Preoperative Prediction of Catheter Ablation Outcome in Persistent Atrial Fibrillation Patients through Spectral Organization Analysis of the Surface Fibrillatory Waves
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
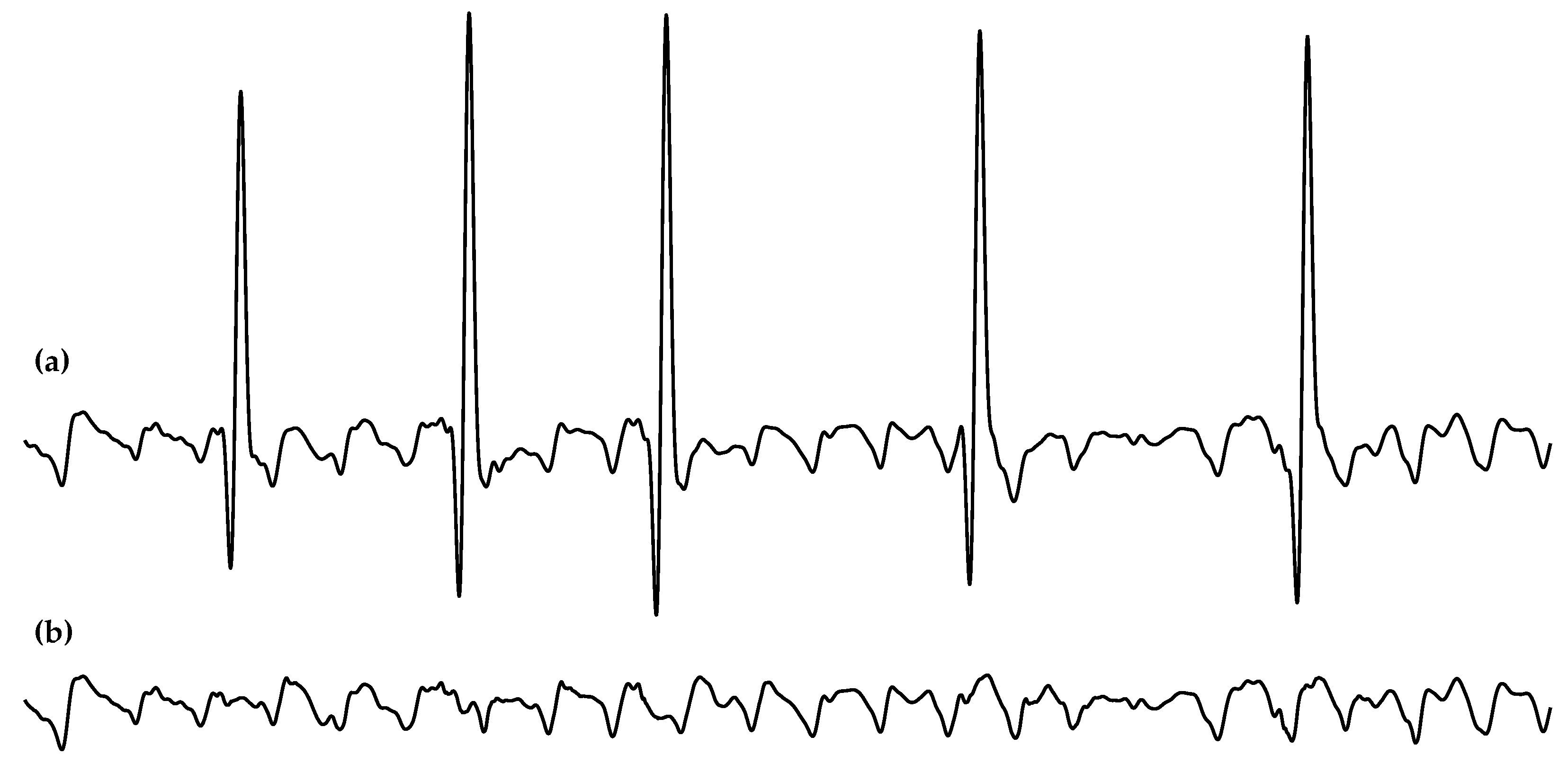
2.2. Data Preprocessing
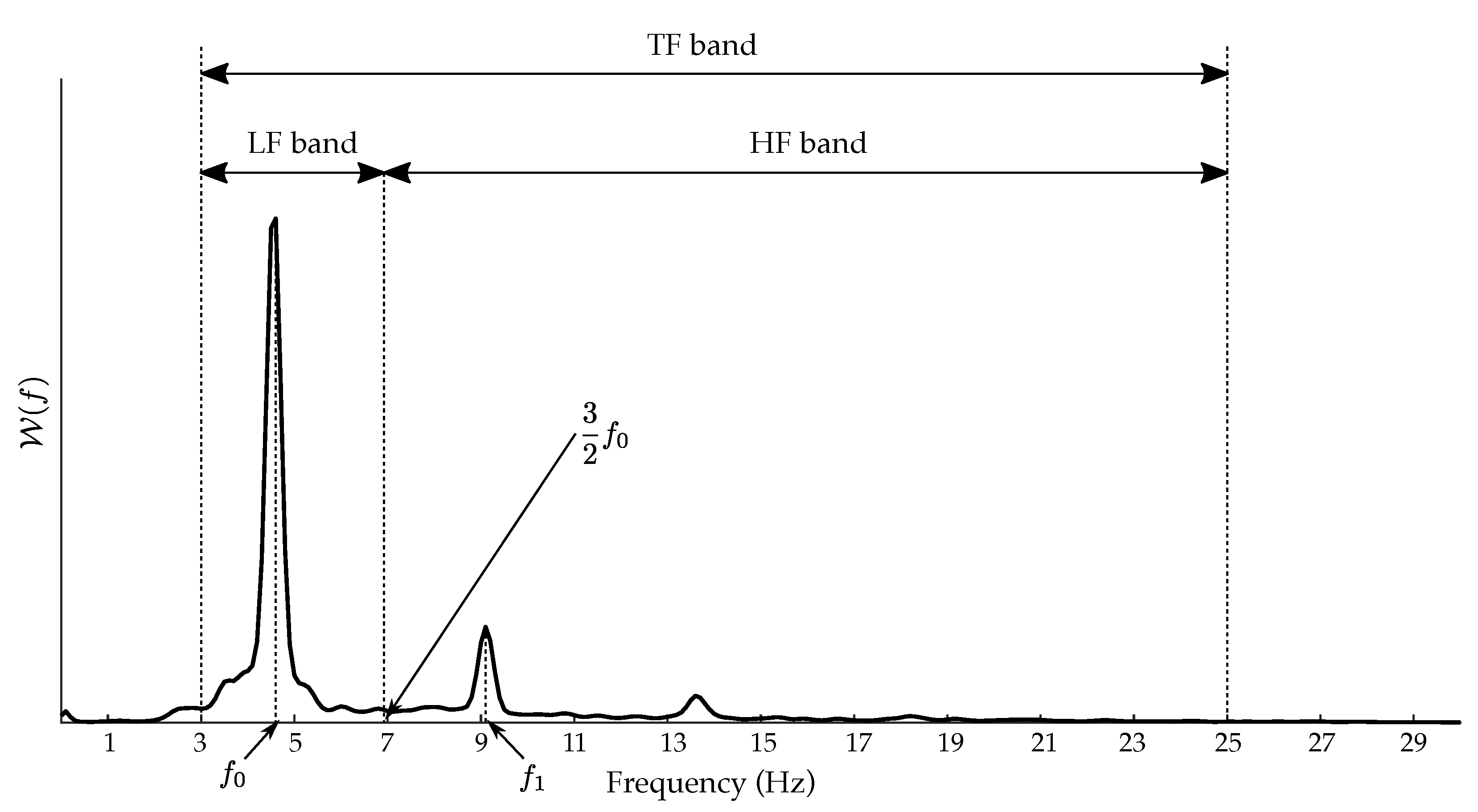
2.3. Spectral Characterization of the f-Waves
2.4. Statistical Analysis and Classification Performance
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Platonov, P.G.; Corino, V.D.A. A Clinical Perspective on Atrial Fibrillation. In Atrial Fibrillation from an Engineering Perspective; Springer International Publishing: Berlin, Germany, 2018; pp. 1–24. [Google Scholar] [CrossRef]
- Lippi, G.; Sanchis-Gomar, F.; Cervellin, G. Global epidemiology of atrial fibrillation: An increasing epidemic and public health challenge. Int. J. Stroke 2021, 16, 217–221. [Google Scholar] [CrossRef] [PubMed]
- Zoni-Berisso, M.; Lercari, F.; Carazza, T.; Domenicucci, S. Epidemiology of atrial fibrillation: European perspective. Clin. Epidemiol. 2014, 6, 213–220. [Google Scholar] [CrossRef] [PubMed]
- Schotten, U.; Dobrev, D.; Platonov, P.G.; Kottkamp, H.; Hindricks, G. Current controversies in determining the main mechanisms of atrial fibrillation. J. Intern. Med. 2016, 279, 428–438. [Google Scholar] [CrossRef] [PubMed]
- Rienstra, M.; Lubitz, S.A.; Mahida, S.; Magnani, J.W.; Fontes, J.D.; Sinner, M.F.; Van Gelder, I.C.; Ellinor, P.T.; Benjamin, E.J. Symptoms and functional status of patients with atrial fibrillation: State of the art and future research opportunities. Circulation 2012, 125, 2933–2943. [Google Scholar] [CrossRef]
- Warmus, P.; Niedziela, N.; Huć, M.; Wierzbicki, K.; Adamczyk-Sowa, M. Assessment of the manifestations of atrial fibrillation in patients with acute cerebral stroke—A single-center study based on 998 patients. Neurol. Res. 2020, 42, 471–476. [Google Scholar] [CrossRef]
- Hindricks, G.; Potpara, T.; Dagres, N.; Arbelo, E.; Bax, J.J.; Blomström-Lundqvist, C.; Boriani, G.; Castella, M.; Dan, G.A.; Dilaveris, P.E.; et al. 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS): The Task Force for the diagnosis and management of atrial fibrillation of the European Society of Cardiology (ESC) Developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Eur. Heart J. 2021, 42, 373–498. [Google Scholar] [CrossRef]
- Nattel, S.; Guasch, E.; Savelieva, I.; Cosio, F.G.; Valverde, I.; Halperin, J.L.; Conroy, J.M.; Al-Khatib, S.M.; Hess, P.L.; Kirchhof, P.; et al. Early management of atrial fibrillation to prevent cardiovascular complications. Eur. Heart J. 2014, 35, 1448–1456. [Google Scholar] [CrossRef]
- Schmidt, B.; Brugada, J.; Arbelo, E.; Laroche, C.; Bayramova, S.; Bertini, M.; Letsas, K.P.; Pison, L.; Romanov, A.; Scherr, D.; et al. Ablation strategies for different types of atrial fibrillation in Europe: Results of the ESC-EORP EHRA Atrial Fibrillation Ablation Long-Term registry. Europace 2020, 22, 558–566. [Google Scholar] [CrossRef]
- Calkins, H.; Reynolds, M.R.; Spector, P.; Sondhi, M.; Xu, Y.; Martin, A.; Williams, C.J.; Sledge, I. Treatment of atrial fibrillation with antiarrhythmic drugs or radiofrequency ablation: Two systematic literature reviews and meta-analyses. Circ. Arrhythm. Electrophysiol. 2009, 2, 349–361. [Google Scholar] [CrossRef]
- Hesselson, A.B. Catheter Ablation in the Treatment of Atrial Fibrillation. Int. J. Angiol. 2020, 29, 108–112. [Google Scholar] [CrossRef]
- Calkins, H.; Hindricks, G.; Cappato, R.; Kim, Y.H.; Saad, E.B.; Aguinaga, L.; Akar, J.G.; Badhwar, V.; Brugada, J.; Camm, J.; et al. 2017 HRS/EHRA/ECAS/APHRS/SOLAECE expert consensus statement on catheter and surgical ablation of atrial fibrillation: Executive summary. J. Arrhythm. 2017, 33, 369–409. [Google Scholar] [CrossRef] [PubMed]
- Walsh, K.; Marchlinski, F. Catheter ablation for atrial fibrillation: Current patient selection and outcomes. Expert Rev. Cardiovasc. Ther. 2018, 16, 679–692. [Google Scholar] [CrossRef] [PubMed]
- Balk, E.M.; Garlitski, A.C.; Alsheikh-Ali, A.A.; Terasawa, T.; Chung, M.; Ip, S. Predictors of atrial fibrillation recurrence after radiofrequency catheter ablation: A systematic review. J. Cardiovasc. Electrophysiol. 2010, 21, 1208–1216. [Google Scholar] [CrossRef] [PubMed]
- Matsuo, S.; Lellouche, N.; Wright, M.; Bevilacqua, M.; Knecht, S.; Nault, I.; Lim, K.T.; Arantes, L.; O’Neill, M.D.; Platonov, P.G.; et al. Clinical predictors of termination and clinical outcome of catheter ablation for persistent atrial fibrillation. J. Am. Coll. Cardiol. 2009, 54, 788–795. [Google Scholar] [CrossRef]
- Cheng, Z.; Deng, H.; Cheng, K.; Chen, T.; Gao, P.; Yu, M.; Fang, Q. The amplitude of fibrillatory waves on leads aVF and V1 predicting the recurrence of persistent atrial fibrillation patients who underwent catheter ablation. Ann. Noninvasive Electrocardiol. 2013, 18, 352–358. [Google Scholar] [CrossRef]
- Nault, I.; Lellouche, N.; Matsuo, S.; Knecht, S.; Wright, M.; Lim, K.T.; Sacher, F.; Platonov, P.; Deplagne, A.; Bordachar, P.; et al. Clinical value of fibrillatory wave amplitude on surface ECG in patients with persistent atrial fibrillation. J. Interv. Card. Electrophysiol. 2009, 26, 11–19. [Google Scholar] [CrossRef]
- Takahashi, Y.; Sanders, P.; Jaïs, P.; Hocini, M.; Dubois, R.; Rotter, M.; Rostock, T.; Nalliah, C.J.; Sacher, F.; Clémenty, J.; et al. Organization of frequency spectra of atrial fibrillation: Relevance to radiofrequency catheter ablation. J. Cardiovasc. Electrophysiol. 2006, 17, 382–388. [Google Scholar] [CrossRef]
- Yoshida, K.; Ulfarsson, M.; Tada, H.; Chugh, A.; Good, E.; Kuhne, M.; Crawford, T.; Sarrazin, J.F.; Chalfoun, N.; Wells, D.; et al. Complex electrograms within the coronary sinus: Time- and frequency-domain characteristics, effects of antral pulmonary vein isolation, and relationship to clinical outcome in patients with paroxysmal and persistent atrial fibrillation. J. Cardiovasc. Electrophysiol. 2008, 19, 1017–1023. [Google Scholar] [CrossRef]
- Atienza, F.; Almendral, J.; Jalife, J.; Zlochiver, S.; Ploutz-Snyder, R.; Torrecilla, E.G.; Arenal, A.; Kalifa, J.; Fernández-Avilés, F.; Berenfeld, O. Real-time dominant frequency mapping and ablation of dominant frequency sites in atrial fibrillation with left-to-right frequency gradients predicts long-term maintenance of sinus rhythm. Heart Rhythm 2009, 6, 33–40. [Google Scholar] [CrossRef]
- Yoshida, K.; Chugh, A.; Good, E.; Crawford, T.; Myles, J.; Veerareddy, S.; Billakanty, S.; Wong, W.S.; Ebinger, M.; Pelosi, F.; et al. A critical decrease in dominant frequency and clinical outcome after catheter ablation of persistent atrial fibrillation. Heart Rhythm 2010, 7, 295–302. [Google Scholar] [CrossRef]
- Sörnmo, L.; Alcaraz, R.; Laguna, P.; Rieta, J.J. Characterization of f Waves. In Atrial Fibrillation from an Engineering Perspective; Springer International Publishing: Berlin, Germany, 2018; pp. 221–279. [Google Scholar] [CrossRef]
- Alcaraz, R.; Hornero, F.; Rieta, J.J. Electrocardiographic Spectral Features for Long-Term Outcome Prognosis of Atrial Fibrillation Catheter Ablation. Ann. Biomed. Eng. 2016, 44, 3307–3318. [Google Scholar] [CrossRef] [PubMed]
- Everett, T.H., 4th; Kok, L.C.; Vaughn, R.H.; Moorman, J.R.; Haines, D.E. Frequency domain algorithm for quantifying atrial fibrillation organization to increase defibrillation efficacy. IEEE Trans. Biomed. Eng. 2001, 48, 969–978. [Google Scholar] [CrossRef] [PubMed]
- Holmqvist, F.; Stridh, M.; Waktare, J.E.P.; Roijer, A.; Sörnmo, L.; Platonov, P.G.; Meurling, C.J. Atrial fibrillation signal organization predicts sinus rhythm maintenance in patients undergoing cardioversion of atrial fibrillation. Europace 2006, 8, 559–565. [Google Scholar] [CrossRef]
- Husser, D.; Stridh, M.; Sornmo, L.; Geller, C.; Klein, H.U.; Olsson, S.B.; Bollmann, A. Time-frequency analysis of the surface electrocardiogram for monitoring antiarrhythmic drug effects in atrial fibrillation. Am. J. Cardiol. 2005, 95, 526–528. [Google Scholar] [CrossRef] [PubMed]
- Michaud, G.; Kumar, S. Pulmonary vein isolation in the treatment of atrial fibrillation. Res. Rep. Clin. Cardiol. 2016, 7, 47–60. [Google Scholar] [CrossRef]
- Morin, D.P.; Bernard, M.L.; Madias, C.; Rogers, P.A.; Thihalolipavan, S.; Estes, N.A.M., 3rd. The State of the Art: Atrial Fibrillation Epidemiology, Prevention, and Treatment. Mayo Clin. Proc. 2016, 91, 1778–1810. [Google Scholar] [CrossRef]
- Petrėnas, A.; Marozas, V.; Sörnmo, L. Lead Systems and Recording Devices. In Atrial Fibrillation from an Engineering Perspective; Springer International Publishing: Berlin, Germany, 2018; pp. 25–48. [Google Scholar] [CrossRef]
- Sörnmo, L.; Laguna, P. Chapter 7—ECG Signal Processing. In Bioelectrical Signal Processing in Cardiac and Neurological Applications; Sörnmo, L., Laguna, P., Eds.; Biomedical Engineering, Academic Press: Burlington, NJ, USA, 2005; pp. 453–566. [Google Scholar] [CrossRef]
- García, M.; Martínez-Iniesta, M.; Ródenas, J.; Rieta, J.J.; Alcaraz, R. A novel wavelet-based filtering strategy to remove powerline interference from electrocardiograms with atrial fibrillation. Physiol. Meas. 2018, 39, 115006. [Google Scholar] [CrossRef]
- Alcaraz, R.; Rieta, J.J. Adaptive singular value cancelation of ventricular activity in single-lead atrial fibrillation electrocardiograms. Physiol. Meas. 2008, 29, 1351–1369. [Google Scholar] [CrossRef]
- Henriksson, M.; García-Alberola, A.; Sörnmo, L. Short-term reproducibility of parameters characterizing atrial fibrillatory waves. Comput. Biol. Med. 2020, 117, 103613. [Google Scholar] [CrossRef]
- Murase, Y.; Inden, Y.; Shibata, R.; Yanagisawa, S.; Fujii, A.; Ando, M.; Otake, N.; Takenaka, M.; Funabiki, J.; Sakamoto, Y.; et al. The impact of the dominant frequency of body surface electrocardiography in patients with persistent atrial fibrillation. Heart Vessel. 2020, 35, 967–976. [Google Scholar] [CrossRef]
- Szilágyi, J.; Walters, T.E.; Marcus, G.M.; Vedantham, V.; Moss, J.D.; Badhwar, N.; Lee, B.; Lee, R.; Tseng, Z.H.; Gerstenfeld, E.P. Surface ECG and intracardiac spectral measures predict atrial fibrillation recurrence after catheter ablation. J. Cardiovasc. Electrophysiol. 2018, 29, 1371–1378. [Google Scholar] [CrossRef]
- Lankveld, T.; Zeemering, S.; Scherr, D.; Kuklik, P.; Hoffmann, B.A.; Willems, S.; Pieske, B.; Haïssaguerre, M.; Jaïs, P.; Crijns, H.J.; et al. Atrial Fibrillation Complexity Parameters Derived From Surface ECGs Predict Procedural Outcome and Long-Term Follow-Up of Stepwise Catheter Ablation for Atrial Fibrillation. Circ. Arrhythm. Electrophysiol. 2016, 9, e003354. [Google Scholar] [CrossRef] [PubMed]
- Eftestol, T.; Sunde, K.; Ole Aase, S.; Husoy, J.H.; Steen, P.A. Predicting outcome of defibrillation by spectral characterization and nonparametric classification of ventricular fibrillation in patients with out-of-hospital cardiac arrest. Circulation 2000, 102, 1523–1529. [Google Scholar] [CrossRef] [PubMed]
- Löfhede, J.; Thordstein, M.; Löfgren, N.; Flisberg, A.; Rosa-Zurera, M.; Kjellmer, I.; Lindecrantz, K. Automatic classification of background EEG activity in healthy and sick neonates. J. Neural Eng. 2010, 7, 16007. [Google Scholar] [CrossRef] [PubMed]
- Uldry, L.; Van Zaen, J.; Prudat, Y.; Kappenberger, L.; Vesin, J.M. Measures of spatiotemporal organization differentiate persistent from long-standing atrial fibrillation. Europace 2012, 14, 1125–1131. [Google Scholar] [CrossRef] [PubMed]
- Julián, M.; Alcaraz, R.; Rieta, J.J. Comparative assessment of nonlinear metrics to quantify organization-related events in surface electrocardiograms of atrial fibrillation. Comput. Biol. Med. 2014, 48, 66–76. [Google Scholar] [CrossRef] [PubMed]
- Xiong, P.Y.; Jahanshahi, H.; Alcaraz, R.; Chu, Y.M.; Gómez-Aguilar, J.; Alsaadi, F.E. Spectral entropy analysis and synchronization of a multi-stable fractional-order chaotic system using a novel neural network-based chattering-free sliding mode technique. Chaos Solitons Fractals 2021, 144, 110576. [Google Scholar] [CrossRef]
- Shen, E.; Cai, Z.; Gu, F. Mathematical foundation of a new complexity measure. Appl. Math. Mech. 2005, 26, 1188–1196. [Google Scholar] [CrossRef]
- Refaeilzadeh, P.; Tang, L.; Liu, H. Cross-Validation. In Encyclopedia of Database Systems; Liu, L., Ozsu, M.T., Eds.; Springer: Berlin, Germany, 2009; pp. 532–538. [Google Scholar] [CrossRef]
- Izenman, A.J. Linear Discriminant Analysis. In Springer Texts in Statistics; Springer: New York, NY, USA, 2013; pp. 237–280. [Google Scholar] [CrossRef]
- Habibzadeh, F.; Habibzadeh, P.; Yadollahie, M. On determining the most appropriate test cut-off value: The case of tests with continuous results. Biochem. Med. 2016, 26, 297–307. [Google Scholar] [CrossRef]
- Rückstieß, T.; Osendorfer, C.; van der Smagt, P. Sequential Feature Selection for Classification. In AI 2011: Advances in Artificial Intelligence; Springer: Berlin/Heidelberg, Germany, 2011; pp. 132–141. [Google Scholar] [CrossRef]
- Fagerland, M.W.; Lydersen, S.; Laake, P. The McNemar test for binary matched-pairs data: Mid-p and asymptotic are better than exact conditional. BMC Med. Res. Methodol. 2013, 13, 91. [Google Scholar] [CrossRef]
- Gerstenfeld, E.P.; Duggirala, S. Atrial fibrillation ablation: Indications, emerging techniques, and follow-up. Prog. Cardiovasc. Dis. 2015, 58, 202–212. [Google Scholar] [CrossRef] [PubMed]
- Dretzke, J.; Chuchu, N.; Agarwal, R.; Herd, C.; Chua, W.; Fabritz, L.; Bayliss, S.; Kotecha, D.; Deeks, J.J.; Kirchhof, P.; et al. Predicting recurrent atrial fibrillation after catheter ablation: A systematic review of prognostic models. Europace 2020, 22, 748–760. [Google Scholar] [CrossRef] [PubMed]
- Lambert, L.; Marek, J.; Fingrova, Z.; Havranek, S.; Kuchynka, P.; Cerny, V.; Simek, J.; Burgetova, A. The predictive value of cardiac morphology for long-term outcome of patients undergoing catheter ablation for atrial fibrillation. J. Cardiovasc. Comput. Tomogr. 2018, 12, 418–424. [Google Scholar] [CrossRef]
- Haïssaguerre, M.; Sanders, P.; Hocini, M.; Hsu, L.F.; Shah, D.C.; Scavée, C.; Takahashi, Y.; Rotter, M.; Pasquié, J.L.; Garrigue, S.; et al. Changes in atrial fibrillation cycle length and inducibility during catheter ablation and their relation to outcome. Circulation 2004, 109, 3007–3013. [Google Scholar] [CrossRef] [PubMed]
- Di Marco, L.Y.; Raine, D.; Bourke, J.P.; Langley, P. Atrial Fibrillation Type Characterization and Catheter Ablation Acute Outcome Prediction: Comparative Analysis of Spectral and Nonlinear Indices from Right Atrium Electrograms. In Proceedings of the 41st Computing in Cardiology Conference (CinC), Cambridge, MA, USA, 7–10 September 2014; Volume 41, pp. 817–820. [Google Scholar]
- Borowska, M. Entropy-based algorithms in the analysis of biomedical signals. Stud. Logic Gramm. Rhetor. 2015, 43, 21–32. [Google Scholar] [CrossRef]
- Sanchez-Somonte, P.; Jiang, C.Y.; Betts, T.R.; Chen, J.; Mantovan, R.; Macle, L.; Morillo, C.A.; Haverkamp, W.; Weerasooriya, R.; Albenque, J.P.; et al. Completeness of Linear or Fractionated Electrogram Ablation in Addition to Pulmonary Vein Isolation on Ablation Outcome: A Substudy of the STAR AF II Trial. Circ. Arrhythm. Electrophysiol. 2021, 14, e010146. [Google Scholar] [CrossRef]
- Verma, A.; Jiang, C.y.; Betts, T.R.; Chen, J.; Deisenhofer, I.; Mantovan, R.; Macle, L.; Morillo, C.A.; Haverkamp, W.; Weerasooriya, R.; et al. Approaches to catheter ablation for persistent atrial fibrillation. N. Engl. J. Med. 2015, 372, 1812–1822. [Google Scholar] [CrossRef]
- Cui, X.; Chang, H.C.; Lin, L.Y.; Yu, C.C.; Hsieh, W.H.; Li, W.; Peng, C.K.; Lin, J.L.; Lo, M.T. Prediction of atrial fibrillation recurrence before catheter ablation using an adaptive nonlinear and non-stationary surface ECG analysis. Phys. A Stat. Mech. Its Appl. 2019, 514, 9–19. [Google Scholar] [CrossRef]
- Hsu, N.W.; Lin, Y.J.; Tai, C.T.; Kao, T.; Chang, S.L.; Wongcharoen, W.; Lo, L.W.; Udyavar, A.R.; Hu, Y.F.; Tso, H.W.; et al. Frequency analysis of the fibrillatory activity from surface ECG lead V1 and intracardiac recordings: Implications for mapping of AF. Europace 2008, 10, 438–443. [Google Scholar] [CrossRef]
- Hidalgo-Muñoz, A.R.; Latcu, D.G.; Meo, M.; Meste, O.; Popescu, I.; Saoudi, N.; Zarzoso, V. Spectral and spatiotemporal variability ECG parameters linked to catheter ablation outcome in persistent atrial fibrillation. Comput. Biol. Med. 2017, 88, 126–131. [Google Scholar] [CrossRef]

| Clinical Feature | Rhythm after Follow-Up | p-Value | |
|---|---|---|---|
| AF | SR | ||
| Number of patients (%) | − | ||
| Male (%) | |||
| Age (years) | |||
| With AF <1 year (%) | |||
| With AF 1–3 years (%) | |||
| With AF >3 years (%) | |||
| Body mass index (kg/m) | |||
| Left atrium diameter (mm) | |||
| Feature | Rhythm after the Follow-Up | p-Value | |
|---|---|---|---|
| AF | SR | ||
| (Hz) | |||
| (mV) | |||
| (Hz) | |||
| (mV) | |||
| <0.001 | |||
| <0.001 | |||
| <0.001 | |||
| <0.001 | |||
| Feature | Se (%) | Sp (%) | Acc (%) | AUC | PPV (%) | NPV (%) |
|---|---|---|---|---|---|---|
| Features in the Model | Se (%) | Sp (%) | Acc (%) | AUC | PPV (%) | NPV (%) |
|---|---|---|---|---|---|---|
| and | ||||||
| and | ||||||
| and | ||||||
| , , and | ||||||
| , , and | ||||||
| , , and |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Escribano, P.; Ródenas, J.; García, M.; Arias, M.A.; Hidalgo, V.M.; Calero, S.; Rieta, J.J.; Alcaraz, R. Preoperative Prediction of Catheter Ablation Outcome in Persistent Atrial Fibrillation Patients through Spectral Organization Analysis of the Surface Fibrillatory Waves. J. Pers. Med. 2022, 12, 1721. https://doi.org/10.3390/jpm12101721
Escribano P, Ródenas J, García M, Arias MA, Hidalgo VM, Calero S, Rieta JJ, Alcaraz R. Preoperative Prediction of Catheter Ablation Outcome in Persistent Atrial Fibrillation Patients through Spectral Organization Analysis of the Surface Fibrillatory Waves. Journal of Personalized Medicine. 2022; 12(10):1721. https://doi.org/10.3390/jpm12101721
Chicago/Turabian StyleEscribano, Pilar, Juan Ródenas, Manuel García, Miguel A. Arias, Víctor M. Hidalgo, Sofía Calero, José J. Rieta, and Raúl Alcaraz. 2022. "Preoperative Prediction of Catheter Ablation Outcome in Persistent Atrial Fibrillation Patients through Spectral Organization Analysis of the Surface Fibrillatory Waves" Journal of Personalized Medicine 12, no. 10: 1721. https://doi.org/10.3390/jpm12101721
APA StyleEscribano, P., Ródenas, J., García, M., Arias, M. A., Hidalgo, V. M., Calero, S., Rieta, J. J., & Alcaraz, R. (2022). Preoperative Prediction of Catheter Ablation Outcome in Persistent Atrial Fibrillation Patients through Spectral Organization Analysis of the Surface Fibrillatory Waves. Journal of Personalized Medicine, 12(10), 1721. https://doi.org/10.3390/jpm12101721






