The Dashboard Vitals of Parkinson’s: Not to Be Missed Yet an Unmet Need
1. Commentary
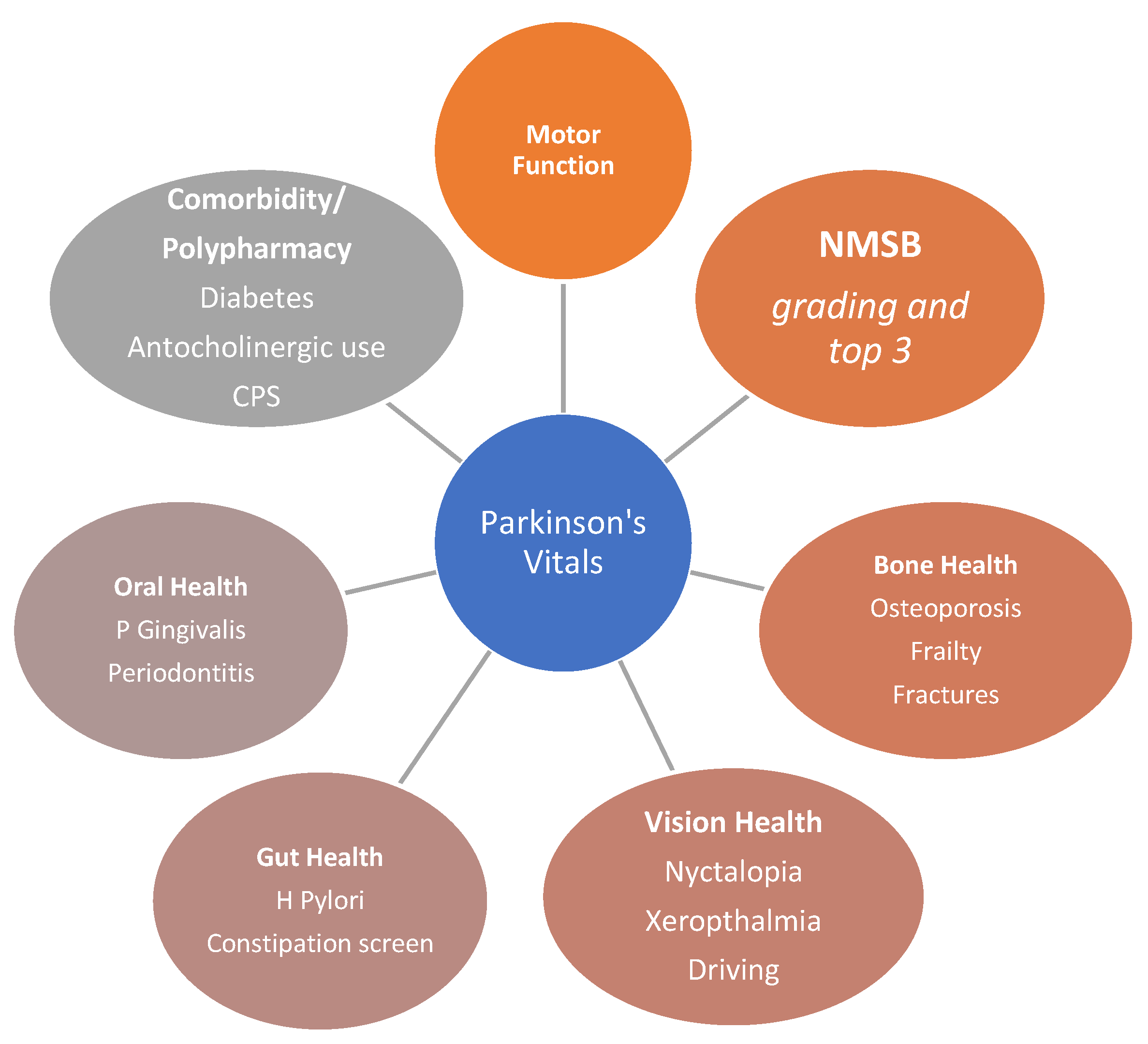
- Specific attention and query about oral health, gum, and gingivitis and an examination by a dentist in all cases. Infection with porphyromonas gingivalis, a Gram-negative anaerobic bacterium, can cause chronic periodontitis and possibly systemic inflammation, together with gingipains, and may have an overall effect on worsening of the Parkinsonian state and even pathogenesis [33]. A recent study suggested that high serum C-reactive protein (CRP) level may be a good indicator of periodontitis and should trigger a referral to a dentist and needs to feature in the dashboard [34].
- Delayed oral drug absorption as well as clinical phenomena of “delayed on” or “no on” or even dyskinesias-related erratic absorption may relate to delayed gastric emptying and “gastric blocks”. Helicobacter pylori (H Pylori) infection, a Gram-negative bacteria, in the stomach is common in PD and several case-control studies report that prevalence of H Pylori infection is five-times higher in older PD patients, specifically those over 80 years of age, and up to three-times higher in PD patients compared to healthy individuals [35].
- Eradication of H Pylori infection using combined antibiotic therapies can improve bioavailability and pharmacokinetics of levodopa and drug bioavailability by increasing its absorption by 21 to 54%, despite one single-centre negative study. The latter study, however, did not address blood levels of levodopa and instead focused on quality of life and motor scores [36]. Any patient with delayed time to ‘ON’ after oral levodopa absorption, as well as upper gastrointestinal symptoms of heartburn, bloating, and reflux, must have H Pylori infection tested and, if positive, be treated [37].
- Severe constipation may arise from chronic dehydration and impacted faeces. This also interferes with oral drug absorption and a simple abdominal X-ray may show dilated bowel loops and impacted faeces [38,39]. Treatment with regular laxatives and even an enema may then be warranted, as part of the vitals, in relevant cases.
2. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Subramanian, I.; Brindle, S.; Perepezko, K.; Chaudhuri, K.R. Wellness, sexual health, and nonmotor Parkinson’s. Int. Rev. Neurobiol. 2022, 162, 171–184. [Google Scholar] [CrossRef] [PubMed]
- LeWitt, P.A.; Chaudhuri, K.R. Unmet needs in Parkinson disease: Motor and non-motor. Park. Relat. Disord. 2020, 80, S7–S12. [Google Scholar] [CrossRef] [PubMed]
- Titova, N.; Chaudhuri, K.R. Personalized Medicine and Nonmotor Symptoms in Parkinson’s Disease. Int. Rev. Neurobiol. 2017, 134, 1257–1281. [Google Scholar] [CrossRef]
- Titova, N.; Chaudhuri, K.R. Personalized medicine in Parkinson’s disease: Time to be precise. Mov. Disord. 2017, 32, 1147–1154. [Google Scholar] [CrossRef] [PubMed]
- Hoehn, M.M.; Yahr, M.D. Parkinsonism: Onset, progression and mortality. Neurology 1967, 17, 427–442. [Google Scholar] [CrossRef] [Green Version]
- Martínez-Martín, P.; Benito-León, J.; Burguera, J.A.; Castro, A.; Linazasoro, G.; Martínez-Castrillo, J.C.; Valldeoriola, F.; Vázquez, A.; Vivancos, F.; del Val, J.; et al. The SCOPA-Motor Scale for assessment of Parkinson’s disease is a consistent and valid measure. J. Clin. Epidemiol. 2005, 58, 674–679. [Google Scholar] [CrossRef]
- Goetz, C.G.; Tilley, B.C.; Shaftman, S.R.; Stebbins, G.T.; Fahn, S.; Martinez-Martin, P.; Poewe, W.; Sampaio, C.; Stern, M.B.; Dodel, R.; et al. Movement Disorder Society-sponsored revision of the Unified Parkinson’s Disease Rating Scale (MDS-UPDRS): Scale presentation and clinimetric testing results. Mov. Disord. 2008, 23, 2129–2170. [Google Scholar] [CrossRef]
- Disease, M.D.S.T.F.o.R.S.f.P.s. The Unified Parkinson’s Disease Rating Scale (UPDRS): Status and recommendations. Mov. Disord. 2003, 18, 738–750. [Google Scholar] [CrossRef]
- Joshi, R.; Bronstein, J.M.; Keener, A.; Alcazar, J.; Yang, D.D.; Joshi, M.; Hermanowicz, N. PKG Movement Recording System Use Shows Promise in Routine Clinical Care of Patients With Parkinson’s Disease. Front. Neurol. 2019, 10, 1027. [Google Scholar] [CrossRef]
- Pahwa, R.; Bergquist, F.; Horne, M.; Minshall, M.E. Objective measurement in Parkinson’s disease: A descriptive analysis of Parkinson’s symptom scores from a large population of patients across the world using the Personal KinetiGraph®. J. Clin. Mov. Disord. 2020, 7, 5. [Google Scholar] [CrossRef]
- Romenets, S.R.; Wolfson, C.; Galatas, C.; Pelletier, A.; Altman, R.; Wadup, L.; Postuma, R.B. Validation of the non-motor symptoms questionnaire (NMS-Quest). Park. Relat. Disord. 2012, 18, 54–58. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Martin, P.; Ray Chaudhuri, K. Comprehensive grading of Parkinson’s disease using motor and non-motor assessments: Addressing a key unmet need. Expert Rev. Neurother. 2018, 18, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Sauerbier, A.; Qamar, M.A.; Rajah, T.; Chaudhuri, K.R. New concepts in the pathogenesis and presentation of Parkinson’s disease. Clin. Med. 2016, 16, 365–370. [Google Scholar] [CrossRef]
- Chaudhuri, K.R.; Sauerbier, A.; Rojo, J.M.; Sethi, K.; Schapira, A.H.; Brown, R.G.; Antonini, A.; Stocchi, F.; Odin, P.; Bhattacharya, K.; et al. The burden of non-motor symptoms in Parkinson’s disease using a self-completed non-motor questionnaire: A simple grading system. Park. Relat. Disord. 2015, 21, 287–291. [Google Scholar] [CrossRef] [PubMed]
- Todorova, A.; Martin, A.; Chaudhuri, K.R. How Do I Examine Nonmotor Aspects of Parkinson’s Disease? What Not to Miss and What to Ignore? Mov. Disord. Clin. Pract. 2014, 1, 274. [Google Scholar] [CrossRef]
- Ekker, M.S.; Janssen, S.; Seppi, K.; Poewe, W.; de Vries, N.M.; Theelen, T.; Nonnekes, J.; Bloem, B.R. Ocular and visual disorders in Parkinson’s disease: Common but frequently overlooked. Park. Relat. Disord. 2017, 40, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Armstrong, R.A. Visual Dysfunction in Parkinson’s Disease. Int. Rev. Neurobiol. 2017, 134, 921–946. [Google Scholar] [CrossRef]
- Armstrong, R.A. Oculo-Visual Dysfunction in Parkinson’s Disease. J. Parkinsons. Dis. 2015, 5, 715–726. [Google Scholar] [CrossRef] [Green Version]
- Borm, C.; Werkmann, M.; Visser, F.; Peball, M.; Putz, D.; Seppi, K.; Poewe, W.; Notting, I.C.; Vlaar, A.; Theelen, T.; et al. Towards seeing the visual impairments in Parkinson’s disease: Protocol for a multicentre observational, cross-sectional study. BMC Neurol. 2019, 19, 141. [Google Scholar] [CrossRef] [Green Version]
- Meppelink, A.M.; de Jong, B.M.; Renken, R.; Leenders, K.L.; Cornelissen, F.W.; van Laar, T. Impaired visual processing preceding image recognition in Parkinson’s disease patients with visual hallucinations. Brain 2009, 132, 2980–2993. [Google Scholar] [CrossRef]
- Sauerbier, A.; Ray Chaudhuri, K. Parkinson’s disease and vision. Basal Ganglia 2013, 3, 159–163. [Google Scholar] [CrossRef]
- Dennison, E.M.; Compston, J.E.; Flahive, J.; Siris, E.S.; Gehlbach, S.H.; Adachi, J.D.; Boonen, S.; Chapurlat, R.; Díez-Pérez, A.; Anderson, F.A., Jr.; et al. Effect of co-morbidities on fracture risk: Findings from the Global Longitudinal Study of Osteoporosis in Women (GLOW). Bone 2012, 50, 1288–1293. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bezza, A.; Ouzzif, Z.; Naji, H.; Achemlal, L.; Mounach, A.; Nouijai, M.; Bourazza, A.; Mossadeq, R.; El Maghraoui, A. Prevalence and risk factors of osteoporosis in patients with Parkinson’s disease. Rheumatol. Int. 2008, 28, 1205–1209. [Google Scholar] [CrossRef] [PubMed]
- Abou-Raya, S.; Helmii, M.; Abou-Raya, A. Bone and mineral metabolism in older adults with Parkinson’s disease. Age Ageing 2009, 38, 675–680. [Google Scholar] [CrossRef] [Green Version]
- Wood, B.; Walker, R. Osteoporosis in Parkinson’s disease. Mov. Disord. 2005, 20, 1636–1640. [Google Scholar] [CrossRef]
- Can, N.U.; Alagöz, A.N. The Relationship Among Bone Mineral Density, Bone Biomarkers and Vitamin D Levels in Patients with Parkinson’s Disease. Clin. Lab. 2020, 66, 8. [Google Scholar] [CrossRef]
- Sharma, J.C.; Lewis, A. Weight in Parkinson’s Disease: Phenotypical Significance. Int. Rev. Neurobiol. 2017, 134, 891–919. [Google Scholar] [CrossRef]
- Sharma, J.C.; Vassallo, M. Prognostic significance of weight changes in Parkinson’s disease: The Park-weight phenotype. Neurodegener. Dis. Manag. 2014, 4, 309–316. [Google Scholar] [CrossRef]
- Lorefält, B.; Ganowiak, W.; Pålhagen, S.; Toss, G.; Unosson, M.; Granérus, A.K. Factors of importance for weight loss in elderly patients with Parkinson’s disease. Acta Neurol. Scand. 2004, 110, 180–187. [Google Scholar] [CrossRef]
- Urso, D.; van Wamelen, D.J.; Batzu, L.; Leta, V.; Staunton, J.; Pineda-Pardo, J.A.; Logroscino, G.; Sharma, J.; Ray Chaudhuri, K. Clinical trajectories and biomarkers for weight variability in early Parkinson’s disease. NPJ Parkinsons Dis. 2022, 8, 95. [Google Scholar] [CrossRef]
- Borda, M.G.; Pérez-Zepeda, M.U.; Jaramillo-Jimenez, A.; Chaudhuri, K.R.; Tovar-Rios, D.A.; Wallace, L.; Batzu, L.; Rockwood, K.; Tysnes, O.B.; Aarsland, D.; et al. Frailty in Parkinson’s disease and its association with early dementia: A longitudinal study. Park. Relat. Disord. 2022, 99, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Metta, V.; Leta, V.; Mrudula, K.R.; Prashanth, L.K.; Goyal, V.; Borgohain, R.; Chung-Faye, G.; Chaudhuri, K.R. Gastrointestinal dysfunction in Parkinson’s disease: Molecular pathology and implications of gut microbiome, probiotics, and fecal microbiota transplantation. J. Neurol. 2022, 269, 1154–1163. [Google Scholar] [CrossRef] [PubMed]
- Auffret, M.; Meuric, V.; Boyer, E.; Bonnaure-Mallet, M.; Vérin, M. Oral Health Disorders in Parkinson’s Disease: More than Meets the Eye. J. Parkinsons Dis. 2021, 11, 1507–1535. [Google Scholar] [CrossRef] [PubMed]
- Lyra, P.; Botelho, J.; Machado, V.; Rota, S.; Walker, R.; Staunton, J.; Proença, L.; Chaudhuri, K.R.; Mendes, J.J. Self-reported periodontitis and C-reactive protein in Parkinson’s disease: A cross-sectional study of two American cohorts. NPJ Parkinsons Dis. 2022, 8, 40. [Google Scholar] [CrossRef] [PubMed]
- Çamcı, G.; Oğuz, S. Association between Parkinson’s Disease and Helicobacter Pylori. J. Clin. Neurol. 2016, 12, 147–150. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tan, A.H.; Mahadeva, S.; Marras, C.; Thalha, A.M.; Kiew, C.K.; Yeat, C.M.; Ng, S.W.; Ang, S.P.; Chow, S.K.; Loke, M.F.; et al. Helicobacter pylori infection is associated with worse severity of Parkinson’s disease. Park. Relat. Disord. 2015, 21, 221–225. [Google Scholar] [CrossRef]
- Nyholm, D.; Hellström, P.M. Effects of Helicobacter pylori on Levodopa Pharmacokinetics. J. Parkinsons Dis. 2021, 11, 61–69. [Google Scholar] [CrossRef]
- Frazzitta, G.; Ferrazzoli, D.; Folini, A.; Palamara, G.; Maestri, R. Severe Constipation in Parkinson’s Disease and in Parkinsonisms: Prevalence and Affecting Factors. Front. Neurol. 2019, 10, 628. [Google Scholar] [CrossRef]
- van Kessel, S.P.; de Jong, H.R.; Winkel, S.L.; van Leeuwen, S.S.; Nelemans, S.A.; Permentier, H.; Keshavarzian, A.; El Aidy, S. Gut bacterial deamination of residual levodopa medication for Parkinson’s disease. BMC Biol. 2020, 18, 137. [Google Scholar] [CrossRef]
- Chohan, H.; Senkevich, K.; Patel, R.K.; Bestwick, J.P.; Jacobs, B.M.; Bandres Ciga, S.; Gan-Or, Z.; Noyce, A.J. Type 2 Diabetes as a Determinant of Parkinson’s Disease Risk and Progression. Mov. Disord. 2021, 36, 1420–1429. [Google Scholar] [CrossRef]
- Brauer, R.; Wei, L.; Ma, T.; Athauda, D.; Girges, C.; Vijiaratnam, N.; Auld, G.; Whittlesea, C.; Wong, I.; Foltynie, T. Diabetes medications and risk of Parkinson’s disease: A cohort study of patients with diabetes. Brain 2020, 143, 3067–3076. [Google Scholar] [CrossRef]
- Xu, Q.; Park, Y.; Huang, X.; Hollenbeck, A.; Blair, A.; Schatzkin, A.; Chen, H. Diabetes and risk of Parkinson’s disease. Diabetes Care 2011, 34, 910–915. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sportelli, C.; Urso, D.; Jenner, P.; Chaudhuri, K.R. Metformin as a Potential Neuroprotective Agent in Prodromal Parkinson’s Disease-Viewpoint. Front. Neurol. 2020, 11, 556. [Google Scholar] [CrossRef] [PubMed]
- Cereda, E.; Barichella, M.; Pedrolli, C.; Klersy, C.; Cassani, E.; Caccialanza, R.; Pezzoli, G. Diabetes and risk of Parkinson’s disease: A systematic review and meta-analysis. Diabetes Care 2011, 34, 2614–2623. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Postuma, R.B.; Gagnon, J.F.; Bertrand, J.A.; Génier Marchand, D.; Montplaisir, J.Y. Parkinson risk in idiopathic REM sleep behavior disorder: Preparing for neuroprotective trials. Neurology 2015, 84, 1104–1113. [Google Scholar] [CrossRef] [Green Version]
- Schenck, C.H.; Montplaisir, J.Y.; Frauscher, B.; Hogl, B.; Gagnon, J.F.; Postuma, R.; Sonka, K.; Jennum, P.; Partinen, M.; Arnulf, I.; et al. Rapid eye movement sleep behavior disorder: Devising controlled active treatment studies for symptomatic and neuroprotective therapy--a consensus statement from the International Rapid Eye Movement Sleep Behavior Disorder Study Group. Sleep Med. 2013, 14, 795–806. [Google Scholar] [CrossRef]
- Xu, Y.; Yang, J.; Shang, H. Meta-analysis of risk factors for Parkinson’s disease dementia. Transl. Neurodegener. 2016, 5, 11. [Google Scholar] [CrossRef] [Green Version]
- Pondal, M.; Marras, C.; Miyasaki, J.; Moro, E.; Armstrong, M.J.; Strafella, A.P.; Shah, B.B.; Fox, S.; Prashanth, L.K.; Phielipp, N.; et al. Clinical features of dopamine agonist withdrawal syndrome in a movement disorders clinic. J. Neurol. Neurosurg. Psychiatry 2013, 84, 130–135. [Google Scholar] [CrossRef] [Green Version]
- Nirenberg, M.J. Dopamine agonist withdrawal syndrome: Implications for patient care. Drugs Aging 2013, 30, 587–592. [Google Scholar] [CrossRef]
- Weintraub, D.; Claassen, D.O. Impulse Control and Related Disorders in Parkinson’s Disease. Int. Rev. Neurobiol. 2017, 133, 679–717. [Google Scholar] [CrossRef]
- Ceravolo, R.; Rossi, C.; Del Prete, E.; Bonuccelli, U. A review of adverse events linked to dopamine agonists in the treatment of Parkinson’s disease. Expert Opin. Drug Saf. 2016, 15, 181–198. [Google Scholar] [CrossRef] [PubMed]
- Fackrell, R.; Carroll, C.B.; Grosset, D.G.; Mohamed, B.; Reddy, P.; Parry, M.; Chaudhuri, K.R.; Foltynie, T. Noninvasive options for ‘wearing-off’ in Parkinson’s disease: A clinical consensus from a panel of UK Parkinson’s disease specialists. Neurodegener. Dis. Manag. 2018, 8, 349–360. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stawicki, S.P.; Kalra, S.; Jones, C.; Justiniano, C.F.; Papadimos, T.J.; Galwankar, S.C.; Pappada, S.M.; Feeney, J.J.; Evans, D.C. Comorbidity polypharmacy score and its clinical utility: A pragmatic practitioner’s perspective. J. Emerg. Trauma Shock 2015, 8, 224–231. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chaudhuri, K.R.; Titova, N.; Qamar, M.A.; Murășan, I.; Falup-Pecurariu, C. The Dashboard Vitals of Parkinson’s: Not to Be Missed Yet an Unmet Need. J. Pers. Med. 2022, 12, 1994. https://doi.org/10.3390/jpm12121994
Chaudhuri KR, Titova N, Qamar MA, Murășan I, Falup-Pecurariu C. The Dashboard Vitals of Parkinson’s: Not to Be Missed Yet an Unmet Need. Journal of Personalized Medicine. 2022; 12(12):1994. https://doi.org/10.3390/jpm12121994
Chicago/Turabian StyleChaudhuri, Kallol Ray, Nataliya Titova, Mubasher A. Qamar, Iulia Murășan, and Cristian Falup-Pecurariu. 2022. "The Dashboard Vitals of Parkinson’s: Not to Be Missed Yet an Unmet Need" Journal of Personalized Medicine 12, no. 12: 1994. https://doi.org/10.3390/jpm12121994
APA StyleChaudhuri, K. R., Titova, N., Qamar, M. A., Murășan, I., & Falup-Pecurariu, C. (2022). The Dashboard Vitals of Parkinson’s: Not to Be Missed Yet an Unmet Need. Journal of Personalized Medicine, 12(12), 1994. https://doi.org/10.3390/jpm12121994





