Level of Estrogen in Females—The Different Impacts at Different Life Stages
Abstract
:1. Introduction
2. Sex Differences in Lifespan and Estrogen
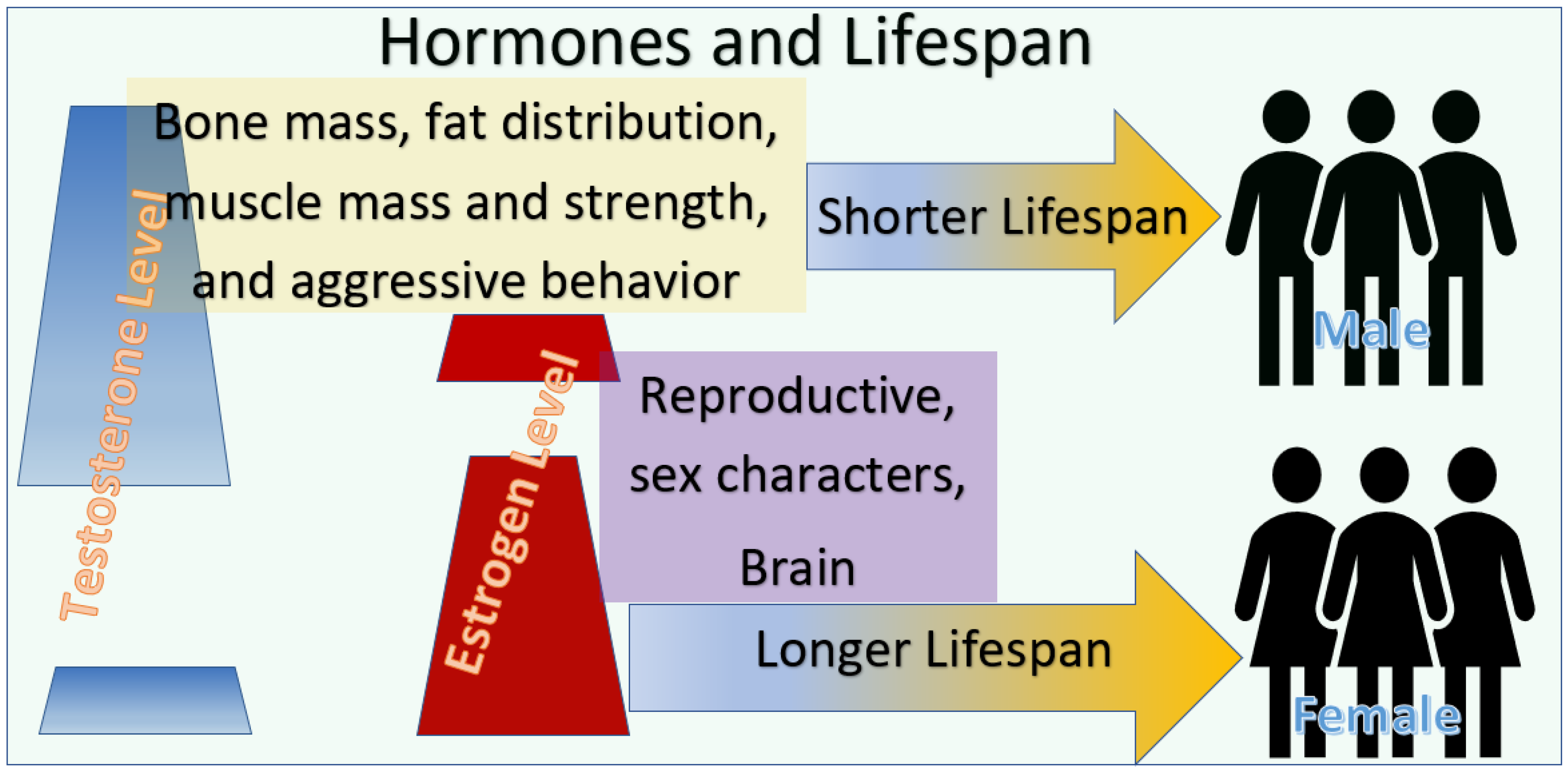
2.1. The Observed Sex Differences in the Lifespan
2.2. The Paradigm of the Impact of Low Estrogen Levels on the Healthy Life of Females
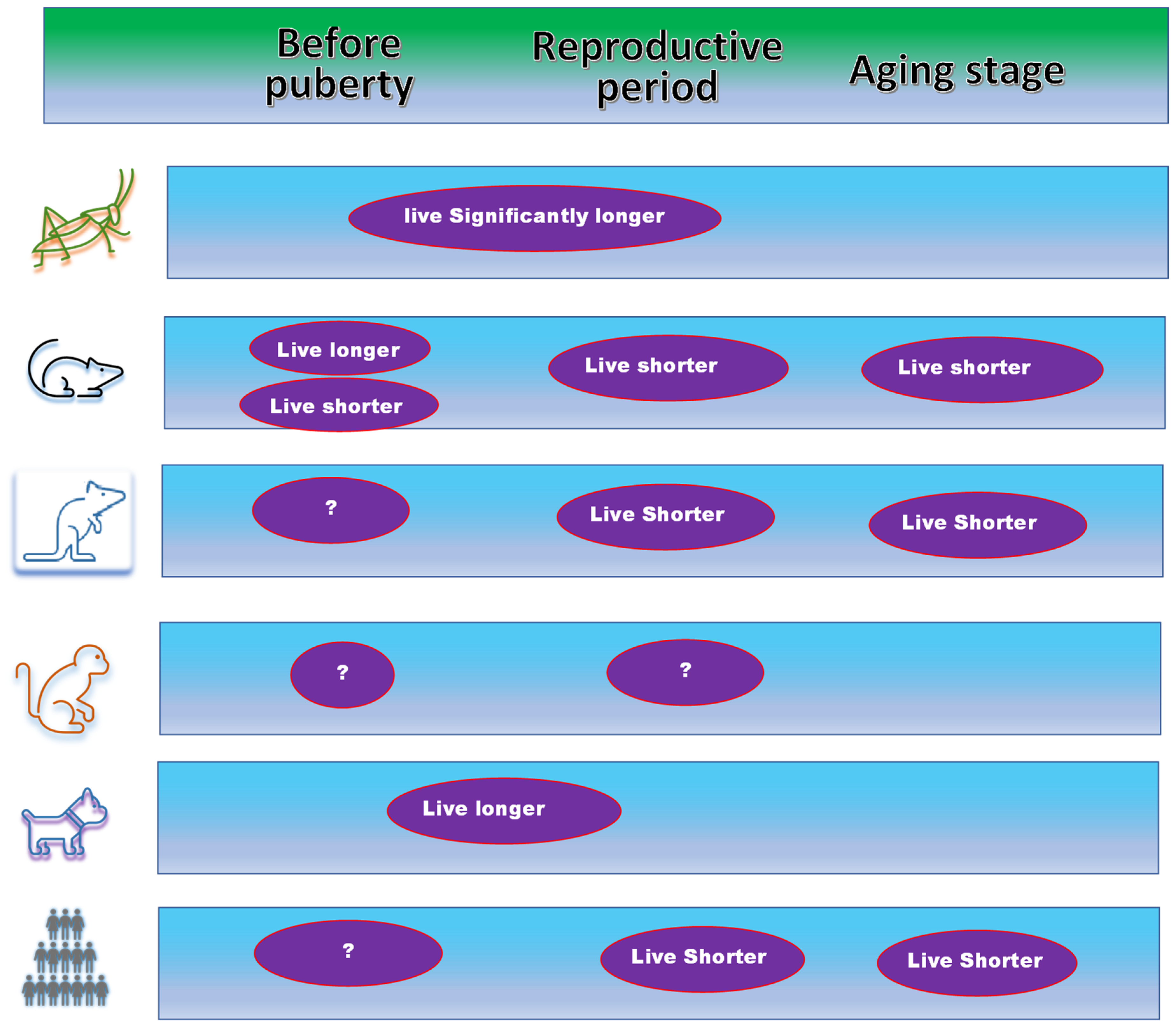
2.3. Confusing Data from Animal Models on the Function of Estrogen
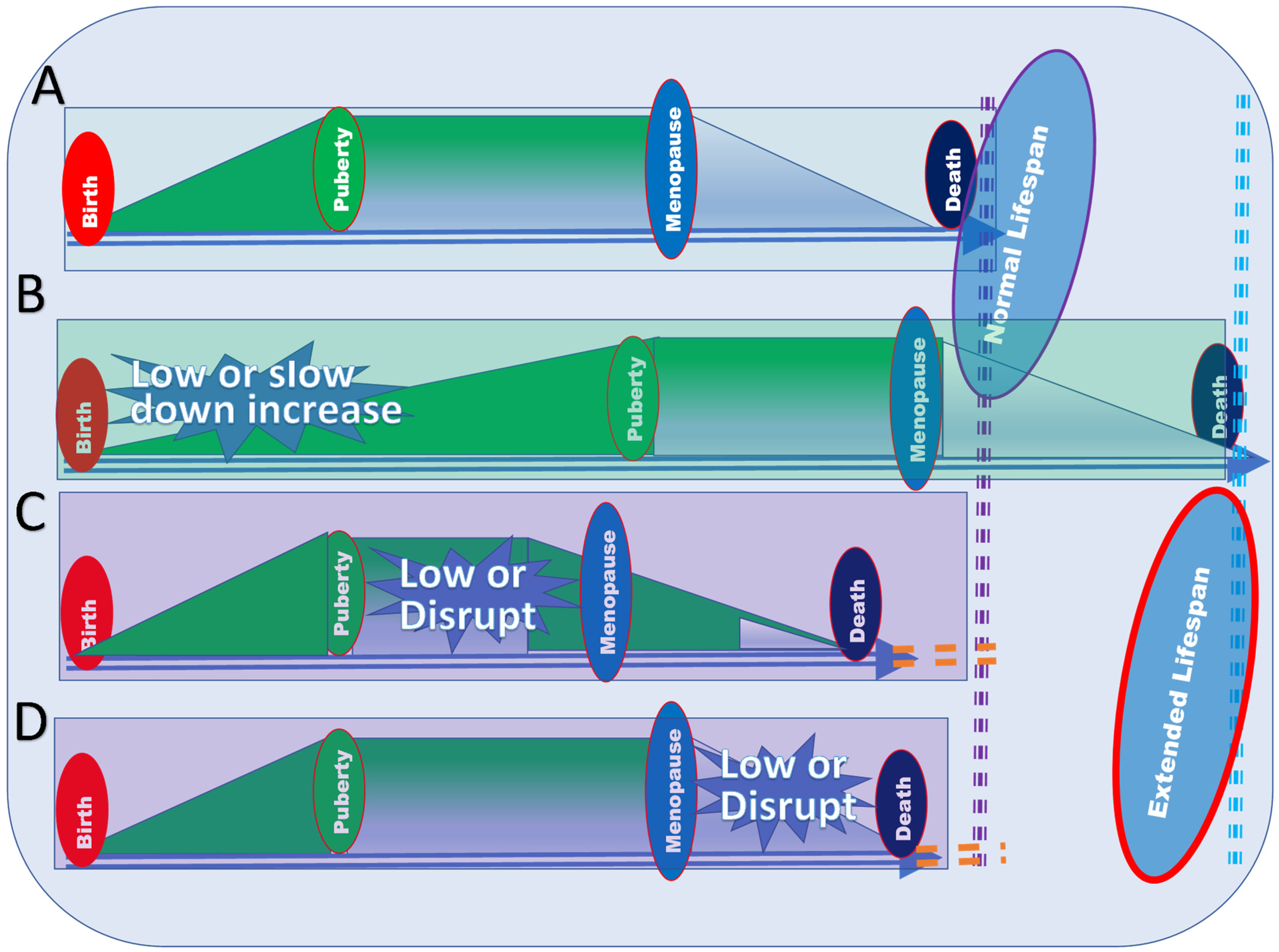
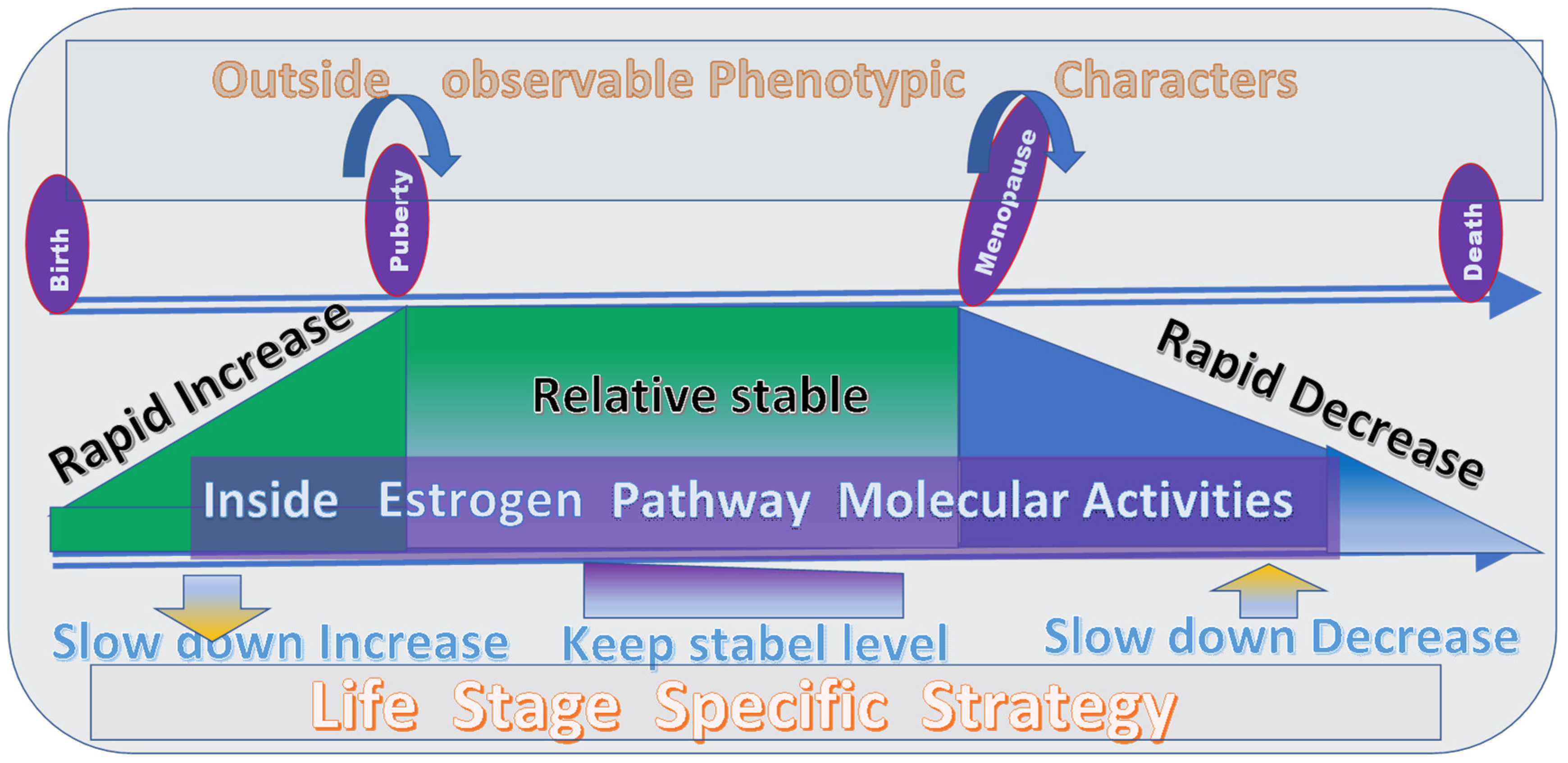
3. Regulation of the Level of Estrogen to Increase the Lifespan Based on the PLOSP
3.1. Explanation of Effects of Estrogen at Different Life Stages Based on the PLOSP
3.2. Differences between Ovariectomy and Orchiectomy in Different Life Stages
3.3. The Impact of Ovariectomy Is Much More than a Decrease in Estrogen
3.4. Manipulation of Estrogen Levels at Different Life Stages Based on the PLOSP
3.5. Hormone Replacement Therapy as Evidence Suporting the Regulation of the Level of Estrogen to Increase the Lifespan Based on the PLOSP
4. Considerations for the Regulation of Estrogen Based on the PLOSP
4.1. Correct Interpretation of Estrogen Levels Based on the PLOSP
4.2. Systematically Testing the Effects of Estrogen Based on the PLOSP Hypothesis
5. Conclusions: Integration and Personalization of Estrogen Levels for Individual Life Stages
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Clegg, D.; Hevener, A.L.; Moreau, K.L.; Morselli, E.; Criollo, A.; Van Pelt, R.E.; Vieira-Potter, V.J. Sex hormones and cardiometabolic health: Role of estrogen and estrogen receptors. Endocrinology 2017, 158, 1095–1105. [Google Scholar] [CrossRef] [PubMed]
- Nieschlag, E.; Nieschlag, S.; Behre, H.M. Lifespan and testosterone. Nature 1993, 366, 215. [Google Scholar] [CrossRef] [PubMed]
- Stanhewicz, A.; Wenner, M.M.; Stachenfeld, N.S. Sex differences in endothelial function important to vascular health and overall cardiovascular disease risk across the lifespan. Am. J. Physiol. Circ. Physiol. 2018, 315, H1569–H1588. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parker, W.H.; Jacoby, V.; Shoupe, D.; Rocca, W. Effect of bilateral oophorectomy on women’s long-term health. Women’s Health 2009, 5, 565–576. [Google Scholar] [CrossRef] [PubMed]
- Faubion, S.S.; Kuhle, C.L.; Shuster, L.T.; Rocca, W.A. Long-term health consequences of premature or early menopause and considerations for management. Climacteric 2015, 18, 483–491. [Google Scholar] [CrossRef] [Green Version]
- Nickels, F.A. Complications of castration and ovariectomy. Vet. Clin. N. Am. Equine Pract. 1988, 4, 515–523. [Google Scholar] [CrossRef]
- Niwano, Y.; Kohzaki, H.; Shirato, M.; Shishido, S.; Nakamura, K. Anti-osteoporotic mechanisms of polyphenols elucidated based on in vivo studies using ovariectomized animals. Antioxidants 2022, 11, 217. [Google Scholar] [CrossRef]
- Gu, W. Healthy long-lived human beings—Working on life stages to break the limitation of human lifespans. Biology 2022, 11, 656. [Google Scholar] [CrossRef]
- Hughes, A.; Kumari, M. Testosterone, risk, and socioeconomic position in British men: Exploring causal directionality. Soc. Sci. Med. 2018, 220, 129–140. [Google Scholar] [CrossRef]
- Morley, J.E.; Haren, M.T.; Kim, M.J.; Kevorkian, R.; Perry, H.M. 3rd. Testosterone, aging and quality of life. J. Endocrinol. Investig. 2005, 28, 76–80. [Google Scholar]
- Kundakovic, M.; Rocks, D. Sex hormone fluctuation and increased female risk for depression and anxiety disorders: From clinical evidence to molecular mechanisms. Front. Neuroendocrinol. 2022, 66, 101010. [Google Scholar] [CrossRef]
- Gu, W. It is time to work on the extension of body growth and reproductive stages. Rejuvenation Res. 2022, 25, 110–115. [Google Scholar] [CrossRef]
- Nappi, R.; Sinforiani, E.; Mauri, M.; Bono, G.; Polatti, F.; Nappi, G. Memory functioning at menopause: Impact of age in ovariectomized women. Gynecol. Obstet. Investig. 1999, 47, 29–36. [Google Scholar] [CrossRef]
- Apelo, S.I.A.; Lin, A.; Brinkman, J.; Meyer, E.; Morrison, M.; Tomasiewicz, J.L.; Pumper, C.P.; Baar, E.L.; Richardson, N.; Alotaibi, M.; et al. Ovariectomy uncouples lifespan from metabolic health and reveals a sex-hormone-dependent role of hepatic mTORC2 in aging. Elife 2020, 9, e56177. [Google Scholar] [CrossRef]
- Tetlak, A.G.; Burnett, J.B.; Hahn, D.A.; Hatle, J.D. Vitellogenin-RNAi and ovariectomy each increase lifespan, increase protein storage, and decrease feeding, but are not additive in grasshoppers. Biogerontology 2015, 16, 761–774. [Google Scholar] [CrossRef] [Green Version]
- Urfer, S.R.; Kaeberlein, M. Desexing dogs: A review of the current literature. Animals 2019, 9, 1086. [Google Scholar] [CrossRef] [Green Version]
- Dutta, S.; Sengupta, P. Men and mice: Relating their ages. Life Sci. 2016, 152, 244–248. [Google Scholar] [CrossRef]
- Sengupta, P. The laboratory rat: Relating its age with human’s. Int. J. Prev. Med. 2013, 4, 624–630. [Google Scholar]
- Benedusi, V.; Martini, E.; Kallikourdis, M.; Villa, A.; Meda, C.; Maggi, A. Ovariectomy shortens the life span of female mice. Oncotarget 2015, 6, 10801–10811. [Google Scholar] [CrossRef] [Green Version]
- Choi, C.-I.; Lee, Y.-D.; Gwag, B.J.; Cho, S.I.; Kim, S.-S.; Suh-Kim, H. Effects of estrogen on lifespan and motor functions in female hSOD1 G93A transgenic mice. J. Neurol. Sci. 2008, 268, 40–47. [Google Scholar] [CrossRef]
- Gresack, J.E.; Kerr, K.M.; Frick, K.M. Life-long environmental enrichment differentially affects the mnemonic response to estrogen in young, middle-aged, and aged female mice. Neurobiol. Learn. Mem. 2007, 88, 393–408. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ponnamperuma, R.M.; Kirchhof, S.M.; Trifiletti, L.; De Luca, L.M. Ovariectomy increases squamous metaplasia of the uterine horns and survival of SENCAR mice fed a vitamin A-deficient diet. Am. J. Clin. Nutr. 1999, 70, 502–508. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cargill, S.L.; Carey, J.R.; Muller, H.-G.; Anderson, G. Age of ovary determines remaining life expectancy in old ovariectomized mice. Aging Cell 2003, 2, 185–190. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Timmermans, S.; Van Montagu, M.; Libert, C. Complete overview of protein-inactivating sequence variations in 36 sequenced mouse inbred strains. Proc. Natl. Acad. Sci. USA 2017, 114, 9158–9163. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dias, D.D.S.; Moraes-Silva, I.C.; Bernardes, N.; Brito-Monzani, J.D.O.; Stoyell-Conti, F.F.; Machi, J.F.; Llesuy, S.; Irigoyen, M.-C.; De Angelis, K. Exercise training initiated at old stage of lifespan attenuates aging-and ovariectomy-induced cardiac and renal oxidative stress: Role of baroreflex. Exp. Gerontol. 2019, 124, 110635. [Google Scholar] [CrossRef]
- Iwasa, T.; Matsuzaki, T.; Yano, K.; Irahara, M. The effects of ovariectomy and lifelong high-fat diet consumption on body weight, appetite, and lifespan in female rats. Horm. Behav. 2017, 97, 25–30. [Google Scholar] [CrossRef]
- Baeza, I.; De Castro, N.M.; Arranz, L.; Fdez-Tresguerres, J.; De la Fuente, M. Ovariectomy causes immunosenescence and oxi-inflamm-ageing in peritoneal leukocytes of aged female mice similar to that in aged males. Biogerontology 2011, 12, 227–238. [Google Scholar] [CrossRef]
- Bame, M.; Pentiak, P.A.; Needleman, R.; Brusilow, W.S. Effect of sex on lifespan, disease progression, and the response to methionine sulfoximine in the SOD1 G93A Mouse Model for ALS. Gend. Med. 2012, 9, 524–535. [Google Scholar] [CrossRef]
- Hatle, J.D.; Paterson, C.S.; Jawaid, I.; Lentz, C.; Wells, S.M.; Fronstin, R.B. Protein accumulation underlying lifespan extension via ovariectomy in grasshoppers is consistent with the disposable soma hypothesis but is not due to dietary restriction. Exp. Gerontol. 2008, 43, 900–908. [Google Scholar] [CrossRef] [Green Version]
- Hatle, J.D.; Awan, A.; Nicholas, J.; Koch, R.; Vokrri, J.R.; McCue, M.D.; Williams, C.M.; Davidowitz, G.; Hahn, D.A. Life-extending dietary restriction and ovariectomy each increase leucine oxidation and alter leucine allocation in grasshoppers. Exp. Gerontol. 2017, 96, 155–161. [Google Scholar] [CrossRef]
- Armstrong, K.; Schwartz, J.S.; Randall, T.; Rubin, S.C.; Weber, B. Hormone Replacement Therapy and Life Expectancy After Prophylactic Oophorectomy in Women with BRCA1/2 Mutations: A Decision Analysis. J. Clin. Oncol. 2004, 22, 1045–1054. [Google Scholar] [CrossRef]
- Panico, S.; Galasso, R.; Celentano, E.; Ciardullo, A.V.; Frova, L.; Capocaccia, R.; Trevisan, M.; Berrino, F. Large-scale hormone replacement therapy and life expectancy: Results from an international comparison among European and North American populations. Am. J. Public Health 2000, 90, 1397–1402. [Google Scholar] [CrossRef]
- Comhaire, F. Hormone replacement therapy and longevity. Andrologia 2015, 48, 65–68. [Google Scholar] [CrossRef]
- Shi, C.; Bao, Y.; Chen, X.; Tian, L. The effectiveness of thyroid hormone replacement therapy on heart failure and low-triiodothyronine syndrome: An updated systematic review and meta-analysis of randomized controlled trials. Endocr. Pract. 2022, 28, 1178–1186. [Google Scholar] [CrossRef]
- De Almeida, M.M.; Dias-Rocha, C.P.; Reis-Gomes, C.F.; Wang, H.; Cordeiro, A.; Pazos-Moura, C.C.; Joss-Moore, L.; Trevenzoli, I.H. Maternal high-fat diet up-regulates type-1 cannabinoid receptor with estrogen signaling changes in a sex- and depot- specific manner in white adipose tissue of adult rat offspring. Eur. J. Nutr. 2020, 60, 1313–1326. [Google Scholar] [CrossRef]
- Thompson, E.E.; Nicodemus-Johnson, J.; Kim, K.W.; Gern, J.E.; Jackson, D.J.; Lemanske, R.F.; Ober, C. Global DNA methylation changes spanning puberty are near predicted estrogen-responsive genes and enriched for genes involved in endocrine and immune processes. Clin. Epigenetics 2018, 10, 62. [Google Scholar] [CrossRef] [Green Version]
- Kang, B.H.; Cho, J.H.; Kim, S.Y.; Jeong, K.; Kim, S.-H.; Kim, C.; Lim, S.-J.; Shim, K.S. Growth and bone mineral density changes in ovariectomized rats treated with estrogen receptor alpha or beta agonists. J. Korean Med. Sci. 2020, 35, e370. [Google Scholar] [CrossRef]
- Leck, I.; Thomson, J.M.; Bocaz, J.; Barja, P.; Bonnar, J.; Daly, L.; Carrol, A.; Coutinho, E.; Goncalves, M.; Tsakok, M.; et al. A multicentre study of coagulation and haemostatic variables during oral contraception: Variations with geographical location and ethnicity. Int. J. Epidemiol. 1991, 20, 913–920. [Google Scholar] [CrossRef]
| Species | Time of Conduct | Approximate Human Age Equivalents * [17,18] | Effect on Lifespan | Reference # and Note |
|---|---|---|---|---|
| Mice | One month, after the first estrous cycle; 5 months of age | 14 years of age and 30 years of age | Shortens the life span | [19] |
| Thirteen months of age | 60 years of age | Immunosenescence and oxi-inflamm-ageing | [20] | |
| At postnatal day 50 | 18 years of age | Correlation of the estrogen levels and the disease progression including lifespan | [21] | |
| Two weeks prior to behavioral testing (at ages of 5,17, and 22 months), mice were ovariectomized | 30, 65, and 68 years of ages | Shortened lifespan in comparison with intact animals. | [22] | |
| At 4 weeks, bilaterally At 3 weeks, bilateral ovariectomies | 13 years of age | Prolongs survival time | [23] | |
| - | 11 years of age | Control/Ox = 265.6/230.5 | [24] With CBA strain. | |
| Rat | 3 and 18 months | 7 and 45 years of age | Inducing complications | [25] |
| At 23 weeks of age | 57 years of age | Alterations in body weight and energy metabolism induced by ovariectomy and high-fat diet consumption might not directly affect the lifespan of female rats. | [26] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, Z.; Jiao, Y.; Zhao, Y.; Gu, W. Level of Estrogen in Females—The Different Impacts at Different Life Stages. J. Pers. Med. 2022, 12, 1995. https://doi.org/10.3390/jpm12121995
Yu Z, Jiao Y, Zhao Y, Gu W. Level of Estrogen in Females—The Different Impacts at Different Life Stages. Journal of Personalized Medicine. 2022; 12(12):1995. https://doi.org/10.3390/jpm12121995
Chicago/Turabian StyleYu, Zhuo, Yan Jiao, Yinhuan Zhao, and Weikuan Gu. 2022. "Level of Estrogen in Females—The Different Impacts at Different Life Stages" Journal of Personalized Medicine 12, no. 12: 1995. https://doi.org/10.3390/jpm12121995
APA StyleYu, Z., Jiao, Y., Zhao, Y., & Gu, W. (2022). Level of Estrogen in Females—The Different Impacts at Different Life Stages. Journal of Personalized Medicine, 12(12), 1995. https://doi.org/10.3390/jpm12121995








