Tumour-Related Parameters as a Prognostic Factor in Patients with Advanced Cervical Cancer: 20-Year Follow-Up of Diagnostic and Treatment Changes during Chemioradiotherapy
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Characteristics: Group 1
2.2. Patient Characteristics: Group 2
2.3. Statistical Analysis
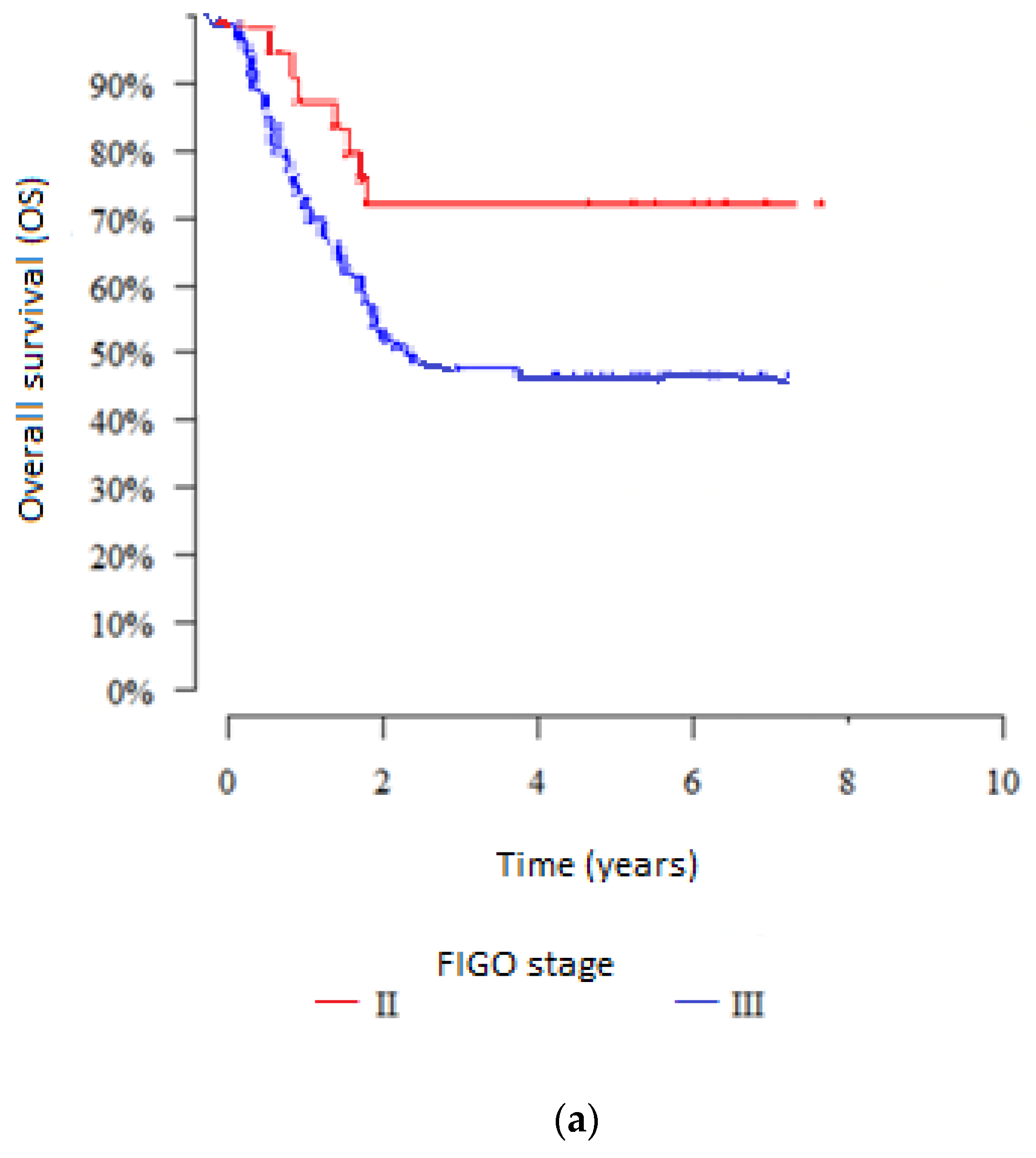
3. Results
3.1. Influence of Clinical-Related Factors on Outcomes in the Groups
3.2. Influence of Treatment-Related Factors on Outcomes in the Groups
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA A Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Whitney, C.W.; Sause, W.; Bundy, B.N.; Malfetano, J.H.; Hannigan, E.V.; Fowler, W.C., Jr.; Clarke-Pearson, D.L.; Liao, S-Y. Randomized comparison of fluorouracil plus cisplatin versus hydroxyurea as an adjunct to radiation therapy in stage IIB-IVA carcinoma of the cervix with negative para-aortic lymph nodes: A Gynecologic Oncology Group and Southwest Oncology Group study. J. Clin. Oncol. 1999, 17, 1339–1348. [Google Scholar] [CrossRef] [PubMed]
- Rose, P.G.; Bundy, B.N.; Watkins, E.B.; Thigpen, J.T.; Deppe, G.; Maiman, M.A.; Clarke-Pearson, D.L.; Insalaco, S. Concurrent cispaltin-base radiotherapy and chemotherapy for locally advanced cervical cancer. N. Engl. J. Med. 1999, 340, 1144–1453. [Google Scholar] [CrossRef] [PubMed]
- Morris, M.; Eifel, P.J.; Lu, J.; Grigsby, P.W.; Levenback, C.; Stevens, R.E.; Rotman, M.; Gershenson, D.M.; Mutch, D.G. Pelvic radiation with concurrent chemotherapy compared with pelvic and para-aortic radiation for high-risk cervical cancer. N. Eng. J. Med. 1999, 349, 1137–1143. [Google Scholar] [CrossRef]
- Peters, W.A., III.; Liu, P.Y.; Barrett, R.J., II.; Stock, R.J.; Monk, B.J.; Berek, J.S.; Souhami, L.; Grigsby, P.; Gordon, W.J.; Alberts, D.S. Concurrent chemotherapy and pelvic radiation therapy compared with pelvic radiation therapy alone ad adjuvant therapy after radical surgery in high-risk early-stage cancer of the cervix. J. Clin. Oncol. 2000, 18, 1606–1613. [Google Scholar] [CrossRef] [PubMed]
- Keys, H.M.; Bundy, B.N.; Stehman, F.B.; Muderspach, L.I.; Chafe, W.E.; Suggs, C.L.; Walker, J.L.; Gersell, D. Cisplatin, radiation, and adjuvant hysterectomy compared with radiation and adjuvant hysterectomy for bulky stage IB cervical carcinoma. N. Eng. J. Med. 1999, 340, 1154–1161. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.J.; Ju, W.; Myung, S.-K.; Kim, Y. Diagnostic performance of computer tomography, magnetic resonance imaging, and positron emission tomography or positron emission tomography/computer tomography for detection of metastatic lymph nodes in patients with cervical cancer: Meta-analysis. Cancer Sci. 2010, 101, 1471–1479. [Google Scholar] [CrossRef]
- Sturdza, A.; Pötter, R.; Fokdal, L.U.; Haie-Meder, C.; Tan, L.T.; Mazeron, R.; Petric, P.; Šegedin, B.; Jurgenliemk-Schulz, I.M.; Nomden, C.; et al. Image guided brachytherapy in locally advanced cervical cancer: Improved pelvic control and survival in RetroEMBRACE, a multicenter cohort study. Radiother. Oncol. 2016, 120, 428–433. [Google Scholar] [CrossRef] [PubMed]
- Cegla, P.; Urbanski, B.; Burchardt, E.; Roszak, A.; Cholewinski, W. Influence of 18F-FDG-PET/CT on staging of cervical cancer. Nuklearmedizin 2019, 58, 17–22. [Google Scholar] [PubMed]
- ICRU Report 38, Dose and Volume Specification for Reporting Intracavitary Therapy in Gynecology. Available online: https://www.icru.org/report/ (accessed on 10 August 2022).
- Haie-Meder, C.; Pötter, R.; Van Limbergen, E.; Briot, E.; De Brabandere, M.; Dimopoulos, J.; Dumas, I.; Hellebust, T.P.; Kirisits, C.; Lang, S.; et al. Recommendations from Gynecological (GYN) GEC-ESTRO Working Group (I): Concepts and terms in 3D image based 3D treatment planning in cervix cancer brachytherapy with emphasis on MRI assessment of GTV and CTV. Radiother. Oncol. 2005, 74, 235–245. [Google Scholar] [CrossRef] [PubMed]
- StatSoft, Inc. STATISTICA (Data Analysis Software), Version 10. 2011. Available online: www.ststsoft.com (accessed on 29 July 2022).
- Chino, J.; Annunziata, C.M.; Beriwal, S.; Bradfield, L.; Erickson, B.A.; Fields, E.C.; Fitch, K.; Harkenrider, M.M.; Holschneider. C.H.; Kamrava, M.; et al. Radiation therapy for cervical cancer: Executive summary of an ASTRO clinical practice guideline. Pract. Radiat. Oncol. 2020, 10, 220–234. [Google Scholar] [CrossRef] [PubMed]
- Chemoradiotherapy for Cervical Cancer Meta-Analysis Collaboration. Reducing uncertainties about the effects of chemoradiotherapy for cervical cancer: A systematic review and meta-analysis of individual patient data from 18 randomized trials. J. Clin. Oncol. 2008, 26, 5802–5812. [Google Scholar] [CrossRef] [PubMed]
- Pearcey, R.; Brundage, M.; Drouin, P.; Jeffrey, J.; Johnston, D.; MacLean, H.L.; Souhami, L.; Stuart, G.; Tu, D. Phase III trial comparing radical radiation therapy with or without cisplatin chemotherapy in patients with advanced squamous cell carcinoma of the cervix. J. Clin. Oncol. 2002, 20, 966–972. [Google Scholar] [CrossRef] [PubMed]
- Einhorn, N.; Tropé, C.; Ridderheim, M.; Boman, K.; Sorbe, B.; Cavallin-Ståhl, E. A systematic overview of radiation therapy effects in cervical cancer (cervix uteri). Acta Oncol. 2003, 42, 546–556. [Google Scholar] [CrossRef] [PubMed]
- Yalman, D.; Aras, A.B.; Ozkök, S.; Duransoy, A.; Celik, O.K.; Ozsaran, Z.; Haydaroğlu, A. Prognostic factor in definitive radiotherapy of uterine cervical cancer. Eur. J. Gynaecol. Oncol. 2003, 24, 309–314. [Google Scholar] [PubMed]
- Park, J.H.; Kim, Y.S.; Ahn, S.D.; Choi, E.K.; Shin, S.S.; Kim, Y.T.; Kim, Y.M.; Kim, J.H.; Yi, S.Y.; Nam, J.H. Concurrent chemoradiotherapy or radiotherapy alone for locally advanced cervical cancer in eldery women. Tumori J. 2010, 96, 959–965. [Google Scholar] [CrossRef]
- Kim, T.-E.; Park, B.-J.; Kwack, H.-S.; Kwon, J.-Y.; Kim, J.-H.; Yoon, S.-C. Outcomes and prognostic factors of cervical cancer after concurrent chemoradiation. J. Obs. Gynecol. Res. 2012, 38, 1315–1320. [Google Scholar] [CrossRef] [PubMed]
- Katanyoo, K.; Sanguanrungsirikul, S.; Monusirivithaya, S. Comparsion of treatment outcomes between squamous cell carcinoma and adenocarcinoma in locally advanced cervical cancer. Gycenol. Oncol. 2012, 125, 292–296. [Google Scholar]
- Pitson, G.; Fyles, A.; Milosevic, M.; Wylie, J.; Pintilie, M.; Hill, R. Tumor size and oxygenation are independent predictors of nodal disease in patients with cervical cancer. Int. J. Radiat. Oncol. Biol. Phys. 2001, 51, 699–703. [Google Scholar] [CrossRef]
- Demirci, S.; Ozsaran, Z.; Ozsaran, A.; Yavas, F.; Demircioglu, B.; Hanhan, M.; Dikmen, Y.; Aras, A.B. Evaluation of treatment result and prognostic factors in Elary-stage cervical carcinoma patient treated with postoperative faiotherapy Or radiochemotherapy. Eur. J. Gynecol. Oncol. 2012, 33, 62–67. [Google Scholar]
- Teh, J.; Yap, S.P.; Tham, I.; Sethi, V.K.; Chua, E.J.; Yeo, R.; Ho, T.H.; Tay, E.H.; Chia, Y.N.; Soh, L.T.; et al. Concurrent chemoradiotherapy incorporating high-dose-rate brachytherapy for locally advanced cervical carcinoma: Survival outcomes, patterns of failure and prognostic factors. Int. J. Gynecol. Oncol. 2010, 20L, 428–433. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.J.; Kim, Y.S.; Shin, S.S.; Nam, J.-H.; Han, S.; Choi, E.K. Long-Term Outcomes of Concomitant Chemoradiotherapy Incorporating High-Dose-Rate Brachytherapy to Treat Locally Advanced Cervical Cancer. Tumori J. 2012, 98, 615–621. [Google Scholar] [CrossRef]
- Kidd, E.A.; Siegel, B.A.; Dehdashti, F.; Rader, J.S.; Mutch, D.G.; Powell, M.A.; Grigsby, P.W. Lymph Node Staging by Positron Emission Tomography in Cervical Cancer: Relationship to Prognosis. J. Clin. Oncol. 2010, 28, 2108–2113. [Google Scholar] [CrossRef] [PubMed]
- Bhatla, N.; Berek, J.S.; Cuello Fredes, M.; Denny, L.A.; Grenman, S.; Karunaratne, K.; Konishi, I.; Olawaiye, A.B.; Prat, J.; Sankaranarayanan, R. Revised FIGO staging for carcinoma of the cervix uteri. Int. J. Gynecol. Obstet. 2019, 145, 129–135. [Google Scholar] [CrossRef] [PubMed]
- McComas, K.N.; Torgeson, A.M.; Ager, B.J.; Hellekson, C.; Burt, L.M.; Maurer, K.A.; Werner, T.L.; Gaffney, D.K. The variable impact of positive lymph nodes in cervical cancer: Implications of the new FIGO staging system. Gynecol. Oncol. 2020, 156, 85–92. [Google Scholar] [CrossRef]
- Wright, J.D.; Matsuo, K.; Huang, Y.; Tergas, A.I.; Hou, J.Y.; Khoury-Collado, F.; St. Clair, C.M.; Ananth, C.V.; Neugut, A.I.; Hershman, D.L. Prognostic Performance of the 2018 International Federation of Gynecology and Obstetrics Cervical Cancer Staging Guidelines. Obstet. Gynecol. 2019, 134, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Sorbe, B.; Bohr, L.; Karlsson, L.; Bermark, B. Combined external and intracavitary irradiation in treatment of advanced cervical carcinomas: Predictive factors for local tumor control and early recurrences. Int. J. Oncol. 2010, 36, 371–378. [Google Scholar] [CrossRef][Green Version]
- Perez, C.A.; Grigsby, P.W.; Chao, K.C.; Mutch, D.G.; Lockett, M.A. Tumor Size, Irradiation Dose, and Long-Term Outcome of Carcinoma of Uterine Cervix. Int. J. Radiat. Oncol. Biol. Physics. 1998, 41, 307–317. [Google Scholar] [CrossRef]
- Tanderup, K.; Lindegaard, J.C.; Kirisits, C.; Haie-Meder, C.; Kirchheiner, K.; de Leeuw, A.; Jürgenliemk-Schulz, I.; van Limbergen, E.; Pötter, R. Image guide adaptive brachytherapy in cervix cancer: A new paradigm changing clinical practice and outcome. Radiother Oncol. 2016, 120, 365–369. [Google Scholar] [CrossRef] [PubMed]
- Pötter, R.; Tanderup, K.; Kirisits, C.; de Leeuw, A.; Kirchheiner, K.; Nout, R.; Tan, L.T.; Haie-Meder, C.; Mahantshetty, U.; Segedin, B.; et al. The EMBRACE II study: The outcome and prospect of two decades of evolution within the GEC-ESTRO GYN working group and the EMBRACE studies. Clin. Transl. Radiat. Oncol. 2018, 9, 48–60. [Google Scholar] [CrossRef] [PubMed]






| Characteristics | Group 1, n = 147 | Group 2, n = 119 | |
|---|---|---|---|
| Treatment Period | 2001–2005 | 2010–2016 | |
| Disease stage | |||
| Stage IIB, n | 32 | 55 | |
| Stage IIIB, n | 115 | 64 | |
| Tumour size in cm (SD) | 4.98 (1.56) | 5.34 (1.48) | |
| Mean (SD) age, years | 51 (8.7) | 52.9 (10.7) | |
| Comorbidities disease, % | 28.6 | 14.2 | |
| Mean BMI (SD) | 25.7 (5.1) | 26.9 (4.9) | |
| Histopathologic diagnosis | Squamous cell ca. | 96.6% | 85.7% |
| Adenocarcinoma | 3.4% | 14.3% | |
| Diagnostic technique | X-ray, USG | PET-CT | |
| EBRT technique | 3D-CRT | 3D-CRT/ IMRT | |
| Brachytherapy | Dose rate | LDR | HDR |
| Technique | 2D | 3D | |
| EBRT dose (SD) | 32.2 (5.6) | 48.6 (1.8) | |
| Brachytherapy dose (SD) | 52.2 (8.6) | 38.9 (0.9) | |
| Mean (SD) number of chemotherapy courses | 4.73 (1.1) | 3.67 (1.96) | |
| Follow-up period, years | 5 | 5 | |
| Number of Metastatic Lymph Nodes | Number of Patients | Overall Survival | |
|---|---|---|---|
| 5-Years (%) | p * | ||
| 1 | 34 | 70.47% | p = 0.677 |
| 2 | 41 | 63.35% | |
| 3 | 21 | 72.43% | |
| 4 | 12 | 55.56% | |
| ≥5 | 10 | 60.00% | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Warenczak-Florczak, Z.; Burchardt, E.; Wilk, A.; Roszak, A. Tumour-Related Parameters as a Prognostic Factor in Patients with Advanced Cervical Cancer: 20-Year Follow-Up of Diagnostic and Treatment Changes during Chemioradiotherapy. J. Pers. Med. 2022, 12, 1722. https://doi.org/10.3390/jpm12101722
Warenczak-Florczak Z, Burchardt E, Wilk A, Roszak A. Tumour-Related Parameters as a Prognostic Factor in Patients with Advanced Cervical Cancer: 20-Year Follow-Up of Diagnostic and Treatment Changes during Chemioradiotherapy. Journal of Personalized Medicine. 2022; 12(10):1722. https://doi.org/10.3390/jpm12101722
Chicago/Turabian StyleWarenczak-Florczak, Zaneta, Ewa Burchardt, Agnieszka Wilk, and Andrzej Roszak. 2022. "Tumour-Related Parameters as a Prognostic Factor in Patients with Advanced Cervical Cancer: 20-Year Follow-Up of Diagnostic and Treatment Changes during Chemioradiotherapy" Journal of Personalized Medicine 12, no. 10: 1722. https://doi.org/10.3390/jpm12101722
APA StyleWarenczak-Florczak, Z., Burchardt, E., Wilk, A., & Roszak, A. (2022). Tumour-Related Parameters as a Prognostic Factor in Patients with Advanced Cervical Cancer: 20-Year Follow-Up of Diagnostic and Treatment Changes during Chemioradiotherapy. Journal of Personalized Medicine, 12(10), 1722. https://doi.org/10.3390/jpm12101722







