Distal Lung Inflammation Assessed by Alveolar Concentration of Nitric Oxide Is an Individualised Biomarker of Severe COVID-19 Pneumonia
Abstract
1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. Exhaled Nitric Oxide Measurement
2.3. Lung Function Measurement
2.4. Statistical Analyses
3. Results
3.1. Study Cohort
3.2. Characteristics of the Studied Population
3.3. Pulmonary Function Tests and Exhaled Nitric Oxide Measurement
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- COVID-19 Map—Johns Hopkins Coronavirus Resource Center. Available online: https://www.jhu.edu/ (accessed on 4 August 2022).
- Kolb, M.; Dinh-Xuan, A.T.; Brochard, L. Guideline-directed management of COVID-19: Do’s and Don’ts. Eur. Respir. J. 2021, 57, 2100753. [Google Scholar] [CrossRef] [PubMed]
- Antoniou, K.M.; Vasarmidi, E.; Russell, A.-M.; Andrejak, C.; Crestani, B.; Delcroix, M.; Dinh-Xuan, A.T.; Poletti, V.; Sverzellati, N.; Vitacca, M.; et al. European Respiratory Society statement on long COVID follow-up. Eur. Respir. J. 2022, 60, 2102174. [Google Scholar] [CrossRef] [PubMed]
- Burnham, E.L.; Janssen, W.J.; Riches, D.W.H.; Moss, M.; Downey, G.P. The fibroproliferative response in acute respiratory distress syndrome: Mechanisms and clinical significance. Eur. Respir. J. 2014, 43, 276–285. [Google Scholar] [CrossRef] [PubMed]
- Torres-Castro, R.; Vasconcello-Castillo, L.; Alsina-Restoy, X.; Solis-Navarro, L.; Burgos, F.; Puppo, H.; Vilaró, J. Respiratory function in patients post-infection by COVID-19: A systematic review and meta-analysis. Pulmonology 2021, 27, 328–337. [Google Scholar] [CrossRef]
- So, M.; Kabata, H.; Fukunaga, K.; Takagi, H.; Kuno, T. Radiological and functional lung sequelae of COVID-19: A systematic review and meta-analysis. BMC Pulm. Med. 2021, 21, 97. [Google Scholar] [CrossRef]
- Lior, Y.; Yatzkan, N.; Brami, I.; Yogev, Y.; Riff, R.; Hekselman, I.; Fremder, M.; Freixo-Lima, G.; Be’Er, M.; Amirav, I.; et al. Fractional exhaled Nitric Oxide (FeNO) level as a predictor of COVID-19 disease severity. Nitric Oxide 2022, 124, 68–73. [Google Scholar] [CrossRef]
- Kerget, B.; Araz, Ö; Akgün, M. The role of exhaled nitric oxide (FeNO) in the evaluation of lung parenchymal involvement in COVID-19 patients. Intern. Emerg. Med. 2022; in press. [Google Scholar] [CrossRef]
- Horváth, I.; Barnes, P.J.; Loukides, S.; Sterk, P.J.; Högman, M.; Olin, A.-C.; Amann, A.; Antus, B.; Baraldi, E.; Bikov, A.; et al. A European Respiratory Society technical standard: Exhaled biomarkers in lung disease. Eur. Respir. J. 2017, 49, 1600965. [Google Scholar] [CrossRef]
- Dinh-Xuan, A.T.; Annesi-Maesano, I.; Berger, P.; Chambellan, A.; Chanez, P.; Chinet, T.; Degano, B.; Delclaux, C.; Demange, V.; Didier, A.; et al. Contribution of exhaled nitric oxide measurement in airway inflammation assessment in asthma. A position paper from the French Speaking Respiratory Society. Rev. Mal. Respir. 2015, 32, 193–215. [Google Scholar] [CrossRef]
- Dinh-Xuan, A.T.; Brusselle, G. FENO as a biomarker guide for inhaled corticosteroid step down in patients with mild-to-moderate well-controlled asthma. Eur. Respir. J. 2020, 55, 2001319. [Google Scholar] [CrossRef]
- Tiev, K.P.; Hua-Huy, T.; Rivière, S.; Le-Dong, N.-N.; Febvre, M.; Cabane, J.; Dinh-Xuan, A.T. High alveolar concentration of nitric oxide is associated with alveolitis in scleroderma. Nitric Oxide 2013, 28, 65–70. [Google Scholar] [CrossRef]
- Högman, M.; Lehtimäki, L.; Dinh-Xuan, A.T. Utilising Exhaled Nitric Oxide Information to Enhance Diagnosis and Therapy of Respiratory Disease—Current Evidence for Clinical Practice and Proposals to Improve the Methodology. Expert Rev. Respir. Med. 2016, 11, 101–109. [Google Scholar] [CrossRef] [PubMed]
- Marshall, M. The Four Most Urgent Questions about Long COVID. Nature 2021, 594, 168–170. [Google Scholar] [CrossRef] [PubMed]
- Hua-Huy, T.; Lorut, C.; Aubourg, F.; Morbieu, C.; Marey, J.; Texereau, J.; Fajac, I.; Mouthon, L.; Roche, N.; Dinh-Xuan, A.T. Persistent Nasal Inflammation 5 Months after Acute Anosmia in Patients with COVID-19. Am. J. Respir. Crit. Care Med. 2021, 203, 1319–1322. [Google Scholar] [CrossRef] [PubMed]
- Hua-Huy, T.; Le-Dong, N.-N.; Duong-Quy, S.; Luchon, L.; Rouhani, S.; Dinh-Xuan, A.T. Increased alveolar nitric oxide concentration is related to nocturnal oxygen desaturation in obstructive sleep apnoea. Nitric Oxide 2015, 45, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Mo, X.; Jian, W.; Su, Z.; Chen, M.; Peng, H.; Peng, P.; Lei, C.; Chen, R.; Zhong, N.; Li, S. Abnormal pulmonary function in COVID-19 patients at time of hospital discharge. Eur. Respir. J. 2020, 55, 2001217. [Google Scholar] [CrossRef] [PubMed]
- ATS/ERS—American Thoracic Society, European Respiratory Society. ATS/ERS Recommendations for Standardized Procedures for the Online and Offline Measurement of Exhaled Lower Respiratory Nitric Oxide and Nasal Nitric Oxide, 2005. Am. J. Respir. Crit. Care Med. 2005, 171, 912–930. [Google Scholar] [CrossRef]
- Tsoukias, N.M.; Shin, H.-W.; Wilson, A.F.; George, S.C. A single-breath technique with variable flow rate to characterize nitric oxide exchange dynamics in the lungs. J. Appl. Physiol. 2001, 91, 477–487. [Google Scholar] [CrossRef]
- Tiev, K.P.; Hua-Huy, T.; Kettaneh, A.; Allanore, Y.; Le-Dong, N.-N.; Duong-Quy, S.; Cabane, J.; Dinh-Xuan, A.T. Alveolar concentration of nitric oxide predicts pulmonary function deterioration in scleroderma. Thorax 2012, 67, 157–163. [Google Scholar] [CrossRef]
- Miller, M.R.; Crapo, R.; Hankinson, J.; Brusasco, V.; Burgos, F.; Casaburi, R.; Coates, A.; Enright, P.; van der Grinten, C.P.M.; Gustafsson, P.; et al. General considerations for lung function testing. Eur. Respir. J. 2005, 26, 153–161. [Google Scholar] [CrossRef]
- Quanjer, P.H.; Stanojevic, S.; Cole, T.J.; Baur, X.; Hall, G.L.; Culver, B.H.; Enright, P.L.; Hankinson, J.L.; Ip, M.S.M.; Zheng, J.; et al. Multi-ethnic reference values for spirometry for the 3–95-yr age range: The global lung function 2012 equations. Eur. Respir. J. 2012, 40, 1324–1343. [Google Scholar] [CrossRef]
- Hall, G.L.; Filipow, N.; Ruppel, G.; Okitika, T.; Thompson, B.; Kirkby, J.; Steenbruggen, I.; Cooper, B.G.; Stanojevic, S. Official ERS technical standard: Global Lung Function Initiative reference values for static lung volumes in individuals of European ancestry. Eur. Respir. J. 2021, 57, 2000289. [Google Scholar] [CrossRef] [PubMed]
- Stanojevic, S.; Graham, B.L.; Cooper, B.G.; Thompson, B.R.; Carter, K.W.; Francis, R.W.; Hall, G.L. Official ERS technical standards: Global Lung Function Initiative reference values for the carbon monoxide transfer factor for Caucasians. Eur. Respir. J. 2017, 50, 1700010. [Google Scholar] [CrossRef] [PubMed]
- Nalbandian, A.; Sehgal, K.; Gupta, A.; Madhavan, M.V.; McGroder, C.; Stevens, J.S.; Cook, J.R.; Nordvig, A.S.; Shalev, D.; Sehrawat, T.S.; et al. Post-acute COVID-19 syndrome. Nat. Med. 2021, 27, 601–615. [Google Scholar] [CrossRef] [PubMed]
- Laveneziana, P.; Straus, C.; Meiners, S. How and to What Extent Immunological Responses to SARS-CoV-2 Shape Pulmonary Function in COVID-19 Patients. Front. Physiol. 2021, 12, 628288. [Google Scholar] [CrossRef]
- Macnaughton, P.D.; Evans, T.W. Measurement of lung volume and DLCO in acute respiratory failure. Am. J. Respir. Crit. Care Med. 1994, 150, 770–775. [Google Scholar] [CrossRef]
- Teuwen, L.-A.; Geldhof, V.; Pasut, A.; Carmeliet, P. COVID-19: The vasculature unleashed. Nat. Rev. Immunol. 2020, 20, 389–391. [Google Scholar] [CrossRef]
- Neff, T.A.; Stocker, R.; Frey, H.-R.; Stein, S.; Russi, E.W. Long-term Assessment of Lung Function in Survivors of Severe ARDS. Chest 2003, 123, 845–853. [Google Scholar] [CrossRef]
- Spagnolo, P.; Balestro, E.; Aliberti, S.; Cocconcelli, E.; Biondini, D.; Casa, G.D.; Sverzellati, N.; Maher, T.M. Pulmonary fibrosis secondary to COVID-19: A call to arms? Lancet Respir. Med. 2020, 8, 750–752. [Google Scholar] [CrossRef]
- Mylvaganam, R.J.; Bailey, J.I.; Sznajder, J.I.; Sala, M.A. Recovering from a pandemic: Pulmonary fibrosis after SARS-CoV-2 infection. Eur. Respir. Rev. 2021, 30, 210194. [Google Scholar] [CrossRef]
- George, P.M.; Wells, A.U.; Jenkins, R.G. Pulmonary fibrosis and COVID-19: The potential role for antifibrotic therapy. Lancet Respir. Med. 2020, 8, 807–815. [Google Scholar] [CrossRef]
- Mack, M. Inflammation and fibrosis. Matrix Biol. 2018, 68-69, 106–121. [Google Scholar] [CrossRef] [PubMed]
- Salem, A.M.; Al Khathlan, N.; Alharbi, A.F.; Alghamdi, T.; AlDuilej, S.; Alghamdi, M.; Alfudhaili, M.; Alsunni, A.; Yar, T.; Latif, R.; et al. The Long-Term Impact of COVID-19 Pneumonia on the Pulmonary Function of Survivors. Int. J. Gen. Med. 2021, 14, 3271–3280. [Google Scholar] [CrossRef] [PubMed]
- Niyatiwatchanchai, N.; Deesomchok, A.; Chaiwong, W.; Duangjit, P.; Pothirat, C.; Liwsrisakun, C.; Bumroongkit, C.; Theerakittikul, T.; Limsukon, A.; Tajarernmuang, P.; et al. Comparative Study of Early Impacts of Post-COVID-19 Pneumonia on Clinical Manifestations, Pulmonary Function, and Chest Radiographs. Medicina 2022, 58, 216. [Google Scholar] [CrossRef] [PubMed]
- Maniscalco, M.; Ambrosino, P.; Poto, R.; Fuschillo, S.; Poto, S.; Matera, M.G.; Cazzola, M. Can FeNO be a biomarker in the post-COVID-19 patients monitoring? Respir. Med. 2022, 193, 106745. [Google Scholar] [CrossRef] [PubMed]
- Betancor, D.; Valverde-Mongue, M.; Gomez-Lopez, A.; Barroso, B.; Ruete, L.; Olaguibel, J.; Sastre, J. Evaluation of Fractional Exhaled Nitric Oxide During SARS-CoV-2 Infection. J. Investig. Allergy Clin. Immunol. 2022, 32, 301–303. [Google Scholar] [CrossRef]
- Hughes, J.M.B.; Pride, N.B. Examination of the Carbon Monoxide Diffusing Capacity (DlCO) in Relation to Its Kco and Va Components. Am. J. Respir. Crit. Care Med. 2012, 186, 132–139. [Google Scholar] [CrossRef]
- Poitevineau, T.; Chassagnon, G.; Bouam, S.; Jaubert, P.; Cheurfa, C.; Regard, L.; Canniff, E.; Dinh-Xuan, A.T.; Revel, M.-P. Computed tomography after severe COVID-19 pneumonia: Findings at 6 months and beyond. ERJ Open Res. 2021, 7, 00488–2021. [Google Scholar] [CrossRef]
- Tiev, K.P.; Coste, J.; Ziani, M.; Aubourg, F.; Cabane, J.; Dinh-Xuan, A.T. Diagnostic value of exhaled nitric oxide to detect interstitial lung disease in systemic sclerosis. Sarcoidosis Vasc. Diffus. lung Dis. Off. J. WASOG 2009, 26, 32–38. [Google Scholar]
- Tiev, K.P.; Cabané, J.; Aubourg, F.; Kettaneh, A.; Ziani, M.; Mouthon, L.; Duong-Quy, S.; Fajac, I.; Guillevin, L.; Dinh-Xuan, A.T. Severity of scleroderma lung disease is related to alveolar concentration of nitric oxide. Eur. Respir. J. 2007, 30, 26–30. [Google Scholar] [CrossRef]
- Cameli, P.; Bargagli, E.; Bergantini, L.; D’Alessandro, M.; Giugno, B.; Gentili, F.; Sestini, P. Alveolar Nitric Oxide as a Biomarker of COVID-19 Lung Sequelae: A Pivotal Study. Antioxidants 2021, 10, 1350. [Google Scholar] [CrossRef]
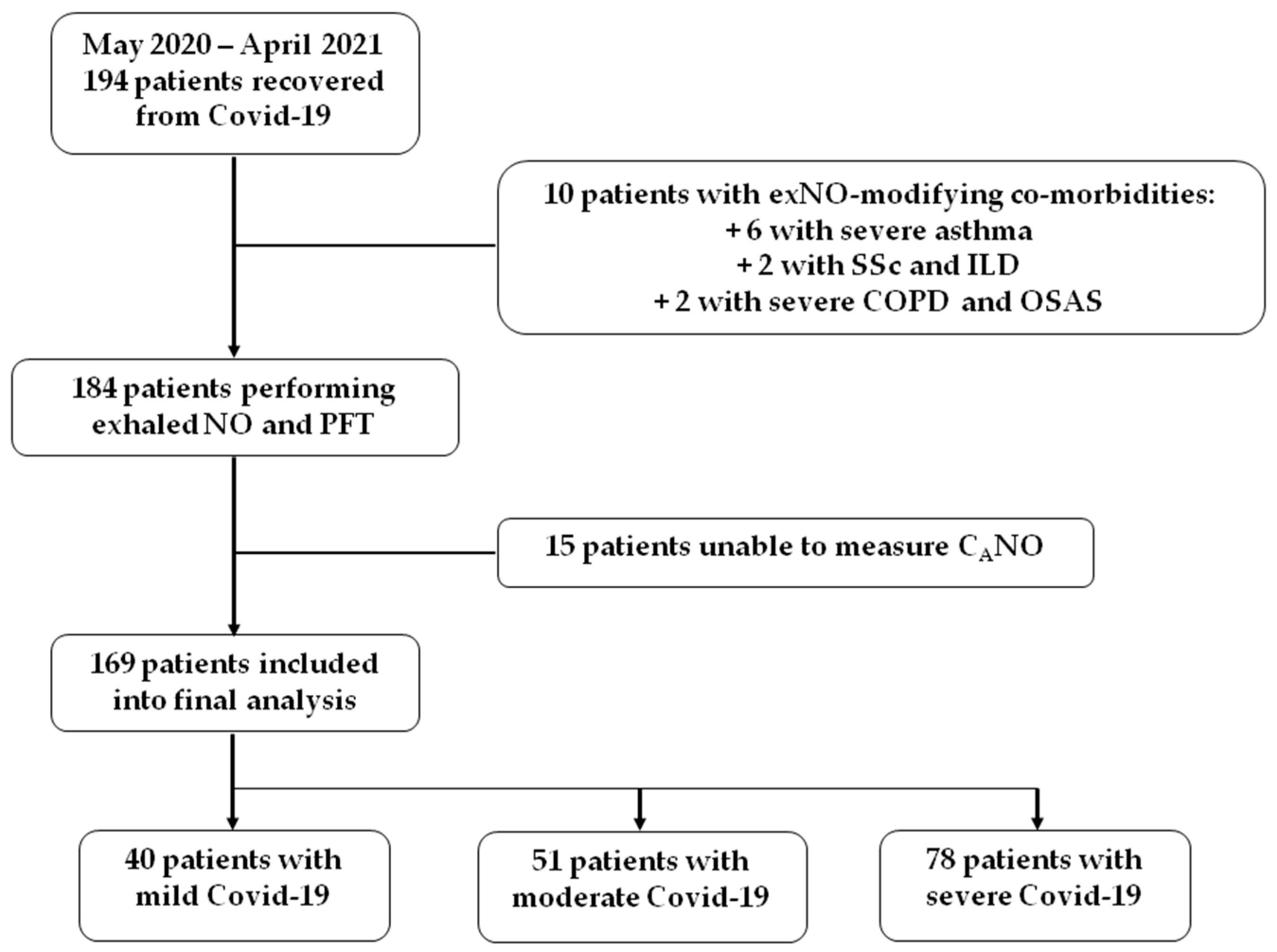

| Mild Disease | Moderate | Severe/Critical | p-Value | |
|---|---|---|---|---|
| N = 40 | N = 51 | N = 78 | (ANOVA) | |
| Age; years | 48.7 ± 14.3 | 58.7 ± 12.7 ## | 60.7 ± 11.7 ### | <0.001 |
| Male; n (%) | 14 (35) | 38 (74.5) | 58 (74) | <0.001 |
| Height; cm | 167 ± 8 | 173 ± 7 | 170 ± 8 ## | 0.011 |
| Weight; kg | 73 ± 17 | 84 ± 15 ## | 79 ± 14 | 0.002 |
| BMI; kg·m−2 | 25.8 ± 5.3 | 28.3 ± 4.4 | 27.4 ± 4.9 | 0.058 |
| Obesity; n (%) | 9 (22.5) | 16 (31) | 15 (19) | 0.279 |
| Smoker | ||||
| 31 (77.5) | 34 (67) | 53 (68) | |
| 4 (10) | 3 (6) | 3 (4) | 0.274 |
| 5 (12.5) | 14 (27) | 22 (28) | |
| Co-morbidities | ||||
| 10 (25) | 12 (24) | 12 (15) | 0.359 |
| 2 (5) | 6 (12) | 12 (15) | 0.255 |
| 6 (15) | 16 (31) | 29 (37) | 0.045 |
| 2 (5) | 9 (18) | 24 (31) | 0.004 |
| 1 (2.5) | 4 (8) | 6 (8) | 0.5 |
| Time from COVID-19; day | 142 ± 41 | 141 ± 52 | 135 ± 48 | 0.712 |
| First thoracic HRCT severity | ||||
| 29 (72.5) | 0 (0) | 0 (0) | |
| 11 (27.5) | 28 (55) | 12 (15) | <0.001 |
| 0 (0) | 23 (45) | 66 (85) | |
| Care modality | ||||
| 39 (97.5) | 2 (4) | 0 (0) | |
| 1 (2.5) | 43 (84) | 12 (15) | <0.001 |
| 0 (0) | 6 (12) | 66 (85) |
| Mild Disease | Moderate | Severe/Critical | p-Value | |
|---|---|---|---|---|
| N = 40 | N = 51 | N = 78 | (ANOVA) | |
| Spirometry | ||||
| 92 ± 16 | 89 ± 17 | 84 ± 16 # | 0.035 |
| 91 ± 14 | 90 ± 16 | 88 ± 18 | 0.666 |
| 0.81 ± 0.07 | 0.79 ± 0.07 | 0.82 ± 0.08 # | 0.044 |
| Obstructive pattern (FEV1/FVC < LLN-GLI) | 1 (2.5) | 2 (4) | 1 (1) | 0.627 |
| Lung volumes | ||||
| 104 ± 14 | 95 ± 18 # | 89 ± 14 ### | < 0.001 |
| 111 ± 22 | 96 ± 18 ## §§ | 84 ± 20 ### | < 0.001 |
| 102 ± 23 | 95 ± 23 | 88 ± 19 ## | 0.003 |
| Restrictive pattern (TLC < LLN-GLI) | 2 (5) | 8 (16) | 21 (27) | 0.012 |
| Lung diffusion capacity (µ) | ||||
| 88 ± 13 | 79 ± 12 # § | 71 ± 17 ### | < 0.001 |
| 92 ± 13 | 92 ± 16 | 91 ± 40 | 0.989 |
| 95 ± 14 | 87 ± 15 ## | 81 ± 13 ### | < 0.001 |
| Lung diffusion impairment (DLCO < LLN-GLI) | 7 (17.5) | 20 (39) | 41 (53) | 0.001 |
| Abnormal PFT | 15 (37.5) | 30 (59) | 52 (67) | 0.005 |
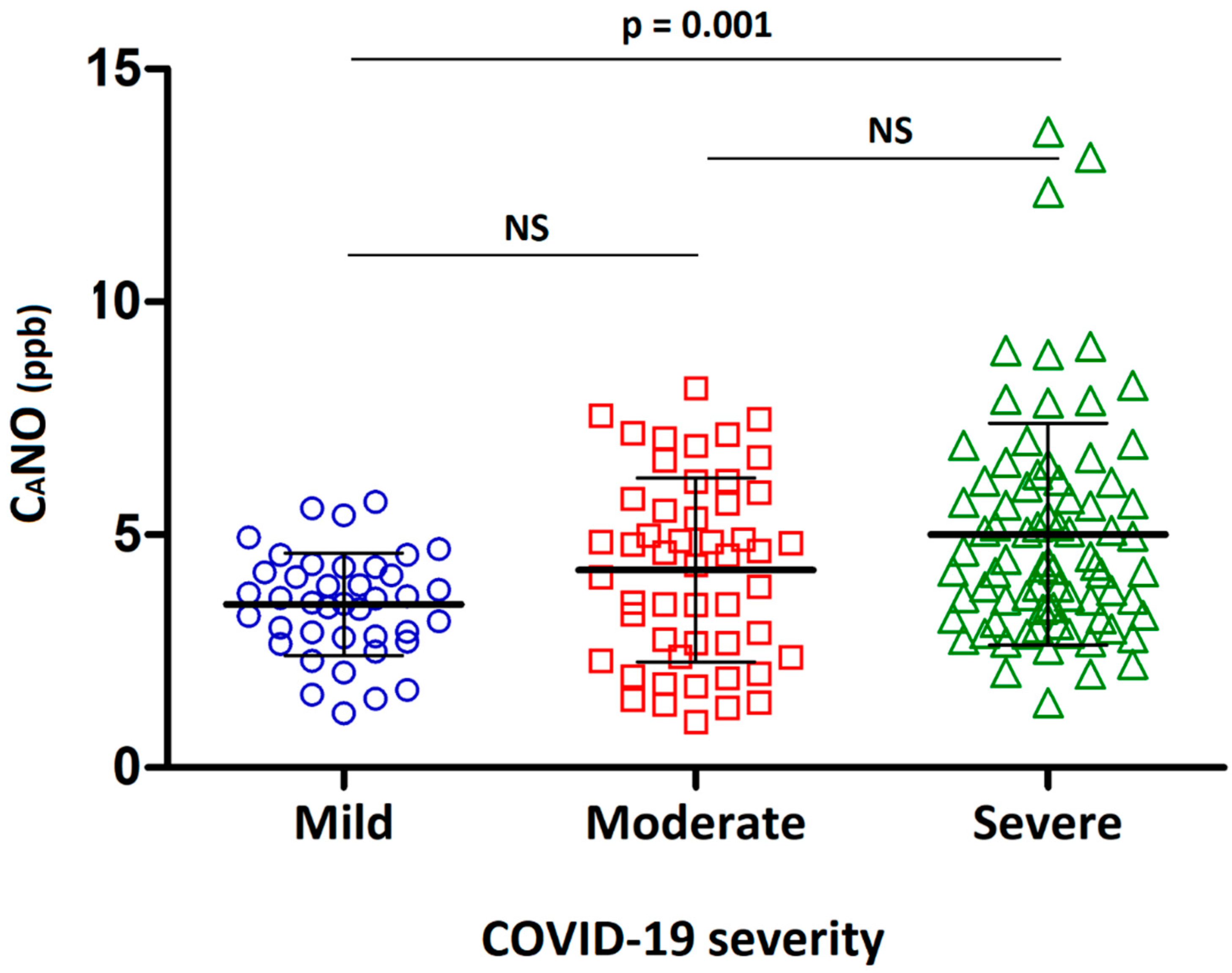
| CaNO; ppb | 3.5 ± 1.1 | 4.2 ± 2.0 | 5.0 ± 2.4 ## | 0.001 |
| 3 (7.5) | 16 (31) | 34 (44) | <0.001 |
| J’awNO; nl/mn | 49.6 ± 23.6 | 55.8 ± 29.5 | 54.9 ± 29.9 | 0.264 |
| FeNO; ppb | 19.4 ± 7.5 | 21.6 ± 9.8 | 22.6 ± 10.8 | 0.541 |
| Mild | Moderate | Extended/Severe | p-Value (ANOVA) | |
|---|---|---|---|---|
| Cases (n) | 29 | 51 | 89 | |
| CaNO; ppb | 3.6 ± 1.1 ## | 4.0 ± 1.7 # | 4.9 ± 2.4 | 0.003 |
| J’awNO; nl/mn | 50.1 ± 23.9 | 56.3 ± 23.9 | 53.8 ± 31.9 | 0.65 |
| FeNO; ppb | 19.6 ± 7.6 | 21.9 ± 8.3 | 22.0 ± 11.2 | 0.5 |
| Home Care | Traditional Hospital | Intensive Care Unit | p-Value (ANOVA) | |
|---|---|---|---|---|
| Cases (n) | 41 | 56 | 72 | |
| CaNO; ppb | 3.5 ± 1.1 | 4.4 ± 2.2 | 5.0 ± 2.3 ### | 0.001 |
| J’awNO; nl/mn | 49.1 ± 23.7 | 58.9 ± 31.3 | 52.8 ± 28.1 | 0.218 |
| FeNO; ppb | 19.2 ± 7.6 | 22.9 ± 10.8 | 21.8 ± 10.1 | 0.18 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hua-Huy, T.; Günther, S.; Lorut, C.; Subileau, M.; Aubourg, F.; Morbieu, C.; Marey, J.; Texereau, J.; Fajac, I.; Mouthon, L.; et al. Distal Lung Inflammation Assessed by Alveolar Concentration of Nitric Oxide Is an Individualised Biomarker of Severe COVID-19 Pneumonia. J. Pers. Med. 2022, 12, 1631. https://doi.org/10.3390/jpm12101631
Hua-Huy T, Günther S, Lorut C, Subileau M, Aubourg F, Morbieu C, Marey J, Texereau J, Fajac I, Mouthon L, et al. Distal Lung Inflammation Assessed by Alveolar Concentration of Nitric Oxide Is an Individualised Biomarker of Severe COVID-19 Pneumonia. Journal of Personalized Medicine. 2022; 12(10):1631. https://doi.org/10.3390/jpm12101631
Chicago/Turabian StyleHua-Huy, Thông, Sven Günther, Christine Lorut, Marielle Subileau, Frédérique Aubourg, Caroline Morbieu, Jonathan Marey, Joëlle Texereau, Isabelle Fajac, Luc Mouthon, and et al. 2022. "Distal Lung Inflammation Assessed by Alveolar Concentration of Nitric Oxide Is an Individualised Biomarker of Severe COVID-19 Pneumonia" Journal of Personalized Medicine 12, no. 10: 1631. https://doi.org/10.3390/jpm12101631
APA StyleHua-Huy, T., Günther, S., Lorut, C., Subileau, M., Aubourg, F., Morbieu, C., Marey, J., Texereau, J., Fajac, I., Mouthon, L., Roche, N., & Dinh-Xuan, A. T. (2022). Distal Lung Inflammation Assessed by Alveolar Concentration of Nitric Oxide Is an Individualised Biomarker of Severe COVID-19 Pneumonia. Journal of Personalized Medicine, 12(10), 1631. https://doi.org/10.3390/jpm12101631








