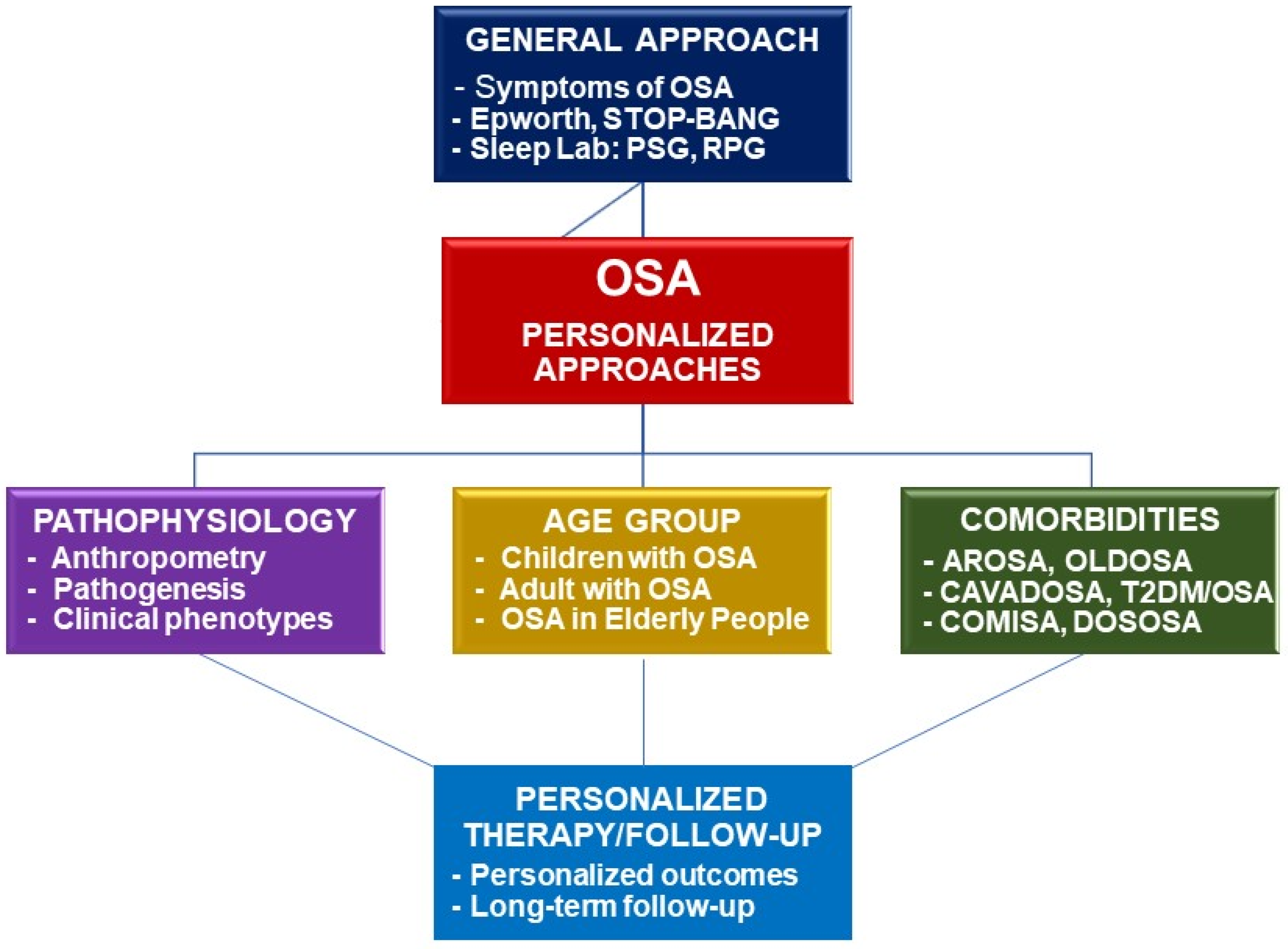
Personalized Medicine and Obstructive Sleep Apnea
Abstract
:1. Introduction
2. Personalization of Diagnosis and Treatment of Children with OSA
2.1. General Consideration
2.2. Personalization of Clinical Approach for Children with OSA
2.2.1. Overview
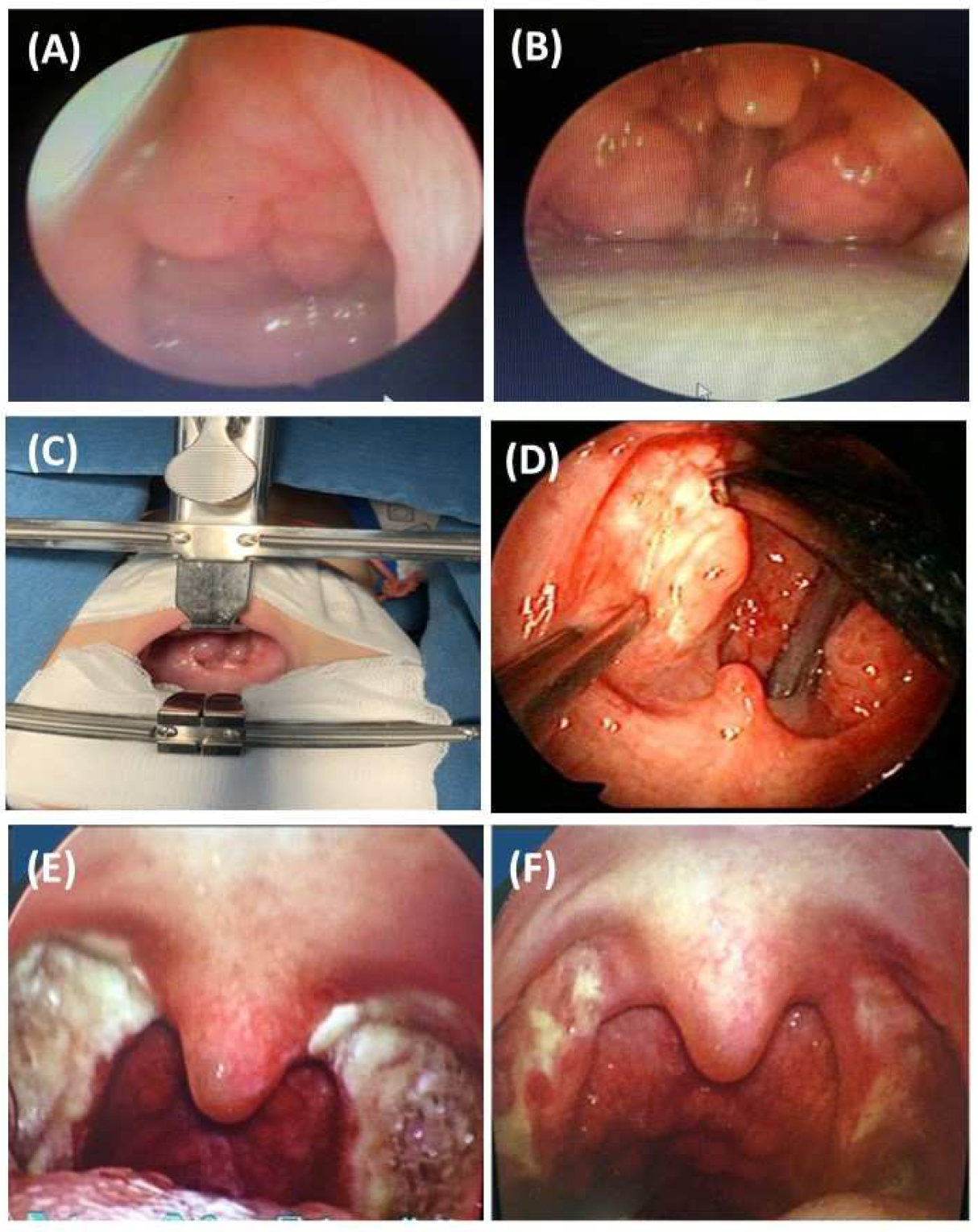
2.2.2. Clinical Manifestations of Children with OSA
2.2.3. Clinical and Laboratory Considerations for Children with OSA
2.3. Personalized Treatment of Children with OSA
3. Personalization of Clinical Approaches for Adults with OSA
3.1. Overview
3.2. Personalization of OSA with Phenotype Approach
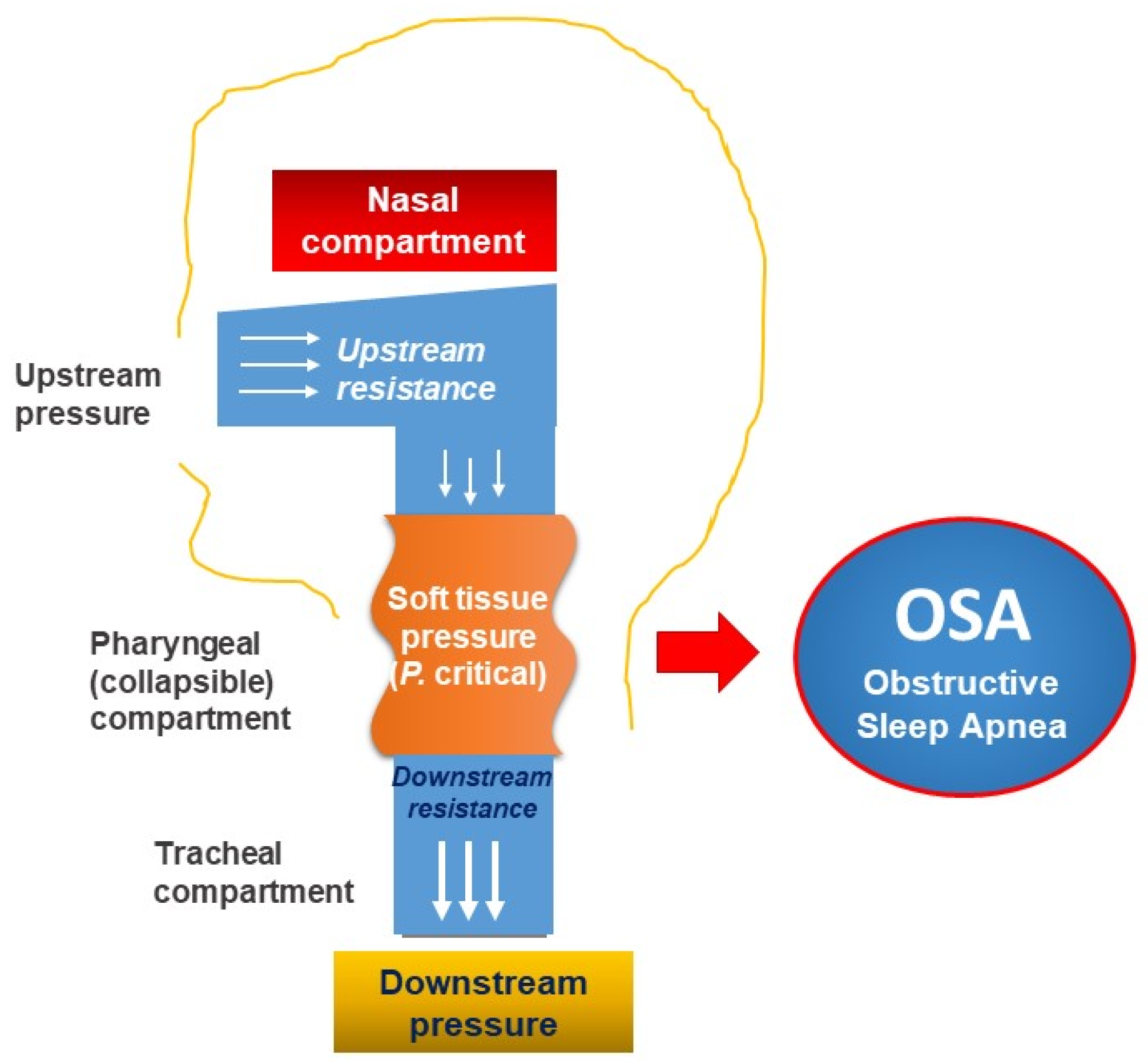
3.3. Personalization of OSA with Function and Pathophysiological Approach
4. Personalization of Clinical Approaches for OSA in Elderly Patients
4.1. General Considerations
4.2. Personalization of OSA with Clinical Approach in Elderly Patients
4.3. Personalized Treatment for OSA in Elderly Patients
5. Personalization of Management for Patients with OSA and Comorbidities
5.1. Background
5.2. Personalized Approaches for Patients with OSA and Airway Diseases
5.2.1. OSA and Allergic Rhinitis
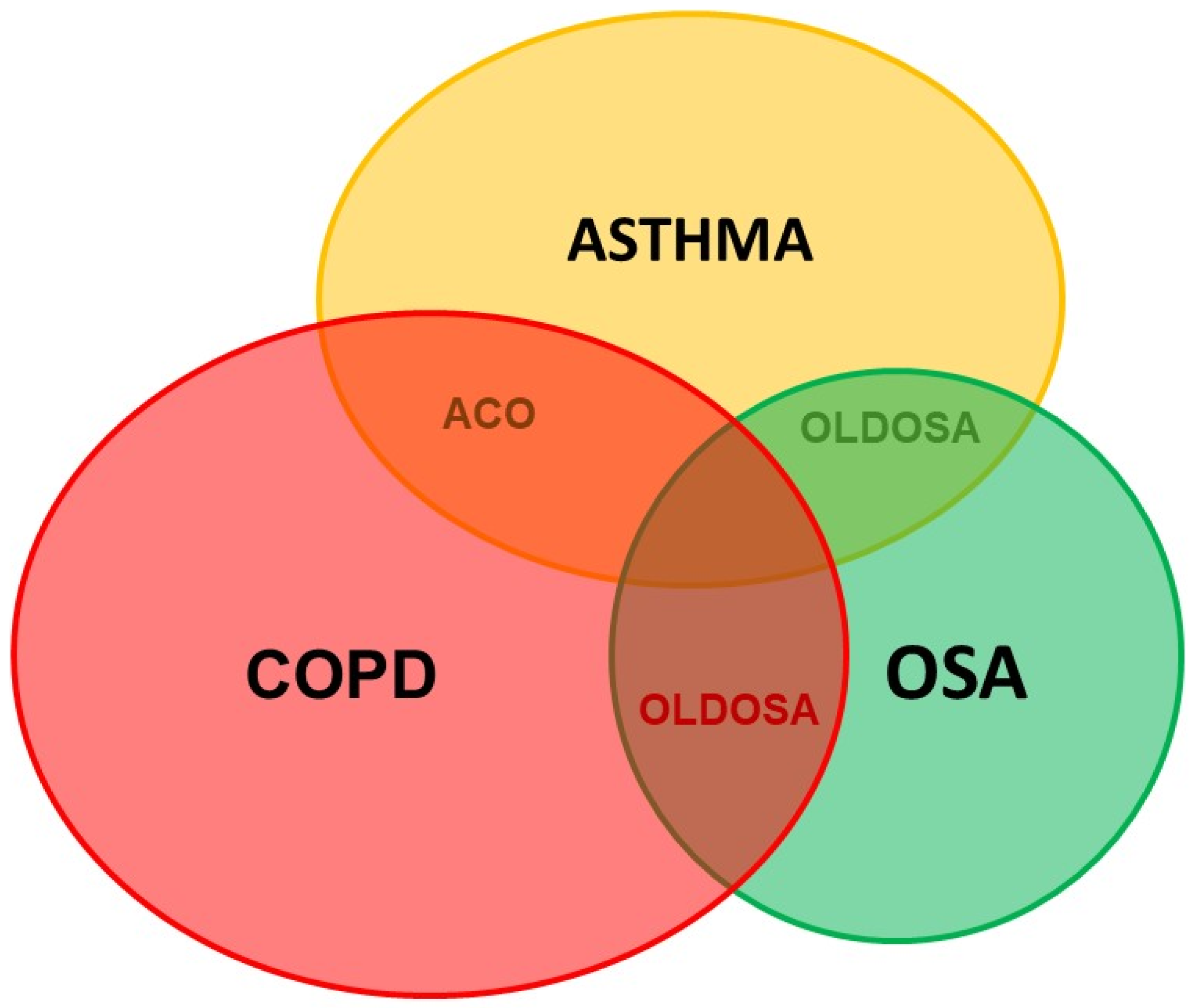
5.2.2. OSA and Obstructive Lung Diseases
5.3. Personalized Approaches for Patients with OSA and Cardiovascular Diseases
5.3.1. General Considerations
5.3.2. Personalization of OSA Diagnosis in Patients with Cardiovascular Diseases
5.3.3. Personalization of OSA Treatment in Patients with Cardiovascular Diseases
5.4. Personalized Approaches for Patients with OSA and Diabetes
5.4.1. General Considerations and Personalized Approach
5.4.2. Personalized Treatment of OSA Patients with Comorbid Diabetes and Metabolic Syndrome
5.5. Personalized Approaches for Patients with OSA and Insomnia
5.5.1. Personalized Diagnosis
5.5.2. Personalized Treatment
5.6. Personalized Approaches for Subjects with OSA and Genetic Defects and Other Disorders
5.6.1. Personalized Approach and Diagnosis
5.6.2. Personalized Treatment
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Capdevila, O.S.; Gozal, L.; Dayyat, E.; Gozal, D. Pediatric Obstructive Sleep Apnea: Complications, Management, and Long-term Outcomes. Proc. Am. Thorac. Soc. 2008, 5, 274–282. [Google Scholar] [CrossRef]
- Garg, R.K.; Afifi, A.M.; Garland, C.B.; Sanchez, R.; Mount, D.L. Pediatric Obstructive Sleep Apnea: Consensus, Controversy, and Craniofacial Considerations. Plast. Reconstr. Surg. 2017, 140, 987–997. [Google Scholar] [CrossRef] [PubMed]
- Bazzano, L.A.; Hu, T.; Bertisch, S.M.; Yao, L.; Harville, E.W.; Gustat, J.; Chen, W.; Webber, L.S.; Redline, S. Childhood obesity patterns and relation to middle-age sleep apnoea risk: The Bogalusa Heart Study. Pediatr. Obes. 2016, 11, 535–542. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schwengel, D.A.; Dalesio, N.M.; Stierer, T.L. Pediatric obstructive sleep apnea. Anesthesiol. Clin. 2014, 32, 237–261. [Google Scholar] [CrossRef] [PubMed]
- Ramirez, F.D.; Groner, J.A.; Ramirez, J.L.; McEvoy, C.T.; Owens, J.A.; McCulloch, C.E.; Cabana, M.D.; Abuabara, K. Prenatal and Childhood Tobacco Smoke Exposure Are Associated with Sleep-Disordered Breathing Throughout Early Childhood. Acad. Pediatr. 2020, 21, 654–662. [Google Scholar] [CrossRef]
- Xiao, L.; Su, S.; Liang, J.; Jiang, Y.; Shu, Y.; Ding, L. Analysis of the Risk Factors Associated with Obstructive Sleep Apnea Syndrome in Chinese Children. Front. Pediatr. 2022, 10, 216. [Google Scholar] [CrossRef]
- Honaker, S.M.; Meltzer, L.J. Sleep in pediatric primary care: A review of the literature. Sleep Med. Rev. 2016, 25, 31–39. [Google Scholar] [CrossRef] [Green Version]
- Li, Z.; Celestin, J.; Lockey, R.F. Pediatric Sleep Apnea Syndrome: An Update. J. Allergy Clin. Immunol. Pr. 2016, 4, 852–861. [Google Scholar] [CrossRef]
- Segù, M.; Pollis, M.; Santagostini, A.; Meola, F.; Manfredini, D. Correlation between Parental-Reported Tooth Grinding and Sleep Disorders: Investigation in a Cohort of 741 Consecutive Children. Pain Res. Manag. 2020, 2020, 1–5. [Google Scholar] [CrossRef]
- El Mallah, M.; Bailey, E.; Trivedi, M.; Kremer, T.; Rhein, L.M. Pediatric Obstructive Sleep Apnea in High-Risk Populations: Clinical Implications. Pediatr. Ann. 2017, 46, e336–e339. [Google Scholar] [CrossRef]
- Bitners, A.C.; Arens, R. Evaluation and Management of Children with Obstructive Sleep Apnea Syndrome. Lung 2020, 198, 257–270. [Google Scholar] [CrossRef] [PubMed]
- Cielo, C.M.; Gungor, A. Treatment Options for Pediatric Obstructive Sleep Apnea. Curr. Problems Pediatr. Adoles. Health Care 2016, 46, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Kheirandish-Gozal, L.; Bandla, H.P.R.; Gozal, D. Montelukast for Children with Obstructive Sleep Apnea: Results of a Double-blind Randomized Placebo-controlled Trial. Ann. Am. Thorac. Soc. 2016, 13, 1736–1741. [Google Scholar] [CrossRef] [PubMed]
- Goldbart, A.D.; Greenberg-Dotan, S.; Tal, A. Montelukast for Children with Obstructive Sleep Apnea: A Double-blind, Placebo-Controlled Study. Pediatrics 2012, 130, e575–e580. [Google Scholar] [CrossRef] [Green Version]
- Goldbart, A.D.; Goldman, J.L.; Veling, M.C.; Gozal, D. Leukotriene Modifier Therapy for Mild Sleep-disordered Breathing in Children. Am. J. Respir. Crit. Care Med. 2005, 172, 364–370. [Google Scholar] [CrossRef] [Green Version]
- Donaldson, A.M.; Choby, G.; Kim, D.H.; Marks, L.A.; Lal, D. Intranasal Corticosteroid Therapy: Systematic Review and Meta-analysis of Reported Safety and Adverse Effects in Children. Otolaryngol. Neck Surg. 2020, 163, 1087–1096. [Google Scholar] [CrossRef]
- Hua, F.; Zhao, T.; Walsh, T.; Sun, Q.; Chen, X.; Worthington, H.; Jiang, F.; He, H. Effects of adenotonsillectomy on the growth of children with obstructive sleep apnoea-hypopnea syndrome (OSAHS): Protocol for a systematic review. BMJ Open 2019, 9, e030866. [Google Scholar] [CrossRef] [Green Version]
- Shan, S.; Wang, S.; Yang, X.; Liu, F.; Xiu, L. Effect of adenotonsillectomy on the growth, development, and comprehensive cognitive abilities of children with obstructive sleep apnea: A prospective single-arm study. BMC Pediatr. 2022, 22, 1–7. [Google Scholar] [CrossRef]
- Uwiera, T.C. Considerations in Surgical Management of Pediatric Obstructive Sleep Apnea: Tonsillectomy and Beyond. Children 2021, 8, 944. [Google Scholar] [CrossRef]
- Gazzaz, M.J.; Isaac, A.; Anderson, S.; Alsufyani, N.; Alrajhi, Y.; El-Hakim, H. Does drug-induced sleep endoscopy change the surgical decision in surgically naïve non-syndromic children with snoring/sleep disordered breathing from the standard adenotonsillectomy? A retrospective cohort study. J. Otolaryngol.-Head Neck Surg. 2017, 46, 1–8. [Google Scholar] [CrossRef]
- Trosman, S.J.; Eleff, D.J.; Krishna, J.; Anne, S. Polysomnography results in pediatric patients with mild obstructive sleep apnea: Adenotonsillectomy vs. watchful waiting. Int. J. Pediatr. Otorhinolaryngol. 2016, 83, 25–30. [Google Scholar] [CrossRef] [PubMed]
- Tran-Minh, D.; Phi-Thi-Quynh, A.; Nguyen-Dinh, P.; Duong-Quy, S. Efficacy of obstructive sleep apnea treatment by antileu-kotriene receptor and surgery therapy in children with adenotonsillar hypertrophy: A descriptive and cohort study. Front. Neurol. 2022, 13, 1008310. [Google Scholar] [CrossRef] [PubMed]
- Guilleminault, C.; Monteyrol, P.-J.; Huynh, N.T.; Pirelli, P.; Quo, S.; Li, K. Adeno-tonsillectomy and rapid maxillary distraction in pre-pubertal children, a pilot study. Sleep Breath. 2011, 15, 173–177. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahn, H.W.; Lee, B.S.; Kim, S.W.; Kim, S.J. Stability of Modified Maxillomandibular Advancement Surgery in a Patient with Preadolescent Refractory Obstructive Sleep Apnea. J. Oral. Maxillofac. Surg. 2015, 73, 1827–1841. [Google Scholar] [CrossRef]
- Li, Y.; Lu, Y.; Li, X.; Zhao, L.; Guo, J.; Yu, L.; Feng, J.; Li, B.; Li, X.; Liu, Y. Efficacy of orthodontic treatment versus adenotonsillectomy in children with moderate obstructive sleep apnoea and mandibular retrognathia: Study design and protocol for a non-inferiority randomised controlled trial. BMJ Open 2022, 12, e055964. [Google Scholar] [CrossRef]
- Fagundes, N.C.F.; Perez-Garcia, A.; Graf, D.; Flores-Mir, C.; Heo, G. Orthodontic interventions as a management option for children with residual obstructive sleep apnea: A cohort study protocol. BMJ Open 2022, 12, e061651. [Google Scholar] [CrossRef]
- McARDLE, N.; Devereux, G.; Heidarnejad, H.; Engleman, H.M.; Mackay, T.W.; Douglas, N.J. Long-term Use of CPAP Therapy for Sleep Apnea/Hypopnea Syndrome. Am. J. Respir. Crit. Care Med. 1999, 159, 1108–1114. [Google Scholar] [CrossRef] [Green Version]
- Krieger, J.; Kurtz, D.; Petiau, C.; Sforza, E.; Trautmann, D. Long-Term Compliance with CPAP Therapy in Obstructive Sleep Apnea Patients and in Snorers. Sleep 1996, 19, S136–S143. [Google Scholar] [CrossRef] [Green Version]
- Engleman, H.M.; E Martin, S.; Douglas, N.J. Compliance with CPAP therapy in patients with the sleep apnoea/hypopnoea syndrome. Thorax 1994, 49, 263–266. [Google Scholar] [CrossRef] [Green Version]
- Hussain, S.F.; Irfan, M.; Waheed, Z.; Alam, N.; Mansoor, S.; Islam, M. Compliance with continuous positive airway pressure (CPAP) therapy for obstructive sleep apnea among privately paying patients- a cross sectional study. BMC Pulm. Med. 2014, 14, 188. [Google Scholar] [CrossRef]
- Zinchuk, A.V.; Gentry, M.J.; Concato, J.; Yaggi, H.K. Phenotypes in obstructive sleep apnea: A definition, examples and evolution of approaches. Sleep Med. Rev. 2017, 35, 113–123. [Google Scholar] [CrossRef] [PubMed]
- Butler, M.P.; Emch, J.T.; Rueschman, M.; Sands, S.A.; Shea, S.A.; Wellman, A.; Redline, S. Apnea–Hypopnea Event Duration Predicts Mortality in Men and Women in the Sleep Heart Health Study. Am. J. Respir. Crit. Care Med. 2019, 199, 903–912. [Google Scholar] [CrossRef] [PubMed]
- Azarbarzin, A.; A Sands, S.; Stone, K.L.; Taranto-Montemurro, L.; Messineo, L.; I Terrill, P.; Ancoli-Israel, S.; Ensrud, K.; Purcell, S.; White, D.P.; et al. The hypoxic burden of sleep apnoea predicts cardiovascular disease-related mortality: The Osteoporotic Fractures in Men Study and the Sleep Heart Health Study. Eur. Hear. J. 2019, 40, 1149–1157. [Google Scholar] [CrossRef] [PubMed]
- Montesi, S.B.; Edwards, B.; Malhotra, A.; Bakker, J.P. The Effect of Continuous Positive Airway Pressure Treatment on Blood Pressure: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. J. Clin. Sleep Med. 2012, 8, 587–596. [Google Scholar] [CrossRef] [PubMed]
- Castro-Grattoni, A.L.; Torres, G.; Martínez-Alonso, M.; Barbé, F.; Turino, C.; Sánchez-De-La-Torre, A.; Cortijo, A.; Duran-Cantolla, J.; Egea, C.; Cao, G.; et al. Blood pressure response to CPAP treatment in subjects with obstructive sleep apnoea: The predictive value of 24-h ambulatory blood pressure monitoring. Eur. Respir. J. 2017, 50, 1700651. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sands, S.A.; Eckert, D.J.; Jordan, A.S.; Edwards, B.A.; Owens, R.L.; Butler, J.P.; Schwab, R.J.; Loring, S.H.; Malhotra, A.; White, D.P.; et al. Enhanced Upper-Airway Muscle Responsiveness Is a Distinct Feature of Overweight/Obese Individuals without Sleep Apnea. Am. J. Respir. Crit. Care Med. 2014, 190, 930–937. [Google Scholar] [CrossRef] [Green Version]
- Eckert, D.J.; White, D.P.; Jordan, A.S.; Malhotra, A.; Wellman, A. Defining phenotypic causes of obstructive sleep apnea. Identif. Nov. Ther. Targets. Am. J. Respir. Crit. Care Med. 2013, 188, 996–1004. [Google Scholar] [CrossRef] [Green Version]
- Owens, R.L.; Edwards, B.A.; Eckert, D.J.; Jordan, A.S.; Sands, S.A.; Malhotra, A.; White, D.P.; Loring, S.H.; Butler, J.P.; Wellman, A. An Integrative Model of Physiological Traits Can be Used to Predict Obstructive Sleep Apnea and Response to Non Positive Airway Pressure Therapy. Sleep 2015, 38, 961–970. [Google Scholar] [CrossRef] [Green Version]
- Azarbarzin, A.; Sands, S.A.; Taranto-Montemurro, L.; Marques, M.D.O.; Genta, P.R.; Edwards, B.A.; Butler, J.; White, D.P.; Wellman, A. Estimation of Pharyngeal Collapsibility During Sleep by Peak Inspiratory Airflow. Sleep 2016, 40, zsw005. [Google Scholar] [CrossRef] [Green Version]
- Kotecha, B.T.; Hannan, S.A.; Khalil, H.M.; Georgalas, C.; Bailey, P. Sleepnasendoscopy: A 10-year retrospective audit study. Eur. Arch. Otorhinolaryngol. 2007, 264, 1361–1367.41. [Google Scholar] [CrossRef]
- MacKay, S.G.; Carney, A.S.; Woods, C.; Antic, N.; McEvoy, R.D.; Chia, M.; Sands, T.; Jones, A.; Hobson, J.; Robinson, S. Modified uvulopalatopharyngoplasty and coblation channeling ofthe tongue for obstructive sleep apnea: A multi-centreaustralian trial. J. Clin. Sleep Med. 2013, 9, 117–124.42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pang, K.P.; Siow, J.K.; Tseng, P. Safety of multilevel surgeryin obstructive sleep apnea: A review of 487 cases. Arch. Otolaryngol. Head Neck Surg. 2012, 138, 353–357.43. [Google Scholar] [PubMed] [Green Version]
- Vicini, C.; Montevecchi, F.; Campanini, A.; Dallan, I.; Hoff, P.T.; Spector, M.E.; Thaler, E.; Ahn, J.; Baptista, P.; Remacle, M.; et al. Clinical outcomes and complications associated with TORS forOSAHS: A benchmark for evaluating an emerging surgicaltechnology in a targeted application for benign disease. ORL J. Otorhinolaryngol. Relat. Spec. 2014, 76, 63–69. [Google Scholar] [CrossRef] [PubMed]
- Koutsourelakis, I.; Safiruddin, F.; Ravesloot, M.; Zakynthinos, S.; de Vries, N. Surgery for obstructive sleep apnea: Sleep endoscopy determinants of outcome. Laryngoscope 2012, 122, 2587–2591. [Google Scholar] [CrossRef]
- Yngkaran, T.; Kanaglingam, J.; Rajeswaran, R.; Georgalas, C.; Kotecha, B. Long-term outcomes of laser-assisted uvulopalatoplasty in 168 patients with snoring. J. Laryngol. Otol. 2006, 120, 932–938. [Google Scholar] [CrossRef]
- Edwards, B.A.; Andara, C.; Landry, S.; Sands, S.A.; Joosten, S.A.; Owens, R.L.; White, D.P.; Hamilton, G.S.; Wellman, A. Upper-Airway Collapsibility and Loop Gain Predict the Response to Oral Appliance Therapy in Patients with Obstructive Sleep Apnea. Am. J. Respir. Crit. Care Med. 2016, 194, 1413–1422. [Google Scholar] [CrossRef] [Green Version]
- Joosten, S.A.; Leong, P.; Landry, S.A.; Sands, S.A.; Terrill, P.I.; Mann, D.; Turton, A.; Rangaswamy, J.; Andara, C.; Burgess, G.; et al. Loop Gain Predicts the Response to Upper Airway Surgery in Patients with Obstructive Sleep Apnea. Sleep 2017, 40, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Ye, J.; Han, D.; Cao, X.; Ding, X.; Zhang, Y.; Xu, W.; Orr, J.; Jen, R.; Sands, S.; et al. Physiology-Based Modeling May Predict Surgical Treatment Outcome for Obstructive Sleep Apnea. J. Clin. Sleep Med. JCSM: Off. Publ. Am. Acad. Sleep Med. 2017, 13, 1029–1037. [Google Scholar] [CrossRef] [Green Version]
- Wellman, A.; Malhotra, A.; Jordan, A.S.; Stevenson, K.E.; Gautam, S.; White, D.P. Effect of oxygen in obstructive sleep apnea: Role of loop gain. Respir. Physiol. Neurobiol. 2008, 162, 144–151. [Google Scholar] [CrossRef] [Green Version]
- Sands, S.A.; Edwards, B.A.; Terrill, P.I.; Butler, J.P.; Owens, R.L.; Taranto-Montemurro, L.; Azarbarzin, A.; Marques, M.; Hess, L.B.; Smales, E.T.; et al. Identifying obstructive sleep apnoea patients responsive to supplemental oxygen therapy. Eur. Respir. J. 2018, 52, 1800674. [Google Scholar] [CrossRef] [PubMed]
- Xie, A.; Teodorescu, M.; Pegelow, D.F.; Teodorescu, M.C.; Gong, Y.; Fedie, J.E.; Dempsey, J.A. Effects of stabilizing or increasing respiratory motor outputs on obstructive sleep apnea. J. Appl. Physiol. 2013, 115, 22–33. [Google Scholar] [CrossRef] [PubMed]
- Taranto-Montemurro, L.; Sands, S.A.; Edwards, B.A.; Azarbarzin, A.; Marques, M.; de Melo, C.; Eckert, D.J.; White, D.P.; Wellman, A. Desipramine improves upper airway collapsibility and reduces OSA severity in patients with minimal muscle compensation. Eur. Respir. J. 2016, 48, 1340–1350. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mediano, O.; González Mangado, N.; Montserrat, J.M.; Alonso-Álvarez, M.L.; Almendros, I.; Alonso-Fernández, A.; Barbé, F.; Borsini, E.; Caballero-Eraso, C.; Cano-Pumarega, I.; et al. Spanish Sleep Network. Int. Consens. Doc. Obstr. Sleep Apnea. Arch. Broconeumol. 2022, 58, 52–68. [Google Scholar] [CrossRef]
- Chang, J.L.; Goldberg, A.N.; Alt, J.A.; Ashbrook, L.; Auckley, D.; Ayappa, I.; Bakhtiar, H.; Barrera, J.E.; Bartley, B.L.; Billings, M.E.; et al. International consensus statement on obstructive sleep apnea. Int. Forum Allergy Rhinol. 2022. [Google Scholar] [CrossRef] [PubMed]
- Duarte, R.L.M.; Togeiro, S.M.G.P.; Palombini, L.O.; Rizzatti, F.P.G.; Fagondes, S.C.; Magalhães-da-Silveira, F.J.; Cabral, M.M.; Genta, P.R.; Lorenzi-Filho, G.; Clímaco, D.C.S.; et al. Brazilian Thoracic Association Consensus on Sleep-disordered Breathing. J. Bras. Pneumol. 2022, 48, e20220106. [Google Scholar] [CrossRef]
- Wellman, A.; Eckert, D.; Jordan, A.; Edwards, B.; Passaglia, C.; Jackson, A.C.; Gautam, S.; Owens, R.L.; Malhotra, A.; White, D.P. A method for measuring and modeling the physiological traits causing obstructive sleep apnea. J. Appl. Physiol. 2011, 110, 1627–1637. [Google Scholar] [CrossRef] [Green Version]
- Wellman, A.; Edwards, B.; Sands, S.; Owens, R.L.; Nemati, S.; Butler, J.; Passaglia, C.; Jackson, A.C.; Malhotra, A.; White, D.P. A simplified method for determining phenotypic traits in patients with obstructive sleep apnea. J. Appl. Physiol. 2013, 114, 911–922. [Google Scholar] [CrossRef] [Green Version]
- Glasser, M.; Bailey, N.; McMillan, A.; Goff, E.; Morrell, M. Sleep apnoea in older people. Breathe 2011, 7, 248–256. [Google Scholar] [CrossRef]
- Young, T.; Shahar, E.; Nieto, F.J.; Redline, S.; Newman, A.B.; Gottlieb, D.J.; Walsleben, J.A.; Finn, L.; Enright, P.; Samet, J.M. Predictors of Sleep-Disordered Breathing in Community-Dwelling AdultsThe Sleep Heart Health Study. Arch. Intern. Med. 2002, 162, 893–900. [Google Scholar] [CrossRef] [Green Version]
- Eikermann, M.; Jordan, A.; Chamberlin, N.L.; Gautam, S.; Wellman, A.; Lo, Y.-L.; White, D.P.; Malhotra, A. The Influence of Aging on Pharyngeal Collapsibility During Sleep. Chest 2007, 131, 1702–1709. [Google Scholar] [CrossRef]
- Malhotra, A.; Huang, Y.; Fogel, R.; Lazic, S.; Pillar, G.; Jakab, M.; Kikinis, R.; White, D.P. Aging Influences on Pharyngeal Anatomy and Physiology: The Predisposition to Pharyngeal Collapse. Am. J. Med. 2006, 119, 72.e9–72.e14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Erskine, R.; Murphy, P.; Langton, J.; Smith, G. Effect of age on the sensitivity of upper airway reflexes. Br. J. Anaesth. 1993, 70, 574–575. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wimms, A.; Woehrle, H.; Ketheeswaran, S.; Ramanan, D.; Armitstead, J. Obstructive Sleep Apnea in Women: Specific Issues and Interventions. BioMed. Res. Int. 2016, 2016, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McMillan, A.; Morrell, M.J. Sleep disordered breathing at the extremes of age: The elderly. Breathe 2016, 12, 50–60. [Google Scholar] [CrossRef]
- Onen, F.; Moreau, T.; Gooneratne, N.S.; Petit, C.; Falissard, B.; Onen, S.H. Limits of the Epworth Sleepiness Scale in older adults. Sleep Breath 2012, 17, 343–350. [Google Scholar] [CrossRef]
- Leng, Y.; McEvoy, C.T.; Allen, I.E.; Yaffe, K. Association of Sleep-Disordered Breathing With Cognitive Function and Risk of Cognitive Impairment: A Systematic Review and Meta-analysis. JAMA Neurol. 2017, 74, 1237–1245. [Google Scholar] [CrossRef]
- Legault, J.; Thompson, C.; Martineau-Dussault, M.; André, C.; Baril, A.-A.; Villar, G.M.; Carrier, J.; Gosselin, N. Obstructive Sleep Apnea and Cognitive Decline: A Review of Potential Vulnerability and Protective Factors. Brain Sci. 2021, 11, 706. [Google Scholar] [CrossRef]
- Born, J.; Wilhelm, I. System consolidation of memory during sleep. Psychol. Res. 2012, 76, 192–203. [Google Scholar] [CrossRef] [Green Version]
- Newman, A.B.; Nieto, F.J.; Guidry, U.; Lind, B.K.; Redline, S.; Pickering, T.G.; Quan, S.F.; Sleep Heart Health Study Research Group. Relation of sleep-disordered breathing to cardiovascular disease risk factors: The Sleep Heart Health Study. Am. J. Ep-idemiol. 2001, 154, 50–59. [Google Scholar] [CrossRef] [Green Version]
- Munoz, R.; Duran-Cantolla, J.; Martinez-Vila, E.; Gallego, J.; Rubio, R.; Aizpuru, F.; De La Torre, G. Severe Sleep Apnea and Risk of Ischemic Stroke in the Elderly. Stroke 2006, 37, 2317–2321. [Google Scholar] [CrossRef]
- McCall, W.; Harding, D.; Orsquo, C. Donovan Correlates of Depressive Symptoms in Patients with Obstructive Sleep Apnea. J. Clin. Sleep Med. 2006, 2, 424–426. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stevens, D.; Jackson, B.; Carberry, J.; McLoughlin, J.; Barr, C.; Mukherjee, S.; Oh, A.; McEvoy, R.D.; Crotty, M.; Vakulin, A. The Impact of Obstructive Sleep Apnea on Balance, Gait, and Falls Risk: A Narrative Review of the Literature. J. Gerontol. Ser. A 2020, 75, 2450–2460. [Google Scholar] [CrossRef] [PubMed]
- Posadas, T.; Oscullo, G.; Zaldívar, E.; Garcia-Ortega, A.; Gómez-Olivas, J.D.; Monteagudo, M.; Martínez-García, M.A. Treatment with CPAP in Elderly Patients with Obstructive Sleep Apnoea. J. Clin. Med. 2020, 9, 546. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lunn, M. Timothy Craig. Rhinitis and sleep. Sleep Med. Rev. 2011, 15, 293–299. [Google Scholar] [CrossRef]
- Pawanker, R.; Canonica, G.W.; Holgate, S.T.; Lockey, R.F. World Health Organization White Book on Allergy 2011–2012 Executive Summary; World Health Organization: Geneva, Switzerland, 2011. [Google Scholar]
- Cao, Y.; Wu, S.; Zhang, L.; Yang, Y.; Cao, S.; Li, Q. Association of allergic rhinitis with obstructive sleep apnea. Medicine 2018, 97, e13783. [Google Scholar] [CrossRef]
- Vo-Thi-Kim, A.; Van-Quang, T.; Nguyen-Thanh, B.; Dao-Van, D.; Duong-Quy, S. The effect of medical treatment on nasal exhaled nitric oxide (NO) in patients with persistent allergic rhinitis: A randomized control study. Adv. Med. Sci. 2020, 65, 182–188. [Google Scholar] [CrossRef]
- Sianturi, M.; Marliyawati, D.; Yusmawan, W.; Yunika, K. The Correlation of Allergic Rhinitis with Obstructive Sleep Apnea Syndrome (OSAS) in Young Adults. Diponegoro. Int. Med. J. 2020, 1, 21–25. [Google Scholar] [CrossRef]
- Olsen, K.D.; Kern, E.B. Nasal Influences on Snoring and Obstructive Sleep Apnea. Mayo Clin. Proc. 1990, 65, 1095–1105. [Google Scholar] [CrossRef]
- Smith, P.L.; Wise, R.A.; Gold, A.R.; Schwartz, A.R.; Permutt, S. Upper airway pressure-flow relationships in obstructive sleep apnea. J. Appl. Physiol. 1988, 64, 789–795. [Google Scholar] [CrossRef]
- Tan, S.N.; Abdullah, B. The Association Between Obstructive Sleep Apnea and Allergic Rhinitis: Current Literature Review. Curr. Respirat. Med. Rev. 2021, 17, 13–19. [Google Scholar] [CrossRef]
- Ioachimescu, O.C.; Teodorescu, M. Integrating the overlap of obstructive lung disease and obstructive sleep apnoea: OLDOSA syndrome. Respirology 2013, 18, 421–431. [Google Scholar] [CrossRef] [PubMed]
- Duong-Quy, S.; Dang Thi Mai, K.; Tran Van, N.; Nguyen Xuan Bich, H.; Hua-Huy, T.; Chalumeau, F.; Dinh-Xuan, A.T.; Soyez, F.; Martin, F. Study about the prevalence of the obstructive sleep apnoea syndrome in Vietnam. Rev. Mal. Respir. 2018, 35, 14–24. [Google Scholar] [CrossRef] [PubMed]
- Duong-Quy, S.; Van, H.T.; Kim, A.V.T.; Huy, Q.P.; Craig, T.J. Clinical and Functional Characteristics of Subjects with Asthma, COPD, and Asthma-COPD Overlap: A Multicentre Study in Vietnam. Can. Respir. J. 2018, 2018, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ioachimescu, O.C.; Janocko, N.J.; Ciavatta, M.-M.; Howard, M.; Warnock, M.V. Obstructive Lung Disease and Obstructive Sleep Apnea (OLDOSA) cohort study: 10-year assessment. J. Clin. Sleep Med. 2020, 16, 267–277. [Google Scholar] [CrossRef] [PubMed]
- Stanchina, M.L.; Welicky, L.M.; Donat, W.; Lee, D.; Corrao, W.; Malhotra, A. Impact of CPAP Use and Age on Mortality in Patients with Combined COPD and Obstructive Sleep Apnea: The Overlap Syndrome. J. Clin. Sleep Med. 2013, 9, 767–772. [Google Scholar] [CrossRef] [Green Version]
- BaHammam, A.S.; Han, F.; Gupta, R.; Duong-Quy, S.; Al-Abri, M.A.; Jahrami, H.A.; Song, P.; Desudchit, T.; Xu, L.; Hong, S.B. Asian accreditation of sleep medicine physicians and technologists: Practice guidelines by the Asian Society of Sleep Medicine. Sleep Med. 2021, 81, 246–252. [Google Scholar] [CrossRef]
- Dinh-Thi-Dieu, H.; Vo-Thi-Kim, A.; Tran-Van, H.; Duong-Quy, S. Efficacy and adherence of auto-CPAP therapy in patients with obstructive sleep apnea: A prospective study. Multidiscip. Respir. Med. 2020, 15, 468. [Google Scholar] [CrossRef] [Green Version]
- Bock, J.; Needham, K.; Gregory, D.A.; Ekono, S.M.M.; Wickwire, E.M.; Somers, V.K.; Lerman, A. CPAP adherence reduces tratment cost in patients with Obstructive Sleep Apnea and Cardiovascular disease. Mayo Clin. Proc. Innov. Qual. Outcomes 2022, 6, 166–175. [Google Scholar] [CrossRef]
- Somers, V.K.; White, D.P.; Amin, R.; Abraham, W.T.; Costa, F.; Culebras, A.; Daniels, S.; Floras, J.S.; Hunt, C.E.; Olson, L.J.; et al. Sleep Apnea and Cardiovascular Disease: An American Heart Association/American College of Cardiology Foundation Scientific Statement From the American Heart Association Council for High Blood Pressure Research Professional Education Committee, Council on Clinical Cardiology, Stroke Council, and Council on Cardiovascular Nursing In Collaboration With the National Heart, Lung, and Blood Institute National Center on Sleep Disorders Research (National Institutes of Health). J. Am. Coll. Cardiol. 2008, 52, 686–717. [Google Scholar] [CrossRef] [Green Version]
- Bitter, T.; Westerheide, N.; Hossain, S.M.; Prinz, C.; Horstkotte, D.; Oldenburg, O. Symptoms of sleep apnoea in chronic heart fail-ure-results from a prospective cohort study in 1500 patients. Sleep Breath 2012, 16, 781–791. [Google Scholar] [CrossRef] [PubMed]
- Bauters, F.; Rietzschel, E.R.; Hertegonne, K.B.C.; Chirinos, J.A. The Link Between Obstructive Sleep Apnea and Cardiovascular Disease. Curr. Atheroscler. Rep. 2016, 18, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Romero-Corral, A.; Caples, S.M.; Lopez-Jimenez, F.; Somers, V.K. Interactions Between Obesity and Obstructive Sleep Apnea: Implications for Treatment. Chest 2010, 137, 711–719. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peppard, P.E.; Young, T.; Palta, M.; Dempsey, J.; Skatrud, J. Longitudinal Study of Moderate Weight Change and Sleep-Disordered Breathing. JAMA 2000, 284, 3015–3021. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Viswanathan, V.; Ramakrishnan, N.; Saboo, B.; Agarwal, S. RSSDI clinical practice recommendations for screening, diagnosis, and treatment in type 2 diabetes mellitus with obstructive sleep apnea. Int. J. Diabetes Dev. Ctries. 2021, 41, 4–21. [Google Scholar] [CrossRef]
- Rajan, P.; Greenberg, H. Obstructive sleep apnea as a risk factor for type 2 diabetes mellitus. Nat. Sci. Sleep 2015, 7, 113–125. [Google Scholar]
- Jehan, S.; Myers, A.; Zizi, F. Obesity, obstructive sleep apnea, and type 2 diabetes mellitus: Epidemiology and pathophysiologic insights. Sleep Med. Dis. Int. J. 2018, 2, 54–60. [Google Scholar]
- Coughlin, S.R.; Mawdsley, L.; Mugarza, J.A.; Calverley, P.M.; Wilding, J.P. Obstructive sleep apnoea is independently associated with an increased prevalence of metabolic syndrome. Eur. Heart J. 2004, 25, 735–741. [Google Scholar] [CrossRef]
- Gruber, A.; Horwood, F.; Sithole, J.; Ali, N.; Idris, I. Obstructive sleep apnoea is independently associated with the metabolic syndrome but not insulin resistance state. Cardiovasc. Diabetol. 2006, 5, 22. [Google Scholar] [CrossRef] [Green Version]
- Shaw, J.; Sicree, R.; Zimmet, P. Global estimates of the prevalence of diabetes for 2010 and 2030. Diabetes Res. Clin. Pr. 2010, 87, 4–14. [Google Scholar] [CrossRef]
- Altaf, Q.-A.A.; Ali, A.; Piya, M.K.; Raymond, N.T.; Tahrani, A.A. The relationship between obstructive sleep apnea and intra-epidermal nerve fiber density, PARP activation and foot ulceration in patients with type 2 diabetes. J. Diabetes Its Complicat. 2016, 30, 1315–1320. [Google Scholar] [CrossRef] [Green Version]
- Mok, Y.; Tan, C.W.; Wong, H.S.; How, C.H.; Tan, K.L.A.; Hsu, P.P. Obstructive sleep apnoea and type 2 diabetes mellitus: Are they connected? Singap. Med. J. 2017, 58, 179. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khalyfa, A.; Wang, Y.; Zhang, S.X.; Qiao, Z.; Abdelkarim, A.; Gozal, D. Sleep Fragmentation in Mice Induces Nicotinamide Adenine Dinucleotide Phosphate Oxidase 2-Dependent Mobilization, Proliferation, and Differentiation of Adipocyte Progenitors in Visceral White Adipose Tissue. Sleep 2014, 37, 999–1009. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Zhang, Z.; Dong, Z.Z. Effects of continuous positive airway pressure therapy on glycaemic control, insulin sensitivity and body mass index in patients with obstructive sleep apnoea and type 2 diabetes: A systematic review and meta-analysis. NPJ Prim. Care Respir. Med. 2015, 25, 15005. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, D.; Liu, Z.; Yang, H.; Luo, Q. Effects of continuous positive airway pressure on glycemic control and insulin resistance in patients with obstructive sleep apnea: A meta-analysis. Sleep Breath 2013, 17, 33–38. [Google Scholar] [CrossRef]
- Babu, A.R.; Herdegen, J.; Fogelfeld, L.; Shott, S.; Mazzone, T. Type 2 Diabetes, Glycemic Control, and Continuous Positive Airway Pressure in Obstructive Sleep Apnea. Arch. Internt. Med. 2005, 165, 447–452. [Google Scholar] [CrossRef] [Green Version]
- Mitchell, L.; Davidson, Z.E.; Bonham, M.; O'Driscoll, D.M.; Hamilton, G.; Truby, H. Weight loss from lifestyle interventions and severity of sleep apnoea: A systematic review and meta-analysis. Sleep Med. 2014, 15, 1173–1183. [Google Scholar] [CrossRef] [PubMed]
- Joosten, S.A.; Hamilton, G.; Naughton, M.T. Impact of Weight Loss Management in OSA. Chest 2017, 152, 194–203. [Google Scholar] [CrossRef]
- Ford, E.S.; Cunningham, T.J.; Giles, W.H.; Croft, J.B. Trends in insomnia and excessive daytime sleepiness among U.S. Adults from 2002 to 2012. Sleep Med. 2015, 16, 372–378. [Google Scholar] [CrossRef] [Green Version]
- Ragnoli, B.; Pochetti, P.; Raie, A.; Malerba, M. Comorbid Insomnia and Obstructive Sleep Apnea (COMISA): Current Concepts of Patient Management. Int. J. Environ. Res. Public Health 2021, 18, 9248. [Google Scholar] [CrossRef]
- Sateia, M.J. International classification of sleep disorders-third edition highlights and modifications. Chest 2014, 146, 1387–1394. [Google Scholar] [CrossRef]
- Sweetman, A.M.; Lack, L.C.; Catcheside, P.; Antic, N.A.; Chai-Coetzer, C.L.; Smith, S.; Douglas, J.A.; McEvoy, D. Developing a successful treatment for co-morbid insomnia and sleep apnoea. Sleep Med. Rev. 2016, 33, 28–38. [Google Scholar] [CrossRef] [PubMed]
- Peppard, P.E.; Young, T.; Barnet, J.H.; Palta, M.; Hagen, E.W.; Hla, K.M. Increased Prevalence of Sleep-Disordered Breathing in Adults. Am. J. Epidemiol. 2013, 177, 1006–1014. [Google Scholar] [CrossRef] [PubMed]
- Trois, M.S.; Capone, G.T.; Lutz, J.A.; Melendres, M.C.; Schwartz, A.R.; A Collop, N.; Marcus, C.L. Obstructive Sleep Apnea in Adults with Down Syndrome. J. Clin. Sleep Med. 2009, 5, 317–323. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murdoch, J.; Ratcliffe, W.; McLarty, D.; Rodger, J.; Ratcliffe, J. Thyroid function in adults with Down's syndrome. J. Clin. Endocrinol. Metab. 1977, 44, 453–458. [Google Scholar] [CrossRef] [PubMed]
- Baynard, T.; Pitetti, K.H.; Guerra, M.; Unnithan, V.B.; Fernhall, B. Age-related changes in aerobic capacity in individuals with mental retardation: A 20-yr review. Med. Sci. Sport. Exerc. 2008, 40, 1984–1989. [Google Scholar] [CrossRef]
- Fernhall, B.; Mendonca, G.V.; Baynard, T. Reduced work capacity in individuals with Down syndrome: A consequence of autonomic dysfunction? Exerc. Sport Sci. Rev. 2013, 41, 138–147. [Google Scholar] [CrossRef]
- Ferri, R.; Curzi-Dascalova, L.; Del Gracco, S.; Elia, M.; Pettinato, S.; Musumeci, S. Sleep Neurophysiopathology in Down syndrome. Down Syndr. Res. Pr. 1998, 5, 105–110. [Google Scholar] [CrossRef]
- Marcus, C.L.; Keens, T.G.; Bautista, D.B.; von Pechmann, W.S.; Ward, S.L. Obstructive sleep apnea in children with Down syn-drome. Pediatrics 1991, 88, 132–139. [Google Scholar] [CrossRef]
- Basil, J.S.; Santoro, S.L.; Martin, L.J.; Healy, K.W.; Chini, B.A.; Saal, H.M. Retrospective Study of Obesity in Children with Down Syndrome. J. Pediatr. 2016, 173, 143–148. [Google Scholar] [CrossRef] [Green Version]
- Chen, C.C.; Ringenbach, S.D.R.; Albert, A.R. Assisted cycling exercise improves fine manual dexterity in persons with Down's syndrome. J. Appl. Res. Intellect. Disabil. 2014, 27, 264–272. [Google Scholar] [CrossRef]
- Skotko, B.G.; Macklin, E.A.; Muselli, M.; Voelz, L.; McDonough, M.E.; Davidson, E.; Allareddy, V.; Jayaratne, Y.S.N.; Bruun, R.; Ching, N.; et al. A predictive model for obstructive sleep apnea and Down syndrome. Am. J. Med. Genet. Part A 2017, 173, 889–896. [Google Scholar] [CrossRef] [PubMed]
- Seo, D.S.; Chau, G.C.; Baek, K.-H.; Um, S.H. A single extra copy of Down syndrome critical region 1–4 results in impaired hepatic glucose homeostasis. Mol. Metab. 2018, 21, 82–89. [Google Scholar] [CrossRef] [PubMed]
- Wiseman, F.K.; Al-Janabi, T.; Hardy, J.; Karmiloff-Smith, A.; Nizetic, D.; Tybulewicz, V.L.J.; Fisher, E.M.C.; Strydom, A. A genetic cause of Alzheimer disease: Mechanistic insights from Down syndrome. Nat. Rev. Neurosci. 2015, 16, 564–574. [Google Scholar] [CrossRef] [Green Version]
- Lal, C.; Strange, C.; Bachman, D. Neurocognitive Impairment in Obstructive Sleep Apnea. Chest 2012, 141, 1601–1610. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.R.; Larkin, E.K.; Redline, S. Shared genetic basis for obstructive sleep apnea and adiposity measures. Int. J. Obes. 2008, 32, 795. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schwartz, A.R.; Patil, S.P.; Laffan, A.M.; Polotsky, V.; Schneider, H.; Smith, P.L. Obesity and Obstructive Sleep Apnea: Pathogenic Mechanisms and Therapeutic Approaches. Proc. Am. Thorac. Soc. 2008, 5, 185–192. [Google Scholar] [CrossRef] [PubMed]
- Redline, S.; Tishler, P.V. The genetics of sleep apnea. Sleep Med. Rev. 2000, 4, 583–602. [Google Scholar] [CrossRef] [PubMed]
- Redline, S.; Tishler, P.V.; Tosteson, T.D.; Williamson, J.; Kump, K.; Browner, I.; Ferrette, V.; Krejci, P. The familial aggregation of obstructive sleep apnea. Am. J. Respir. Crit. Care Med. 1995, 151, 682–687. [Google Scholar] [CrossRef]
- McEvoy, R.D.; Antic, N.A.; Heeley, E.; Luo, Y.; Ou, Q.; Zhang, X.; Mediano, O.; Chen, R.; Drager, L.F.; Liu, Z.; et al. CPAP for Prevention of Cardiovascular Events in Obstructive Sleep Apnea. N. Engl. J. Med. 2016, 375, 919–931. [Google Scholar] [CrossRef]
- Nguyen, T.D.; Baillieul, S.; Guinot, M.; Doutreleau, S.; Bricout, V.-A. Classification of Factors Effect on Sleep in Individuals with Down Syndrome. Brain Sci. 2021, 11, 1500. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Duong-Quy, S.; Nguyen-Huu, H.; Hoang-Chau-Bao, D.; Tran-Duc, S.; Nguyen-Thi-Hong, L.; Nguyen-Duy, T.; Tang-Thi-Thao, T.; Phan, C.; Bui-Diem, K.; Vu-Tran-Thien, Q.; et al. Personalized Medicine and Obstructive Sleep Apnea. J. Pers. Med. 2022, 12, 2034. https://doi.org/10.3390/jpm12122034
Duong-Quy S, Nguyen-Huu H, Hoang-Chau-Bao D, Tran-Duc S, Nguyen-Thi-Hong L, Nguyen-Duy T, Tang-Thi-Thao T, Phan C, Bui-Diem K, Vu-Tran-Thien Q, et al. Personalized Medicine and Obstructive Sleep Apnea. Journal of Personalized Medicine. 2022; 12(12):2034. https://doi.org/10.3390/jpm12122034
Chicago/Turabian StyleDuong-Quy, Sy, Hoang Nguyen-Huu, Dinh Hoang-Chau-Bao, Si Tran-Duc, Lien Nguyen-Thi-Hong, Thai Nguyen-Duy, Tram Tang-Thi-Thao, Chandat Phan, Khue Bui-Diem, Quan Vu-Tran-Thien, and et al. 2022. "Personalized Medicine and Obstructive Sleep Apnea" Journal of Personalized Medicine 12, no. 12: 2034. https://doi.org/10.3390/jpm12122034
APA StyleDuong-Quy, S., Nguyen-Huu, H., Hoang-Chau-Bao, D., Tran-Duc, S., Nguyen-Thi-Hong, L., Nguyen-Duy, T., Tang-Thi-Thao, T., Phan, C., Bui-Diem, K., Vu-Tran-Thien, Q., Nguyen-Ngoc-Phuong, T., Nguyen-Nhu, V., Le-Thi-Minh, H., & Craig, T. (2022). Personalized Medicine and Obstructive Sleep Apnea. Journal of Personalized Medicine, 12(12), 2034. https://doi.org/10.3390/jpm12122034







