognitive Impairments in COVID-19 Patients: Assessment Instruments and Main Results
3.3.1. Assessment Instruments
3.3.2. Main Results of Cognitive Assessment
3.4. Outcomes of Neuroimaging and Neuroinflammatory Studies
4. Discussion
4.1. Main Findings and Characteristics of the Study Design and of Included Samples: Possible Bias of the Collected Outcomes
4.1.1. Main Findings and Methodological Limitations
4.1.2. Characteristics of the Subjects Included and Possible Selection Bias
4.2. Cognitive Impairment in COVID-19: Possible Pathophysiological Mechanisms
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Miners, S.; Kehoe, P.G.; Love, S. Cognitive impact of COVID-19: Looking beyond the short term. Alzheimer’s Res. Ther. 2020, 12, 170. [Google Scholar] [CrossRef] [PubMed]
- WHO. World Health Organization: COVID-19 Dashboard. Available online: https://covid19.who.int/ (accessed on 1 October 2022).
- Leung, T.Y.M.; Chan, A.Y.L.; Chan, E.W.; Chan, V.K.Y.; Chui, C.S.L.; Cowling, B.J.; Gao, L.; Ge, M.Q.; Hung, I.F.N.; Ip, M.S.M.; et al. Short- and potential long-term adverse health outcomes of COVID-19: A rapid review. Emerg. Microbes Infect. 2020, 9, 2190–2199. [Google Scholar] [CrossRef] [PubMed]
- Kamal, M.; Abo Omirah, M.; Hussein, A.; Saeed, H. Assessment and characterisation of post-COVID-19 manifestations. Int. J. Clin. Pract. 2021, 75, e13746. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.F.; Cotler, J.; Jason, L.A. Post-viral fatigue and COVID-19: Lessons from past epidemics. Fatigue Biomed. Health Behav. 2020, 8, 61–69. [Google Scholar] [CrossRef]
- World Health Organization. A Clinical Case Definition of Post COVID-19 Condition by a Delphi Consensus; WHO: Geneva, Switzerland, 2021. [Google Scholar]
- Crook, H.; Raza, S.; Nowell, J.; Young, M.; Edison, P. Long covid—Mechanisms, risk factors, and management. BMJ 2021, 374, n1648. [Google Scholar] [CrossRef]
- Nalbandian, A.; Sehgal, K.; Gupta, A.; Madhavan, M.V.; McGroder, C.; Stevens, J.S.; Cook, J.R.; Nordvig, A.S.; Shalev, D.; Sehrawat, T.S.; et al. Post-acute COVID-19 syndrome. Nat. Med. 2021, 27, 601–615. [Google Scholar] [CrossRef] [PubMed]
- Woo, M.S.; Malsy, J.; Pöttgen, J.; Seddiq Zai, S.; Ufer, F.; Hadjilaou, A.; Schmiedel, S.; Addo, M.M.; Gerloff, C.; Heesen, C.; et al. Frequent neurocognitive deficits after recovery from mild COVID-19. Brain Commun. 2020, 2, fcaa205. [Google Scholar] [CrossRef]
- Wu, Y.; Xu, X.; Chen, Z.; Duan, J.; Hashimoto, K.; Yang, L.; Liu, C.; Yang, C. Nervous system involvement after infection with COVID-19 and other coronaviruses. Brain Behav. Immun. 2020, 87, 18–22. [Google Scholar] [CrossRef]
- Ritchie, K.; Chan, D. The emergence of cognitive COVID. World Psychiatry 2021, 20, 52–53. [Google Scholar] [CrossRef]
- Coleman, B.; Casiraghi, E.; Blau, H.; Chan, L.; Haendel, M.A.; Laraway, B.; Callahan, T.J.; Deer, R.R.; Wilkins, K.J.; Reese, J.; et al. Risk of new-onset psychiatric sequelae of COVID-19 in the early and late post-acute phase. World Psychiatry 2022, 21, 319–320. [Google Scholar] [CrossRef]
- De Hert, M.; Mazereel, V.; Detraux, J.; Van Assche, K. Prioritizing COVID-19 vaccination for people with severe mental illness. World Psychiatry 2021, 20, 54–55. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Yang, Z.; Qiu, H.; Wang, Y.; Jian, L.; Ji, J.; Li, K. Anxiety and depression among general population in China at the peak of the COVID-19 epidemic. World Psychiatry 2020, 19, 249–250. [Google Scholar] [CrossRef] [PubMed]
- Tyrer, P. COVID-19 health anxiety. World Psychiatry 2020, 19, 307–308. [Google Scholar] [CrossRef] [PubMed]
- Wasserman, D.; Iosue, M.; Wuestefeld, A.; Carli, V. Adaptation of evidence-based suicide prevention strategies during and after the COVID-19 pandemic. World Psychiatry 2020, 19, 294–306. [Google Scholar] [CrossRef] [PubMed]
- McIntyre, R.S.; Lee, Y. Preventing suicide in the context of the COVID-19 pandemic. World Psychiatry 2020, 19, 250–251. [Google Scholar] [CrossRef]
- Rooksby, M.; Furuhashi, T.; McLeod, H.J. Hikikomori: A hidden mental health need following the COVID-19 pandemic. World Psychiatry 2020, 19, 399–400. [Google Scholar] [CrossRef]
- Rogers, J.P.; Chesney, E.; Oliver, D.; Pollak, T.A.; McGuire, P.; Fusar-Poli, P.; Zandi, M.S.; Lewis, G.; David, A.S. Psychiatric and neuropsychiatric presentations associated with severe coronavirus infections: A systematic review and meta-analysis with comparison to the COVID-19 pandemic. Lancet Psychiatry 2020, 7, 611–627. [Google Scholar] [CrossRef]
- Kępińska, A.P.; Iyegbe, C.O.; Vernon, A.C.; Yolken, R.; Murray, R.M.; Pollak, T.A. Schizophrenia and Influenza at the Centenary of the 1918-1919 Spanish Influenza Pandemic: Mechanisms of Psychosis Risk. Front. Psychiatry 2020, 11, 72. [Google Scholar] [CrossRef]
- Adhanom Ghebreyesus, T. Addressing mental health needs: An integral part of COVID-19 response. World Psychiatry 2020, 19, 129–130. [Google Scholar] [CrossRef]
- Unützer, J.; Kimmel, R.J.; Snowden, M. Psychiatry in the age of COVID-19. World Psychiatry 2020, 19, 130–131. [Google Scholar] [CrossRef]
- Ceban, F.; Ling, S.; Lui, L.M.W.; Lee, Y.; Gill, H.; Teopiz, K.M.; Rodrigues, N.B.; Subramaniapillai, M.; Di Vincenzo, J.D.; Cao, B.; et al. Fatigue and cognitive impairment in Post-COVID-19 Syndrome: A systematic review and meta-analysis. Brain Behav. Immun. 2022, 101, 93–135. [Google Scholar] [CrossRef] [PubMed]
- Crivelli, L.; Palmer, K.; Calandri, I.; Guekht, A.; Beghi, E.; Carroll, W.; Frontera, J.; García-Azorín, D.; Westenberg, E.; Winkler, A.S.; et al. Changes in cognitive functioning after COVID-19: A systematic review and meta-analysis. Alzheimers Dement. 2022, 18, 1047–1066. [Google Scholar] [CrossRef] [PubMed]
- Schou, T.M.; Joca, S.; Wegener, G.; Bay-Richter, C. Psychiatric and neuropsychiatric sequelae of COVID-19—A systematic review. Brain Behav. Immun. 2021, 97, 328–348. [Google Scholar] [CrossRef]
- Mucci, A.; Galderisi, S.; Green, M.F.; Nuechterlein, K.; Rucci, P.; Gibertoni, D.; Rossi, A.; Rocca, P.; Bertolino, A.; Bucci, P.; et al. Familial aggregation of MATRICS Consensus Cognitive Battery scores in a large sample of outpatients with schizophrenia and their unaffected relatives. Psychol. Med. 2018, 48, 1359–1366. [Google Scholar] [CrossRef] [PubMed]
- Elliott, A.F.; McGwin, G., Jr.; Owsley, C. Health-related quality of life and visual and cognitive impairment among nursing-home residents. Br. J. Ophthalmol. 2009, 93, 240–243. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Huang, L.; Li, X.; Gu, X.; Zhang, H.; Ren, L.; Guo, L.; Liu, M.; Wang, Y.; Cui, D.; Wang, Y.; et al. Health outcomes in people 2 years after surviving hospitalisation with COVID-19: A longitudinal cohort study. Lancet Respir. Med. 2022, 10, 863–876. [Google Scholar] [CrossRef]
- Saraçlı, Ö.; Akca, A.S.; Atasoy, N.; Önder, Ö.; Şenormancı, Ö.; Kaygisız, İ.; Atik, L. The Relationship between Quality of Life and Cognitive Functions, Anxiety and Depression among Hospitalized Elderly Patients. Clin. Psychopharmacol. Neurosci. 2015, 13, 194–200. [Google Scholar] [CrossRef]
- Tavares-Júnior, J.W.L.; de Souza, A.C.C.; Borges, J.W.P.; Oliveira, D.N.; Siqueira-Neto, J.I.; Sobreira-Neto, M.A.; Braga-Neto, P. COVID-19 associated cognitive impairment: A systematic review. Cortex 2022, 152, 77–97. [Google Scholar] [CrossRef]
- Wlodarczyk, J.H.; Brodaty, H.; Hawthorne, G. The relationship between quality of life, Mini-Mental State Examination, and the Instrumental Activities of Daily Living in patients with Alzheimer’s disease. Arch. Gerontol. Geriatr. 2004, 39, 25–33. [Google Scholar] [CrossRef]
- Mei, Q.; Wang, F.; Bryant, A.; Wei, L.; Yuan, X.; Li, J. Mental health problems among COVID-19 survivors in Wuhan, China. World Psychiatry 2021, 20, 139–140. [Google Scholar] [CrossRef]
- Marazziti, D.; Stahl, S.M. The relevance of COVID-19 pandemic to psychiatry. World Psychiatry 2020, 19, 261. [Google Scholar] [CrossRef] [PubMed]
- Galderisi, S.; Rossi, A.; Rocca, P.; Bertolino, A.; Mucci, A.; Bucci, P.; Rucci, P.; Gibertoni, D.; Aguglia, E.; Amore, M.; et al. The influence of illness-related variables, personal resources and context-related factors on real-life functioning of people with schizophrenia. World Psychiatry 2014, 13, 275–287. [Google Scholar] [CrossRef] [PubMed]
- Kuiper, J.S.; Oude Voshaar, R.C.; Zuidema, S.U.; Stolk, R.P.; Zuidersma, M.; Smidt, N. The relationship between social functioning and subjective memory complaints in older persons: A population-based longitudinal cohort study. Int J. Geriatr. Psychiatry 2017, 32, 1059–1071. [Google Scholar] [CrossRef] [PubMed]
- Aretouli, E.; Brandt, J. Everyday functioning in mild cognitive impairment and its relationship with executive cognition. Int. J. Geriatr. Psychiatry 2010, 25, 224–233. [Google Scholar] [CrossRef]
- Galderisi, S.; Rucci, P.; Mucci, A.; Rossi, A.; Rocca, P.; Bertolino, A.; Aguglia, E.; Amore, M.; Bellomo, A.; Bozzatello, P.; et al. The interplay among psychopathology, personal resources, context-related factors and real-life functioning in schizophrenia: Stability in relationships after 4 years and differences in network structure between recovered and non-recovered patients. World Psychiatry 2020, 19, 81–91. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.C.; Bai, W.Z.; Hashikawa, T. The neuroinvasive potential of SARS-CoV2 may play a role in the respiratory failure of COVID-19 patients. J. Med. Virol. 2020, 92, 552–555. [Google Scholar] [CrossRef]
- Jason, L.A.; Islam, M.F.; Conroy, K.; Cotler, J.; Torres, C.; Johnson, M.; Mabie, B. COVID-19 symptoms over time: Comparing long-haulers to ME/CFS. Fatigue Biomed. Health Behav. 2021, 9, 59–68. [Google Scholar] [CrossRef]
- Miskowiak, K.W.; Johnsen, S.; Sattler, S.M.; Nielsen, S.; Kunalan, K.; Rungby, J.; Lapperre, T.; Porsberg, C.M. Cognitive impairments four months after COVID-19 hospital discharge: Pattern, severity and association with illness variables. Eur. Neuropsychopharmacol. 2021, 46, 39–48. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef]
- Liu, Y.-H.; Wang, Y.-R.; Wang, Q.-H.; Chen, Y.; Chen, X.; Li, Y.; Cen, Y.; Xu, C.; Hu, T.; Liu, X.-D.; et al. Post-infection cognitive impairments in a cohort of elderly patients with COVID-19. Mol. Neurodegener. 2021, 16, 48. [Google Scholar] [CrossRef]
- Ortelli, P.; Ferrazzoli, D.; Sebastianelli, L.; Engl, M.; Romanello, R.; Nardone, R.; Bonini, I.; Koch, G.; Saltuari, L.; Quartarone, A.; et al. Neuropsychological and neurophysiological correlates of fatigue in post-acute patients with neurological manifestations of COVID-19: Insights into a challenging symptom. J. Neurol. Sci. 2021, 420, 117271. [Google Scholar] [CrossRef]
- Negrini, F.; Ferrario, I.; Mazziotti, D.; Berchicci, M.; Bonazzi, M.; de Sire, A.; Negrini, S.; Zapparoli, L. Neuropsychological Features of Severe Hospitalized Coronavirus Disease 2019 Patients at Clinical Stability and Clues for Postacute Rehabilitation. Arch. Phys. Med. Rehabil. 2021, 102, 155–158. [Google Scholar] [CrossRef] [PubMed]
- Versace, V.; Sebastianelli, L.; Ferrazzoli, D.; Romanello, R.; Ortelli, P.; Saltuari, L.; D’Acunto, A.; Porrazzini, F.; Ajello, V.; Oliviero, A.; et al. Intracortical GABAergic dysfunction in patients with fatigue and dysexecutive syndrome after COVID-19. Clin. Neurophysiol. 2021, 132, 1138–1143. [Google Scholar] [CrossRef]
- Yesilkaya, U.H.; Sen, M.; Balcioglu, Y.H. COVID-19-related cognitive dysfunction may be associated with transient disruption in the DLPFC glutamatergic pathway. J. Clin. Neurosci. 2021, 87, 153–155. [Google Scholar] [CrossRef] [PubMed]
- Beaud, V.; Crottaz-Herbette, S.; Dunet, V.; Vaucher, J.; Bernard-Valnet, R.; Du Pasquier, R.; Bart, P.-A.; Clarke, S. Pattern of cognitive deficits in severe COVID-19. J. Neurol. Neurosurg. Psychiatry 2021, 92, 567–568. [Google Scholar] [CrossRef] [PubMed]
- Gautam, N.; Madathil, S.; Tahani, N.; Bolton, S.; Parekh, D.; Stockley, J.; Goyal, S.; Qureshi, H.; Yasmin, S.; Cooper, B.G.; et al. Medium-Term Outcomes in Severely to Critically Ill Patients With Severe Acute Respiratory Syndrome Coronavirus 2 Infection. Clin. Infect. Dis. 2022, 74, 301–308. [Google Scholar] [CrossRef] [PubMed]
- Groiss, S.J.; Balloff, C.; Elben, S.; Brandenburger, T.; Müttel, T.; Kindgen-Milles, D.; Vollmer, C.; Feldt, T.; Kunstein, A.; Ole Jensen, B.-E.; et al. Prolonged Neuropsychological Deficits, Central Nervous System Involvement, and Brain Stem Affection After COVID-19—A Case Series. Front. Neurol. 2020, 11, 574004. [Google Scholar] [CrossRef]
- Hellmuth, J.; Barnett, T.A.; Asken, B.M.; Kelly, J.D.; Torres, L.; Stephens, M.L.; Greenhouse, B.; Martin, J.N.; Chow, F.C.; Deeks, S.G.; et al. Persistent COVID-19-associated neurocognitive symptoms in non-hospitalized patients. J. NeuroVirol. 2021, 27, 191–195. [Google Scholar] [CrossRef]
- Tolentino, J.C.; Gjorup, A.L.T.; Schmidt, G.J.; Schmidt, S.L. Early attention impairment in a patient with COVID-19. Psychiatry Clin. Neurosci. 2021, 75, 66–67. [Google Scholar] [CrossRef]
- Whiteside, D.M.; Oleynick, V.; Holker, E.; Waldron, E.J.; Porter, J.; Kasprzak, M. Neurocognitive deficits in severe COVID-19 infection: Case series and proposed model. Clin. Neuropsychol. 2021, 35, 799–818. [Google Scholar] [CrossRef]
- Vannorsdall, T.D.; Brigham, E.; Fawzy, A.; Raju, S.; Gorgone, A.; Pletnikova, A.; Lyketsos, C.G.; Parker, A.M.; Oh, E.S. Cognitive Dysfunction, Psychiatric Distress, and Functional Decline After COVID-19. J. Acad. Consult. Liaison Psychiatry 2022, 63, 133–143. [Google Scholar] [CrossRef] [PubMed]
- Henneghan, A.M.; Lewis, K.A.; Gill, E.; Kesler, S.R. Cognitive Impairment in Non-critical, Mild-to-Moderate COVID-19 Survivors. Front. Psychol. 2022, 13, 770459. [Google Scholar] [CrossRef]
- Graham, E.L.; Clark, J.R.; Orban, Z.S.; Lim, P.H.; Szymanski, A.L.; Taylor, C.; DiBiase, R.M.; Jia, D.T.; Balabanov, R.; Ho, S.U.; et al. Persistent neurologic symptoms and cognitive dysfunction in non-hospitalized Covid-19 “long haulers”. Ann. Clin. Transl. Neurol. 2021, 8, 1073–1085. [Google Scholar] [CrossRef]
- Del Brutto, O.H.; Wu, S.; Mera, R.M.; Costa, A.F.; Recalde, B.Y.; Issa, N.P. Cognitive decline among individuals with history of mild symptomatic SARS-CoV-2 infection: A longitudinal prospective study nested to a population cohort. Eur. J. Neurol. 2021, 28, 3245–3253. [Google Scholar] [CrossRef] [PubMed]
- Abdelghani, M.; Atwa, S.A.; Said, A.; Zayed, N.E.; Abdelmoaty, A.A.; Hassan, M.S. Cognitive after-effects and associated correlates among post-illness COVID-19 survivors: A cross-sectional study, Egypt. Egypt. J. Neurol. Psychiatry Neurosurg. 2022, 58, 77. [Google Scholar] [CrossRef] [PubMed]
- Miskowiak, K.W.; Fugledalen, L.; Jespersen, A.E.; Sattler, S.M.; Podlekareva, D.; Rungby, J.; Porsberg, C.M.; Johnsen, S. Trajectory of cognitive impairments over 1 year after COVID-19 hospitalisation: Pattern, severity, and functional implications. Eur. Neuropsychopharmacol. 2022, 59, 82–92. [Google Scholar] [CrossRef] [PubMed]
- Rubega, M.; Ciringione, L.; Bertuccelli, M.; Paramento, M.; Sparacino, G.; Vianello, A.; Masiero, S.; Vallesi, A.; Formaggio, E.; Del Felice, A. High-density EEG sleep correlates of cognitive and affective impairment at 12-month follow-up after COVID-19. Clin. Neurophysiol. 2022, 140, 126–135. [Google Scholar] [CrossRef]
- Vialatte de Pémille, C.; Ray, A.; Michel, A.; Stefano, F.; Yim, T.; Bruel, C.; Zuber, M. Prevalence and prospective evaluation of cognitive dysfunctions after SARS due to SARS-CoV-2 virus. The COgnitiVID study. Rev. Neurol. 2022, 178, 802–807. [Google Scholar] [CrossRef]
- Almeria, M.; Cejudo, J.C.; Sotoca, J.; Deus, J.; Krupinski, J. Cognitive profile following COVID-19 infection: Clinical predictors leading to neuropsychological impairment. Brain Behav. Immun. Health 2020, 9, 100163. [Google Scholar] [CrossRef]
- Cian, V.; De Laurenzis, A.; Siri, C.; Gusmeroli, A.; Canesi, M. Cognitive and Neuropsychiatric Features of COVID-19 Patients After Hospital Dismission: An Italian Sample. Front. Psychol. 2022, 13, 908363. [Google Scholar] [CrossRef]
- Cecchetti, G.; Agosta, F.; Canu, E.; Basaia, S.; Barbieri, A.; Cardamone, R.; Bernasconi, M.P.; Castelnovo, V.; Cividini, C.; Cursi, M.; et al. Cognitive, EEG, and MRI features of COVID-19 survivors: A 10-month study. J. Neurol. 2022, 269, 3400–3412. [Google Scholar] [CrossRef] [PubMed]
- Johnsen, S.; Sattler, S.M.; Miskowiak, K.W.; Kunalan, K.; Victor, A.; Pedersen, L.; Andreassen, H.F.; Jørgensen, B.J.; Heebøll, H.; Andersen, M.B.; et al. Descriptive analysis of long COVID sequelae identified in a multidisciplinary clinic serving hospitalised and non-hospitalised patients. ERJ Open Res. 2021, 7, 00205–02021. [Google Scholar] [CrossRef]
- Zhou, H.; Lu, S.; Chen, J.; Wei, N.; Wang, D.; Lyu, H.; Shi, C.; Hu, S. The landscape of cognitive function in recovered COVID-19 patients. J. Psychiatr. Res. 2020, 129, 98–102. [Google Scholar] [CrossRef]
- Aiello, E.N.; Radici, A.; Mora, G.; Pain, D. Cognitive phenotyping of post-infectious SARS-CoV-2 patients. Neurol. Sci. 2022, 43, 4599–4604. [Google Scholar] [CrossRef]
- Frontera, J.A.; Yang, D.; Lewis, A.; Patel, P.; Medicherla, C.; Arena, V.; Fang, T.; Andino, A.; Snyder, T.; Madhavan, M.; et al. A prospective study of long-term outcomes among hospitalized COVID-19 patients with and without neurological complications. J. Neurol. Sci. 2021, 426, 117486. [Google Scholar] [CrossRef] [PubMed]
- Manera, M.R.; Fiabane, E.; Pain, D.; Aiello, E.N.; Radici, A.; Ottonello, M.; Padovani, M.; Wilson, B.A.; Fish, J.; Pistarini, C. Clinical features and cognitive sequelae in COVID-19: A retrospective study on N=152 patients. Neurol. Sci. 2022, 43, 45–50. [Google Scholar] [CrossRef] [PubMed]
- Serrano-Castro, P.J.; Garzón-Maldonado, F.J.; Casado-Naranjo, I.; Ollero-Ortiz, A.; Mínguez-Castellanos, A.; Iglesias-Espinosa, M.; Baena-Palomino, P.; Sánchez-Sanchez, V.; Sánchez-Pérez, R.M.; Rubi-Callejon, J.; et al. The cognitive and psychiatric subacute impairment in severe Covid-19. Sci. Rep. 2022, 12, 3563. [Google Scholar] [CrossRef] [PubMed]
- Amalakanti, S.; Arepalli, K.V.R.; Jillella, J.P. Cognitive assessment in asymptomatic COVID-19 subjects. VirusDisease 2021, 32, 146–149. [Google Scholar] [CrossRef]
- Guo, P.; Benito Ballesteros, A.; Yeung, S.P.; Liu, R.; Saha, A.; Curtis, L.; Kaser, M.; Haggard, M.P.; Cheke, L.G. COVCOG 2: Cognitive and Memory Deficits in Long COVID: A Second Publication From the COVID and Cognition Study. Front. Aging Neurosci. 2022, 14, 804937. [Google Scholar] [CrossRef]
- Holdsworth, D.A.; Chamley, R.; Barker-Davies, R.; O’Sullivan, O.; Ladlow, P.; Mitchell, J.L.; Dewson, D.; Mills, D.; May, S.L.J.; Cranley, M.; et al. Comprehensive clinical assessment identifies specific neurocognitive deficits in working-age patients with long-COVID. PLoS ONE 2022, 17, e0267392. [Google Scholar] [CrossRef]
- Mattioli, F.; Stampatori, C.; Righetti, F.; Sala, E.; Tomasi, C.; De Palma, G. Neurological and cognitive sequelae of Covid-19: A four month follow-up. J. Neurol. 2021, 268, 4422–4428. [Google Scholar] [CrossRef] [PubMed]
- Raman, B.; Cassar, M.P.; Tunnicliffe, E.M.; Filippini, N.; Griffanti, L.; Alfaro-Almagro, F.; Okell, T.; Sheerin, F.; Xie, C.; Mahmod, M.; et al. Medium-term effects of SARS-CoV-2 infection on multiple vital organs, exercise capacity, cognition, quality of life and mental health, post-hospital discharge. EClinicalMedicine 2021, 31, 100683. [Google Scholar] [CrossRef] [PubMed]
- Ermis, U.; Rust, M.I.; Bungenberg, J.; Costa, A.; Dreher, M.; Balfanz, P.; Marx, G.; Wiesmann, M.; Reetz, K.; Tauber, S.C.; et al. Neurological symptoms in COVID-19: A cross-sectional monocentric study of hospitalized patients. Neurol. Res. Pract. 2021, 3, 17. [Google Scholar] [CrossRef] [PubMed]
- Jaywant, A.; Vanderlind, W.M.; Alexopoulos, G.S.; Fridman, C.B.; Perlis, R.H.; Gunning, F.M. Frequency and profile of objective cognitive deficits in hospitalized patients recovering from COVID-19. Neuropsychopharmacology 2021, 46, 2235–2240. [Google Scholar] [CrossRef]
- Patel, R.; Savrides, I.; Cahalan, C.; Doulatani, G.; O’Dell, M.W.; Toglia, J.; Jaywant, A. Cognitive impairment and functional change in COVID-19 patients undergoing inpatient rehabilitation. Int. J. Rehabil. Res. 2021, 44, 285–288. [Google Scholar] [CrossRef]
- Albu, S.; Zozaya, N.R.; Murillo, N.; García-Molina, A.; Chacón, C.A.F.; Kumru, H. What’s going on following acute covid-19? Clinical characteristics of patients in an out-patient rehabilitation program. NeuroRehabilitation 2021, 48, 469–480. [Google Scholar] [CrossRef]
- Bolattürk, Ö.F.; Soylu, A.C. Evaluation of cognitive, mental, and sleep patterns of post-acute COVID-19 patients and their correlation with thorax CT. Acta Neurol. Belg. 2022. [Google Scholar] [CrossRef] [PubMed]
- De Lorenzo, R.; Conte, C.; Lanzani, C.; Benedetti, F.; Roveri, L.; Mazza, M.G.; Brioni, E.; Giacalone, G.; Canti, V.; Sofia, V.; et al. Residual clinical damage after COVID-19: A retrospective and prospective observational cohort study. PLoS ONE 2020, 15, e0239570. [Google Scholar] [CrossRef]
- Dressing, A.; Bormann, T.; Blazhenets, G.; Schroeter, N.; Walter, L.I.; Thurow, J.; August, D.; Hilger, H.; Stete, K.; Gerstacker, K.; et al. Neuropsychologic Profiles and Cerebral Glucose Metabolism in Neurocognitive Long COVID Syndrome. J. Nucl. Med. 2022, 63, 1058–1063. [Google Scholar] [CrossRef]
- Evans, R.A.; McAuley, H.; Harrison, E.M.; Shikotra, A.; Singapuri, A.; Sereno, M.; Elneima, O.; Docherty, A.B.; Lone, N.I.; Leavy, O.C.; et al. Physical, cognitive, and mental health impacts of COVID-19 after hospitalisation (PHOSP-COVID): A UK multicentre, prospective cohort study. Lancet Respir. Med. 2021, 9, 1275–1287. [Google Scholar] [CrossRef]
- Ferrucci, R.; Dini, M.; Groppo, E.; Rosci, C.; Reitano, M.R.; Bai, F.; Poletti, B.; Brugnera, A.; Silani, V.; D’Arminio Monforte, A.; et al. Long-Lasting Cognitive Abnormalities after COVID-19. Brain Sci. 2021, 11, 235. [Google Scholar] [CrossRef] [PubMed]
- García-Sánchez, C.; Calabria, M.; Grunden, N.; Pons, C.; Arroyo, J.A.; Gómez-Anson, B.; Lleó, A.; Alcolea, D.; Belvís, R.; Morollón, N.; et al. Neuropsychological deficits in patients with cognitive complaints after COVID-19. Brain Behav. 2022, 12, e2508. [Google Scholar] [CrossRef] [PubMed]
- Hadad, R.; Khoury, J.; Stanger, C.; Fisher, T.; Schneer, S.; Ben-Hayun, R.; Possin, K.; Valcour, V.; Aharon-Peretz, J.; Adir, Y. Cognitive dysfunction following COVID-19 infection. J. Neurovirol. 2022, 28, 430–437. [Google Scholar] [CrossRef] [PubMed]
- Hellgren, L.; Birberg Thornberg, U.; Samuelsson, K.; Levi, R.; Divanoglou, A.; Blystad, I. Brain MRI and neuropsychological findings at long-term follow-up after COVID-19 hospitalisation: An observational cohort study. BMJ Open 2021, 11, e055164. [Google Scholar] [CrossRef]
- Leth, S.; Gunst, J.D.; Mathiasen, V.; Hansen, K.; Søgaard, O.; Østergaard, L.; Jensen-Fangel, S.; Storgaard, M.; Agergaard, J. Persistent Symptoms in Patients Recovering From COVID-19 in Denmark. Open Forum Infect. Dis. 2021, 8, ofab042. [Google Scholar] [CrossRef] [PubMed]
- Mazza, M.G.; Palladini, M.; De Lorenzo, R.; Magnaghi, C.; Poletti, S.; Furlan, R.; Ciceri, F.; Rovere-Querini, P.; Benedetti, F. Persistent psychopathology and neurocognitive impairment in COVID-19 survivors: Effect of inflammatory biomarkers at three-month follow-up. Brain Behav. Immun. 2021, 94, 138–147. [Google Scholar] [CrossRef]
- Méndez, R.; Balanzá-Martínez, V.; Luperdi, S.C.; Estrada, I.; Latorre, A.; González-Jiménez, P.; Feced, L.; Bouzas, L.; Yépez, K.; Ferrando, A.; et al. Short-term neuropsychiatric outcomes and quality of life in COVID-19 survivors. J. Intern. Med. 2021, 290, 621–631. [Google Scholar] [CrossRef]
- Monti, G.; Leggieri, C.; Fominskiy, E.; Scandroglio, A.M.; Colombo, S.; Tozzi, M.; Moizo, E.; Mucci, M.; Crivellari, M.; Pieri, M.; et al. Two-months quality of life of COVID-19 invasively ventilated survivors; an Italian single-center study. Acta Anaesthesiol. Scand. 2021, 65, 912–920. [Google Scholar] [CrossRef]
- Puchner, B.; Sahanic, S.; Kirchmair, R.; Pizzini, A.; Sonnweber, B.; Wöll, E.; Mühlbacher, A.; Garimorth, K.; Dareb, B.; Ehling, R.; et al. Beneficial effects of multi-disciplinary rehabilitation in postacute COVID-19: An observational cohort study. Eur. J. Phys. Rehabil. Med. 2021, 57, 189–198. [Google Scholar] [CrossRef]
- Rass, V.; Beer, R.; Schiefecker, A.J.; Kofler, M.; Lindner, A.; Mahlknecht, P.; Heim, B.; Limmert, V.; Sahanic, S.; Pizzini, A.; et al. Neurological outcome and quality of life 3 months after COVID-19: A prospective observational cohort study. Eur. J. Neurol. 2021, 28, 3348–3359. [Google Scholar] [CrossRef]
- Soldati, A.B.; Almeida, C.; Lima, M.; Araujo, A.; Araujo-Leite, M.A.; Silva, M.T.T. Telephone Screening of Cognitive Status (TICS) in severe COVID-19 patients: Utility in the era of social isolation. eNeurologicalSci 2021, 22, 100322. [Google Scholar] [CrossRef] [PubMed]
- van den Borst, B.; Peters, J.B.; Brink, M.; Schoon, Y.; Bleeker-Rovers, C.P.; Schers, H.; van Hees, H.W.H.; van Helvoort, H.; van den Boogaard, M.; van der Hoeven, H.; et al. Comprehensive Health Assessment 3 Months After Recovery From Acute Coronavirus Disease 2019 (COVID-19). Clin. Infect. Dis. 2021, 73, e1089–e1098. [Google Scholar] [CrossRef] [PubMed]
- Group, T.W.C.f.t.C.S. Four-Month Clinical Status of a Cohort of Patients After Hospitalization for COVID-19. JAMA 2021, 325, 1525–1534. [Google Scholar] [CrossRef]
- Hosp, J.A.; Dressing, A.; Blazhenets, G.; Bormann, T.; Rau, A.; Schwabenland, M.; Thurow, J.; Wagner, D.; Waller, C.; Niesen, W.D.; et al. Cognitive impairment and altered cerebral glucose metabolism in the subacute stage of COVID-19. Brain 2021, 144, 1263–1276. [Google Scholar] [CrossRef]
- Latronico, N.; Peli, E.; Calza, S.; Rodella, F.; Novelli, M.P.; Cella, A.; Marshall, J.; Needham, D.M.; Rasulo, F.A.; Piva, S. Physical, cognitive and mental health outcomes in 1-year survivors of COVID-19-associated ARDS. Thorax 2022, 77, 300–303. [Google Scholar] [CrossRef]
- Venturelli, S.; Benatti, S.V.; Casati, M.; Binda, F.; Zuglian, G.; Imeri, G.; Conti, C.; Biffi, A.M.; Spada, M.S.; Bondi, E.; et al. Surviving COVID-19 in Bergamo province: A post-acute outpatient re-evaluation. Epidemiol. Infect. 2021, 149, e32. [Google Scholar] [CrossRef] [PubMed]
- Weihe, S.; Mortensen, C.B.; Haase, N.; Andersen, L.P.K.; Mohr, T.; Siegel, H.; Ibsen, M.; Jørgensen, V.R.L.; Buck, D.L.; Pedersen, H.B.S.; et al. Long-term cognitive and functional status in Danish ICU patients with COVID-19. Acta Anaesthesiol. Scand. 2022, 66, 978–986. [Google Scholar] [CrossRef]
- Becker, J.H.; Lin, J.J.; Doernberg, M.; Stone, K.; Navis, A.; Festa, J.R.; Wisnivesky, J.P. Assessment of Cognitive Function in Patients After COVID-19 Infection. JAMA Netw. Open 2021, 4, e2130645. [Google Scholar] [CrossRef]
- Bonizzato, S.; Ghiggia, A.; Ferraro, F.; Galante, E. Cognitive, behavioral, and psychological manifestations of COVID-19 in post-acute rehabilitation setting: Preliminary data of an observational study. Neurol. Sci. 2022, 43, 51–58. [Google Scholar] [CrossRef]
- Walle-Hansen, M.M.; Ranhoff, A.H.; Mellingsæter, M.; Wang-Hansen, M.S.; Myrstad, M. Health-related quality of life, functional decline, and long-term mortality in older patients following hospitalisation due to COVID-19. BMC Geriatr. 2021, 21, 199. [Google Scholar] [CrossRef]
- Sardella, A.; Chiara, E.; Alibrandi, A.; Bellone, F.; Catalano, A.; Lenzo, V.; Quattropani, M.C.; Basile, G. Changes in Cognitive and Functional Status and in Quality of Life of Older Outpatients during the COVID-19 Pandemic. Gerontology 2022, 68, 1285–1290. [Google Scholar] [CrossRef] [PubMed]
- Buonsenso, D.; Munblit, D.; De Rose, C.; Sinatti, D.; Ricchiuto, A.; Carfi, A.; Valentini, P. Preliminary evidence on long COVID in children. Acta Paediatr. 2021, 110, 2208–2211. [Google Scholar] [CrossRef] [PubMed]
- Giordano, G.M.; Brando, F.; Pezzella, P.; De Angelis, M.; Mucci, A.; Galderisi, S. Factors influencing the outcome of integrated therapy approach in schizophrenia: A narrative review of the literature. Front. Psychiatry 2022, 13, 970210. [Google Scholar] [CrossRef] [PubMed]
- Giuliani, L.; Giordano, G.M.; Bucci, P.; Pezzella, P.; Brando, F.; Galderisi, S. Improving Knowledge on Pathways to Functional Outcome in Schizophrenia: Main Results From the Italian Network for Research on Psychoses. Front. Psychiatry 2021, 12, 791117. [Google Scholar] [CrossRef] [PubMed]
- Penninx, B. Psychiatric symptoms and cognitive impairment in “Long COVID”: The relevance of immunopsychiatry. World Psychiatry 2021, 20, 357–358. [Google Scholar] [CrossRef] [PubMed]
- Greenberg, N.; Rafferty, L. Post-traumatic stress disorder in the aftermath of COVID-19 pandemic. World Psychiatry 2021, 20, 53–54. [Google Scholar] [CrossRef] [PubMed]
- Holt-Lunstad, J. A pandemic of social isolation? World Psychiatry 2021, 20, 55–56. [Google Scholar] [CrossRef]
- Wang, Q.; Xu, R.; Volkow, N.D. Increased risk of COVID-19 infection and mortality in people with mental disorders: Analysis from electronic health records in the United States. World Psychiatry 2021, 20, 124–130. [Google Scholar] [CrossRef]
- Stewart, D.E.; Appelbaum, P.S. COVID-19 and psychiatrists’ responsibilities: A WPA position paper. World Psychiatry 2020, 19, 406–407. [Google Scholar] [CrossRef]
- Pinto, T.C.C.; Machado, L.; Bulgacov, T.M.; Rodrigues-Júnior, A.L.; Costa, M.L.G.; Ximenes, R.C.C.; Sougey, E.B. Is the Montreal Cognitive Assessment (MoCA) screening superior to the Mini-Mental State Examination (MMSE) in the detection of mild cognitive impairment (MCI) and Alzheimer’s Disease (AD) in the elderly? Int. Psychogeriatr. 2019, 31, 491–504. [Google Scholar] [CrossRef]
- Tsoi, K.K.; Chan, J.Y.; Hirai, H.W.; Wong, S.Y.; Kwok, T.C. Cognitive Tests to Detect Dementia: A Systematic Review and Meta-analysis. JAMA Intern. Med. 2015, 175, 1450–1458. [Google Scholar] [CrossRef] [PubMed]
- Aboujaoude, E.; Gega, L.; Saltarelli, A.J. The retention challenge in remote therapy and learning seen through the lens of the COVID-19 pandemic. World Psychiatry 2021, 20, 138–139. [Google Scholar] [CrossRef] [PubMed]
- Maleki Dana, P.; Sadoughi, F.; Hallajzadeh, J.; Asemi, Z.; Mansournia, M.A.; Yousefi, B.; Momen-Heravi, M. An Insight into the Sex Differences in COVID-19 Patients: What are the Possible Causes? Prehosp. Disaster Med. 2020, 35, 438–441. [Google Scholar] [CrossRef]
- Michelutti, M.; Furlanis, G.; Buoite Stella, A.; Bellavita, G.; Frezza, N.; Torresin, G.; Ajčević, M.; Manganotti, P. Sex-dependent characteristics of Neuro-Long-COVID: Data from a dedicated neurology ambulatory service. J. Neurol. Sci. 2022, 441, 120355. [Google Scholar] [CrossRef] [PubMed]
- Bai, F.; Tomasoni, D.; Falcinella, C.; Barbanotti, D.; Castoldi, R.; Mulè, G.; Augello, M.; Mondatore, D.; Allegrini, M.; Cona, A.; et al. Female gender is associated with long COVID syndrome: A prospective cohort study. Clin. Microbiol. Infect. 2022, 28, P611.e9–P611.e16. [Google Scholar] [CrossRef]
- Pletzer, B.; Harris, T.-A.; Scheuringer, A.; Hidalgo-Lopez, E. The cycling brain: Menstrual cycle related fluctuations in hippocampal and fronto-striatal activation and connectivity during cognitive tasks. Neuropsychopharmacology 2019, 44, 1867–1875. [Google Scholar] [CrossRef]
- Eshkoor, S.A.; Hamid, T.A.; Mun, C.Y.; Ng, C.K. Mild cognitive impairment and its management in older people. Clin. Interv. Aging 2015, 10, 687–693. [Google Scholar] [CrossRef]
- Reynolds, C.F., 3rd; Jeste, D.V.; Sachdev, P.S.; Blazer, D.G. Mental health care for older adults: Recent advances and new directions in clinical practice and research. World Psychiatry 2022, 21, 336–363. [Google Scholar] [CrossRef]
- Solomon, T. Neurological infection with SARS-CoV-2—The story so far. Nat. Rev. Neurol. 2021, 17, 65–66. [Google Scholar] [CrossRef]
- Biagianti, B.; Di Liberto, A.; Nicolò Edoardo, A.; Lisi, I.; Nobilia, L.; de Ferrabonc, G.D.; Zanier, E.R.; Stocchetti, N.; Brambilla, P. Cognitive Assessment in SARS-CoV-2 Patients: A Systematic Review. Front. Aging Neurosci. 2022, 14, 909661. [Google Scholar] [CrossRef]
- Sasannejad, C.; Ely, E.W.; Lahiri, S. Long-term cognitive impairment after acute respiratory distress syndrome: A review of clinical impact and pathophysiological mechanisms. Crit. Care 2019, 23, 352. [Google Scholar] [CrossRef]
- Bandala, C.; Cortes-Altamirano, J.L.; Reyes-Long, S.; Lara-Padilla, E.; Ilizaliturri-Flores, I.; Alfaro-Rodríguez, A. Putative mechanism of neurological damage in COVID-19 infection. Acta Neurobiol. Exp. 2021, 81, 69–79. [Google Scholar] [CrossRef] [PubMed]
- Hawkins, M.; Sockalingam, S.; Bonato, S.; Rajaratnam, T.; Ravindran, M.; Gosse, P.; Sheehan, K.A. A rapid review of the pathoetiology, presentation, and management of delirium in adults with COVID-19. J. Psychosom. Res. 2021, 141, 110350. [Google Scholar] [CrossRef] [PubMed]
- Grasby, K.L.; Jahanshad, N.; Painter, J.N.; Colodro-Conde, L.; Bralten, J.; Hibar, D.P.; Lind, P.A.; Pizzagalli, F.; Ching, C.R.K.; McMahon, M.A.B.; et al. The genetic architecture of the human cerebral cortex. Science 2020, 367, eaay6690. [Google Scholar] [CrossRef] [PubMed]
- Areza-Fegyveres, R.; Kairalla, R.A.; Carvalho, C.R.R.; Nitrini, R. Cognition and chronic hypoxia in pulmonary diseases. Dement. Neuropsychol. 2010, 4, 14–22. [Google Scholar] [CrossRef]
- Menon, V. Brain networks and cognitive impairment in psychiatric disorders. World Psychiatry 2020, 19, 309–310. [Google Scholar] [CrossRef]
- Alemanno, F.; Houdayer, E.; Parma, A.; Spina, A.; Del Forno, A.; Scatolini, A.; Angelone, S.; Brugliera, L.; Tettamanti, A.; Beretta, L.; et al. COVID-19 cognitive deficits after respiratory assistance in the subacute phase: A COVID-rehabilitation unit experience. PLoS ONE 2021, 16, e0246590. [Google Scholar] [CrossRef]
- Vanderlind, W.M.; Rabinovitz, B.B.; Miao, I.Y.; Oberlin, L.E.; Bueno-Castellano, C.; Fridman, C.; Jaywant, A.; Kanellopoulos, D. A systematic review of neuropsychological and psychiatric sequalae of COVID-19: Implications for treatment. Curr. Opin. Psychiatry 2021, 34, 420–433. [Google Scholar] [CrossRef]
- Altuna, M.; Sánchez-Saudinós, M.B.; Lleó, A. Cognitive symptoms after COVID-19. Neurol. Perspect. 2021, 1, S16–S24. [Google Scholar] [CrossRef]
- Daroische, R.; Hemminghyth, M.S.; Eilertsen, T.H.; Breitve, M.H.; Chwiszczuk, L.J. Cognitive Impairment After COVID-19—A Review on Objective Test Data. Front. Neurol. 2021, 12, 699582. [Google Scholar] [CrossRef]
- Rabinovitz, B.; Jaywant, A.; Fridman, C.B. Neuropsychological functioning in severe acute respiratory disorders caused by the coronavirus: Implications for the current COVID-19 pandemic. Clin. Neuropsychol. 2020, 34, 1453–1479. [Google Scholar] [CrossRef] [PubMed]
| Cognitive Domain (and Subdomains if Present) | Assessment Scales | Studies | |
|---|---|---|---|
| Executive Functions | FAB | [43,44,45,46,47,59,60,63,66,101] | |
| TMT-B | [40,46,50,52,53,54,58,59,61,63,64,65,69,81,84,96,100,101] | ||
| Stroop Color-Word Test | [43,54,59,60,61,84,96,101] | ||
| Tower of London, | [73] | ||
| CDT | [62] | ||
| WCST | [71] | ||
| Similarities test from the Wechsler Adult Intelligence Scale (WAIS IV), | [60] | ||
| Brixton test | [60] | ||
| D-KEFS | [50] | ||
| Speed Of Processing | TMT-A | [46,50,52,53,54,59,61,63,65,69,81,84,96,100,101] | |
| SDMT | [49,59,61,63,81,83,96,101] | ||
| SCT | [65] | ||
| SYMBOL SEARCH | [84] | ||
| PASAT | [83] | ||
| Memory | Episodic Memory | FCRST | [69] |
| List Learning Test | [54] | ||
| Dubois five words test | [60] | ||
| Logical Memory I e II from the Weschler Memory Scale (WMS-IV) | [91] | ||
| Category Cued Verbal Fluency | [53] | ||
| Verbal Memory | Word List Recognition Memory Test | [71] | |
| VVM | [91] | ||
| Pictorial Associative Memory Test | [71] | ||
| Visuospatial Memory | ROCFT/RCFT | [50,69,73,84] | |
| DRT | [69] | ||
| BVMT-R | [81] | ||
| VOSP | [63] | ||
| NAB (visual discrimination task) | [50] | ||
| SPART | [83] | ||
| 2D-Mental Rotation Test | [71] | ||
| Working Memory | Digit Span Forward and Reverse | [50,53,59,60,62,63,65,78,81,84,89,96,101]. | |
| Attention/ Vigilance | RBANS | [52,86] | |
| Digit Span Forward and Reverse | [50,53,59,60,62,63,65,78,81,84,89,96,101]. | ||
| CPT-II | [65,84] | ||
| Tea Attention Test | [73] | ||
| D2-R Test | [95] | ||
| Attentional Matrices | [66] | ||
| TAP | [91] | ||
| Vigilance Task | [43] | ||
| CVAT | [51] | ||
| Language | Global Evaluation | BNT | [69,84] |
| 40 Words oral naming test | [60] | ||
| SAND | [63] | ||
| BDAE (Language subtest) | [52] | ||
| Categorical and lexical verbal fluencies during two-minute test | [60] | ||
| Verbal Fluency | FAS | [69,78,101] | |
| Letter Cued Verbal Fluency | [53] | ||
| Semantic Fluency Test | [61,62,81,96] | ||
| Phonemic Fluency Test | [61,96,100] | ||
| Category Fluency Test | [53,59,71,100] | ||
| WLG | [83] | ||
| COWA | [73,89] | ||
| Verbal Learning | RAVLT | [53,59,62,63,78,84], | |
| HVLT-R | [52,81,96] | ||
| TAVEC | [61,69] | ||
| SRT | [83] | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Perrottelli, A.; Sansone, N.; Giordano, G.M.; Caporusso, E.; Giuliani, L.; Melillo, A.; Pezzella, P.; Bucci, P.; Mucci, A.; Galderisi, S. Cognitive Impairment after Post-Acute COVID-19 Infection: A Systematic Review of the Literature. J. Pers. Med. 2022, 12, 2070. https://doi.org/10.3390/jpm12122070
Perrottelli A, Sansone N, Giordano GM, Caporusso E, Giuliani L, Melillo A, Pezzella P, Bucci P, Mucci A, Galderisi S. Cognitive Impairment after Post-Acute COVID-19 Infection: A Systematic Review of the Literature. Journal of Personalized Medicine. 2022; 12(12):2070. https://doi.org/10.3390/jpm12122070
Chicago/Turabian StylePerrottelli, Andrea, Noemi Sansone, Giulia Maria Giordano, Edoardo Caporusso, Luigi Giuliani, Antonio Melillo, Pasquale Pezzella, Paola Bucci, Armida Mucci, and Silvana Galderisi. 2022. "Cognitive Impairment after Post-Acute COVID-19 Infection: A Systematic Review of the Literature" Journal of Personalized Medicine 12, no. 12: 2070. https://doi.org/10.3390/jpm12122070
APA StylePerrottelli, A., Sansone, N., Giordano, G. M., Caporusso, E., Giuliani, L., Melillo, A., Pezzella, P., Bucci, P., Mucci, A., & Galderisi, S. (2022). Cognitive Impairment after Post-Acute COVID-19 Infection: A Systematic Review of the Literature. Journal of Personalized Medicine, 12(12), 2070. https://doi.org/10.3390/jpm12122070








