The Impact of Radiographic, Metabolic and Demographic Characteristics on Kidney Stone Recurrence
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Study Outcomes and Variables
- (1)
- Demographic data: Gender, age at first CT with stones.
- (2)
- Baseline CT stone data:
- (a)
- Stone burden: Several stone burden measurements tools are available which correlate to each other [7]. Here, we employed the widely accepted ellipsoid formula of the European Association of Urology [8]: Stone volume = length × width × depth × (π/6). Dimensions were measured on three axes. In cases of multiple stones, a calculation was performed for each stone separately and addition was performed afterwards. Units are in mm3.
- (b)
- Stone density: Measured in Hounsfield units (HU) at the region of interest (ROI) of the largest stone, including at least 60% of the stone volume.
- (c)
- Stone location: lower pole, renal pelvis, ureter, elsewhere, multiple.
- (3)
- Baseline CT metabolic data:
- (a)
- Visceral fat area (VFA): Measured at the level of the umbilicus, by marking and calculating the fat area internal to the abdominal muscles, found by HU matching fat tissue. We used computer software designed specifically to track these data (Philips Workstation segmentation and tissue measurement tools, Version 12.2.6.2000019, Best, The Netherlands).
- (b)
- Liver steatosis: Measured in HU for the liver and spleen. To measure the density of the liver parenchyma, we placed an ROI circle in the right lobe of the liver. The circle was sufficiently large to include a large portion of the liver parenchyma while excluding blood vessels. Hepatic attenuation of less than 45 HU is considered in the literature as moderate to severe steatosis [9,10]. The attenuation of the splenic parenchyma was measured in a similar fashion. We also measured the liver–spleen difference (in HU). In the literature, a difference of 19 HU or more is considered as liver steatosis [9].
- (c)
- Vertebral bone density: We measured ROI attenuation of the body of the L1 vertebra in HU 24.
- (4)
- Baseline blood sample values: Creatinine, uric acid, and calcium, obtained 1 year before or after the baseline NCCT, and at least 4 weeks from the day of NCCT to minimize its effect on these parameters.
2.3. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sorokin, I.; Mamoulakis, C.; Miyazawa, K.; Rodgers, A.; Talati, J.; Lotan, Y. Epidemiology of stone disease across the world. World J. Urol. 2017, 35, 1301–1320. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhu, W.; Lam, W.; Yue, Y.; Duan, H.; Zeng, G. Outcomes of long-term follow-up of asymptomatic renal stones and prediction of stone-related events. BJU Int. 2018, 123, 485–492. [Google Scholar] [CrossRef] [PubMed]
- Rule, A.D.; Lieske, J.C.; Li, X.; Melton, L.J.; Krambeck, A.E.; Bergstralh, E.J. The ROKS nomogram for predicting a second symptomatic stone episode. J. Am. Soc. Nephrol. 2014, 25, 2878–2886. [Google Scholar] [CrossRef] [PubMed]
- D’Costa, M.R.; Pais, V.M.; Rule, A.D. Leave no stone unturned. Curr. Opin. Nephrol. Hypertens. 2019, 28, 148–153. [Google Scholar] [CrossRef]
- Ahlstrand, C.; Tiselius, H.G. Recurrences during a 10-year follow-up after first renal stone episode. Urol. Res. 1990, 18, 397–399. [Google Scholar] [CrossRef]
- Trinchieri, A.; Ostini, F.; Nespoli, R.; Rovera, F.; Montanari, E.; Zanetti, G. A prospective study of recurrence rate and risk factors for recurrence after a first renal stone. J. Urol. 1999, 162, 27–30. [Google Scholar] [CrossRef]
- Cui, H.W.; Tan, T.K.; Christiansen, F.E.; Osther, P.J.S.; Turney, B.W. The utility of automated volume analysis of renal stones before and after shockwave lithotripsy treatment. Urolithiasis 2021, 49, 219–226. [Google Scholar] [CrossRef]
- Tiselius, H.G.; Ackermann, D.; Alken, P.; Buck, C.; Conart, P.; Gallucci, M. Guidelines on Urolithiasis. Eur. Assoc. Urol. 2008, 40, 362–371. [Google Scholar] [CrossRef]
- Graffy, P.M.; Pickhardt, P.J. Quantification of hepatic and visceral fat by CT and MR imaging: Relevance to the obesity epidemic, metabolic syndrome and NAFLD. Br. J. Radiol. 2016, 89, 20151024. [Google Scholar] [CrossRef]
- Pickhardt, P.J.; Jee, Y.; O’Connor, S.D.; del Rio, A.M. Visceral adiposity and hepatic steatosis at abdominal CT: Association with the metabolic syndrome. Am. J. Roentgenol. 2012, 198, 1100–1107. [Google Scholar] [CrossRef]
- Ljunghall, S.; Danielson, B.G. A prospective study of renal stone recurrences. Br. J. Urol. 1984, 56, 122–124. [Google Scholar] [CrossRef] [PubMed]
- D’Costa, M.R.; Haley, W.E.; Mara, K.C.; Enders, F.T.; Vrtiska, T.J.; Pais, V.M.; Jacobsen, S.J.; McCollough, C.H.; Lieske, J.C.; Rule, A.D. Symptomatic and radiographic manifestations of kidney stone recurrence and their prediction by risk factors: A prospective cohort study. J. Am. Soc. Nephrol. 2019, 30, 1251–1260. [Google Scholar] [CrossRef] [PubMed]
- Darrad, M.P.; Yallappa, S.; Metcalfe, J.; Subramonian, K. The natural history of asymptomatic calyceal stones. BJU Int. 2018, 122, 263–269. [Google Scholar] [CrossRef] [PubMed]
- Unal, D.; Yeni, E.; Verit, A.; Karatas, O.F. Prognostic Factors Effecting on Recurrence of Urinary Stone Disease: A Multivariate Analysis of Everyday Patient Parameters. Int. Urol. Nephrol. 2005, 37, 447–452. [Google Scholar] [CrossRef]
- Vaughan, L.E.; Enders, F.T.; Lieske, J.C.; Pais, V.M.; Rivera, M.E.; Mehta, R.A.; Vrtiska, T.J.; Rule, A.D. Predictors of symptomatic kidney stone recurrence after the first and subsequent episodes. Mayo Clin. Proc. 2019, 94, 202–210. [Google Scholar] [CrossRef]
- Ferraro, P.M.; Curhan, G.C.; D’Addessi, A.; Gambaro, G. Risk of recurrence of idiopathic calcium kidney stones: Analysis of data from the literature. J. Nephrol. 2016, 30, 227–233. [Google Scholar] [CrossRef]
- Iremashvili, V.; Li, S.; Penniston, K.; Best, S.; Hedican, S.; Nakada, S.Y. External validation of the recurrence of kidney stone nomogram in a surgical cohort. J. Endourol. 2019, 33, 475–479. [Google Scholar] [CrossRef]
- Craven, B.L.; Passman, C.; Assimos, D.G. Hypercalcemic states associated with nephrolithiasis. Rev. Urol. 2008, 10, 218–226. [Google Scholar]
- Kim, Y.G.; Kim, C.H.; Sung, E.J.; Kim, S.R.; Shin, H.C.; Jung, W.J. Association of nephrolithiasis with metabolic syndrome and its components. Metabolism 2013, 62, 808–813. [Google Scholar] [CrossRef]
- Lee, S.C.; Kim, Y.J.; Kim, T.H.; Yun, S.J.; Lee, N.K.; Kim, W.J. Impact of obesity in patients with urolithiasis and its prognostic usefulness in stone recurrence. J. Urol. 2008, 179, 570–574. [Google Scholar] [CrossRef]
- Shah, R.V.; Murthy, V.L.; Abbasi, S.A.; Blankstein, R.; Kwong, R.Y.; Goldfine, A.B.; Jerosch-Herold, M.; Lima, J.A.C.; Ding, J.; Allison, M.A. Visceral adiposity and the risk of metabolic syndrome across body mass index: The MESA Study. JACC Cardiovasc. Imaging 2014, 7, 1221–1235. [Google Scholar] [CrossRef] [PubMed]
- Oka, R.; Kobayashi, J.; Yagi, K.; Tanii, H.; Miyamoto, S.; Asano, A.; Hagishita, T.; Mori, M.; Moriuchi, T.; Kobayashi, M.; et al. Reassessment of the cutoff values of waist circumference and visceral fat area for identifying Japanese subjects at risk for the metabolic syndrome. Diabetes Res. Clin. Pract. 2008, 79, 474–481. [Google Scholar] [CrossRef] [PubMed]
- Caudarella, R.; Vescini, F.; Buffa, A.; La Manna, G.; Stefoni, S. Osteoporosis and urolithiasis. Urol. Int. 2004, 72, 17–19. [Google Scholar] [CrossRef]
- Sakhaee, K.; Maalouf, N.M.; Kumar, R.; Pasch, A.; Moe, O.W. Nephrolithiasis-associated bone disease: Pathogenesis and treatment options. Kidney Int. 2011, 79, 393–403. [Google Scholar] [CrossRef] [PubMed]
- Pickhardt, P.J.; Pooler, B.D.; Lauder, T.; del Rio, A.M.; Bruce, R.J.; Binkley, N. Opportunistic screening for osteoporosis using abdominal computed tomography scans obtained for other indications. Ann. Intern. Med. 2013, 158, 588–595. [Google Scholar] [CrossRef]
- Taylor, E.N.; Stampfer, M.J.; Curhan, G.C. Obesity, weight gain, and the risk of kidney stones. JAMA 2005, 293, 455–462. [Google Scholar] [CrossRef] [PubMed]
| Characteristic | Value |
|---|---|
| Gender | |
| Male, n (%) | 167 (70.5%) |
| Female, n (%) | 70 (29.5%) |
| Age, years, mean (SD) | 53.15 (15.58) |
| Stone location | |
| Ureter, n (%) | 85 (35.9%) |
| Lower pole, n (%) | 21 (8.9%) |
| Renal pelvis, n (%) | 9 (3.8%) |
| Elsewhere, n (%) | 27 (11.4%) |
| Multiple, n (%) | 95 (40.1%) |
| Underwent stone-related procedure, n (%) | 66 (27.8%) |
| 2nd imaging modality | |
| NCCT, n (%) | 85 (35.9%) |
| CCT, n (%) | 21 (8.9%) |
| US, n (%) | 124 (52.3%) |
| KUB, n (%) | 7 (3.0%) |
| Baseline creatinine, mg/dL, mean (SD)† | 0.96 (0.27) |
| Baseline calcium, mg/dL, mean (SD)† | 9.27 (0.48) |
| Baseline uric acid, mg/dL, mean (SD)† | 5.52 (1.46) |
| Stone burden volume, mm3, mean (SD) | 513.5 (1720.60) |
| Stone density at ROI, HU, mean (SD) | 723.12 (307.05) |
| VFA, cm2, mean (SD) † | 172.99 (69.27) |
| Hepatic attenuation, HU, mean (SD | 48.62 (13.31) |
| Splenic attenuation, HU, mean (SD)† | 42.37 (7.06) |
| Liver attenuation index, HU, mean (SD)† | 6.25 (11.84) |
| Bone density at L1, HU, mean (SD) | 149.60 (51.64 |
| Variable | Recurrence | p-Value | |
|---|---|---|---|
| No, n (%) | Yes, n (%) | ||
| Gender | |||
| Male, n (%) | 104 (62.3%) | 63 (37.7%) | 0.77 |
| Female, n (%) | 45 (64.3%) | 25 (35.7%) | |
| Stone Location | |||
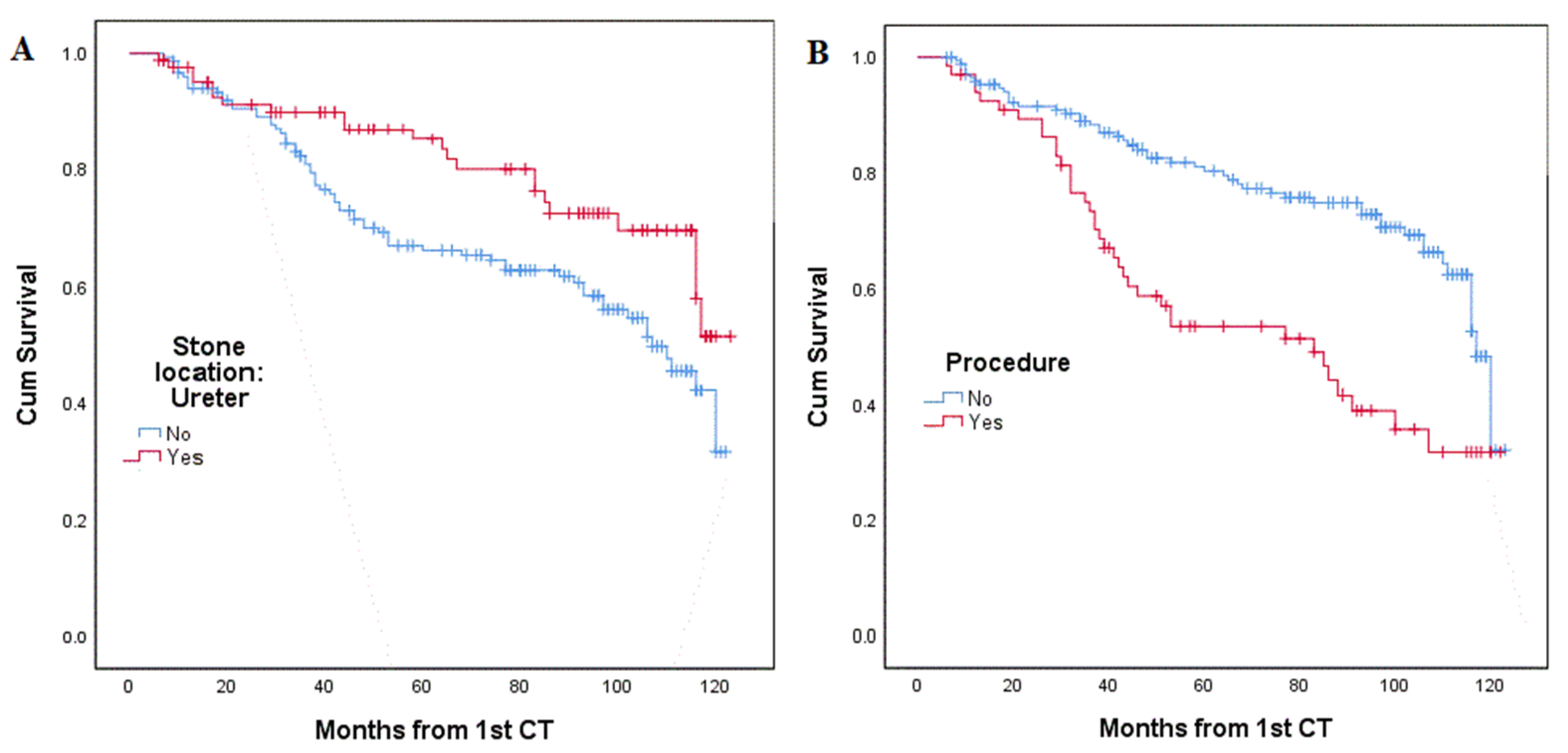
| Kidney, n (%) | 86 (56.6%) | 66 (43%) | 0.007 |
| Ureter, n (%) | 63 (74.1%) | 22 (25.9%) | |
| Underwent stone-related procedure | |||
| No, n (%) | 120 (70.2%) | 51 (29.8%) | <0.001 |
| Yes, n (%) | 29 (43.9%) | 37 (56.1%) | |
| Normal Distribution Assumed, t-Test, 2-Tailed | |||
| Variable | Recurrence | p-Value | |
| No, Mean | Yes, Mean | ||
| Age at 1st CT | 54.17 years | 51.43 years | 0.192 |
| VFA | 171.76 cm2 | 175.10 cm2 | 0.721 |
| Hepatic attenuation | 49.09 HU | 49.09 HU | 0.481 |
| Splenic attenuation | 42.54 HU | 42.08 HU | 0.626 |
| Liver attenuation index | 6.55 HU | 5.75 HU | 0.617 |
| Bone density at L1 | 147.13 HU | 153.78 HU | 0.339 |
| Baseline creatinine | 0.95 mg/dL | 0.98 mg/dL | 0.550 |
| Baseline calcium | 9.19 mg/dL | 9.42 mg/dL | 0.001 |
| Baseline uric acid | 5.31 mg/dL | 5.86 mg/dL | 0.011 |
| Non-normal distribution, Mann-Whitney test, 2-tailed | |||
| Variable | p-Value | ||
| Stone burden volume | 0.044 | ||
| Stone density at ROI | 0.254 | ||
| Variable | p-Value | Hazard Ratio (CI 95%) |
|---|---|---|
| Age at 1st CT | 0.166 | |
| VFA | 0.584 | |
| Hepatic attenuation | 0.269 | |
| Splenic attenuation | 0.643 | |
| Liver attenuation index | 0.141 | |
| Baseline creatinine | 0.530 | |
| Baseline calcium | 0.002 | 1.935 (1.27–2.96) |
| Baseline uric acid | 0.035 | 1.174 (1.012–1.362) |
| Stone burden volume | 0.661 | |
| Stone density at ROI | 0.228 |
| Variable | p-Value | OR (CI 95%) |
|---|---|---|
| Underwent stone-related procedure | 0.001 | 3.071 (1.560–6.046) |
| Baseline calcium | 0.011 | 2.564 (1.243–5.290) |
| Baseline uric acid | 0.021 | 1.303 (1.040–1.633) |
| Stone burden volume | 0.663 | --- |
| Stone location in kidney † | 0.012 | 2.163 (1.181–3.962) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shpunt, I.; Pratt Aloni, H.; Khanukaeva, N.; Herskovitz, P.; Dror, I.; Berkowitz, B.; Leibovici, D.; Shilo, Y. The Impact of Radiographic, Metabolic and Demographic Characteristics on Kidney Stone Recurrence. J. Pers. Med. 2022, 12, 1632. https://doi.org/10.3390/jpm12101632
Shpunt I, Pratt Aloni H, Khanukaeva N, Herskovitz P, Dror I, Berkowitz B, Leibovici D, Shilo Y. The Impact of Radiographic, Metabolic and Demographic Characteristics on Kidney Stone Recurrence. Journal of Personalized Medicine. 2022; 12(10):1632. https://doi.org/10.3390/jpm12101632
Chicago/Turabian StyleShpunt, Igal, Hadar Pratt Aloni, Nelli Khanukaeva, Pearl Herskovitz, Ishai Dror, Brian Berkowitz, Dan Leibovici, and Yaniv Shilo. 2022. "The Impact of Radiographic, Metabolic and Demographic Characteristics on Kidney Stone Recurrence" Journal of Personalized Medicine 12, no. 10: 1632. https://doi.org/10.3390/jpm12101632
APA StyleShpunt, I., Pratt Aloni, H., Khanukaeva, N., Herskovitz, P., Dror, I., Berkowitz, B., Leibovici, D., & Shilo, Y. (2022). The Impact of Radiographic, Metabolic and Demographic Characteristics on Kidney Stone Recurrence. Journal of Personalized Medicine, 12(10), 1632. https://doi.org/10.3390/jpm12101632





