MicroRNA Expression Profiles in Autism Spectrum Disorder: Role for miR-181 in Immunomodulation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Cell Lines and Culture
2.3. Library Construction and Sequencing
2.4. Bioinformatics Analysis
2.5. Target Prediction and Enrichment Analysis
2.6. Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR)
2.7. Statistical Analysis
3. Results
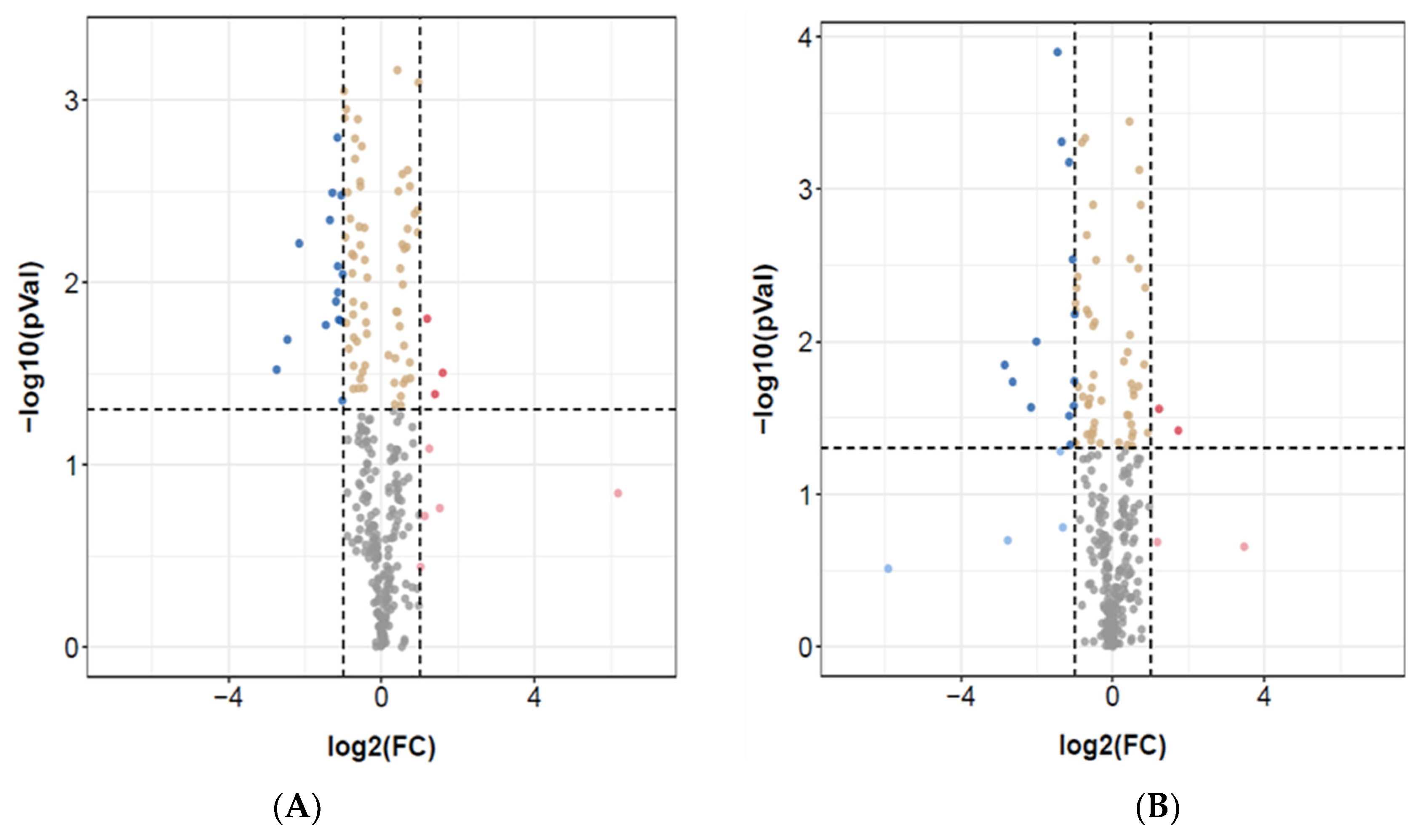
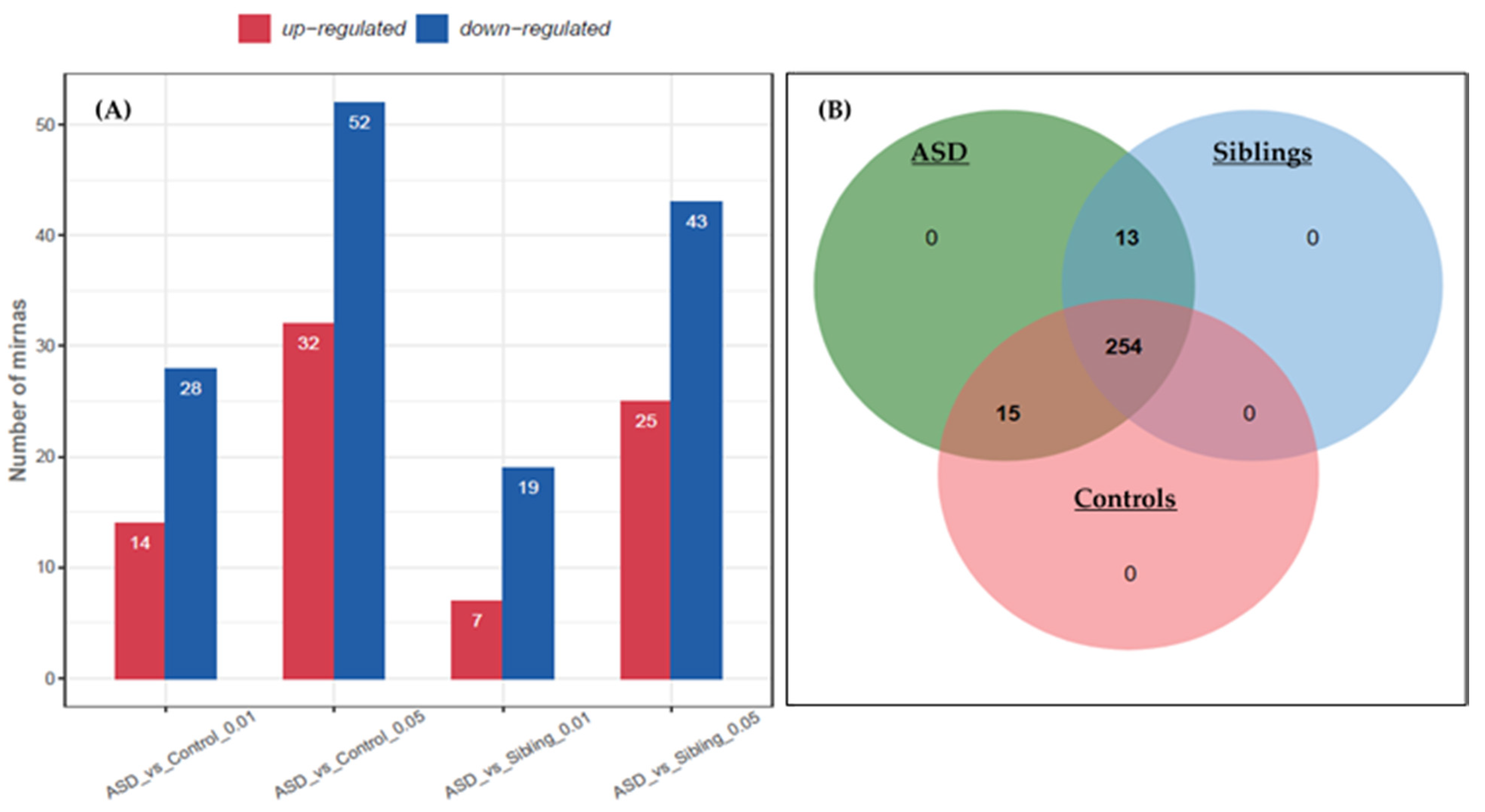
3.1. Identification of Differentially Expressed miRNAs
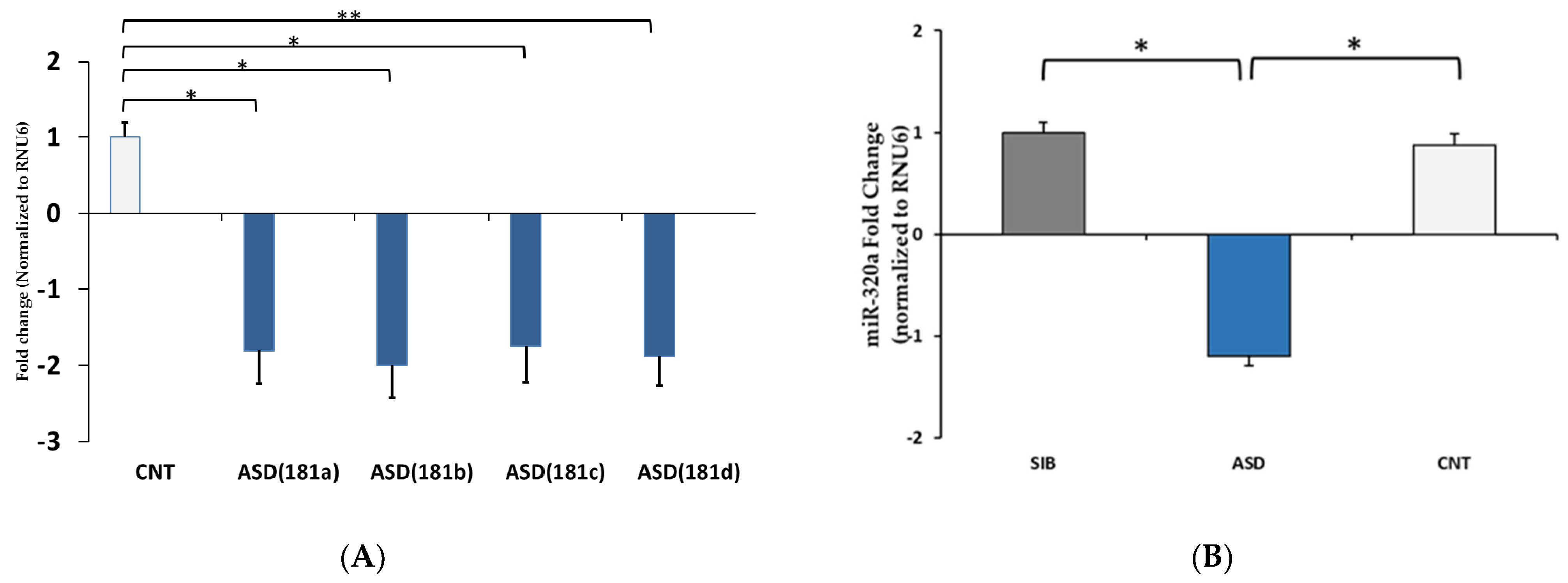
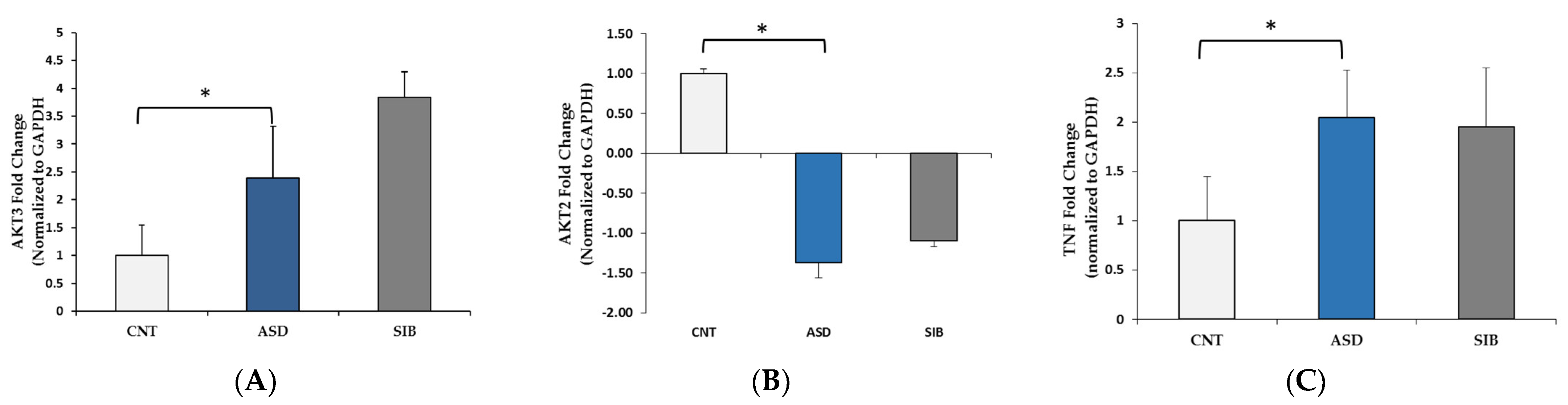
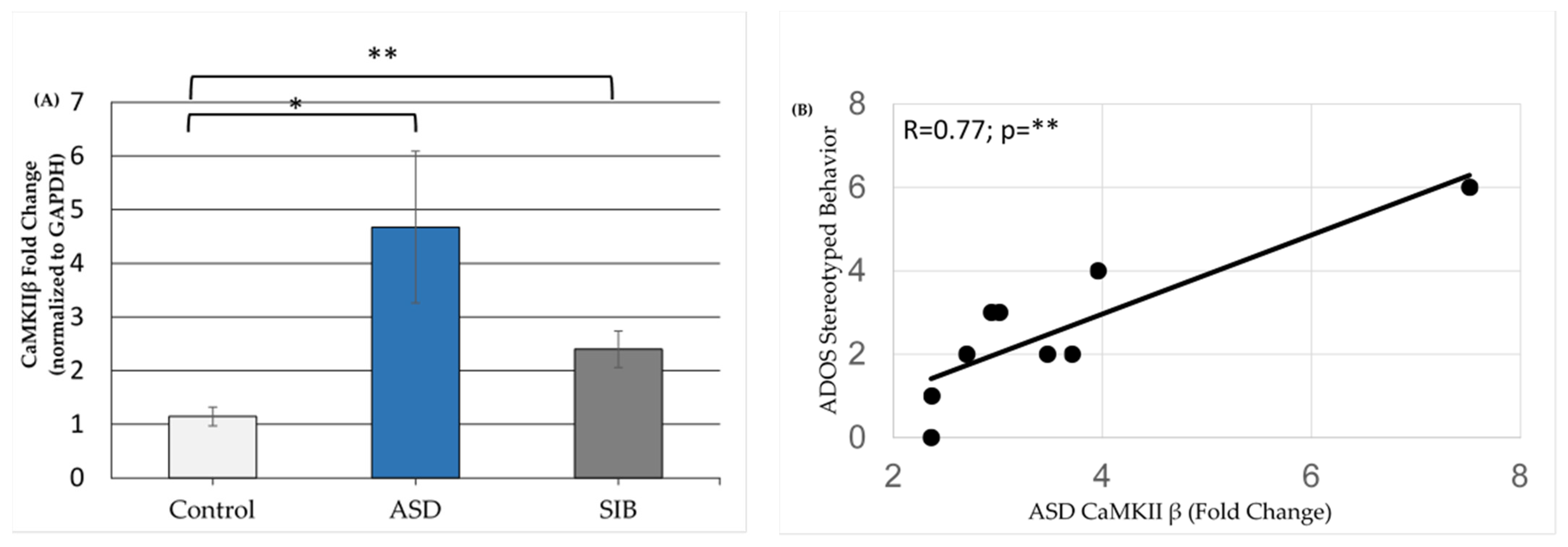
3.2. qPCR Validation of Differentially Expressed miRNAs
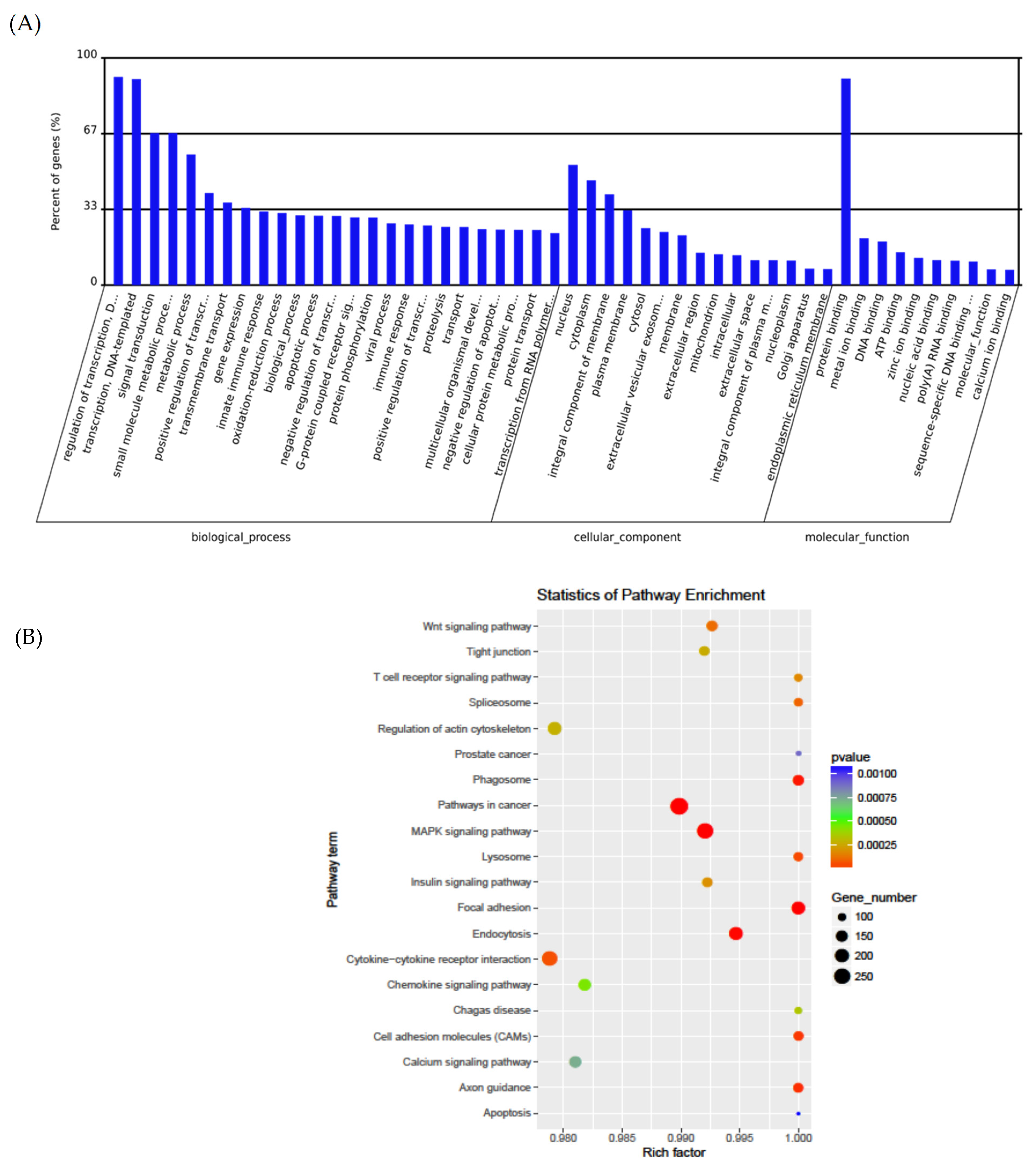
3.3. Pathway Analysis
4. Discussion
5. Conclusions
6. Patents
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- APA. Diagnostic and Statistical Manual of Mental Disorders, 5th ed.; American Psychiatric Association: Washington, DC, USA, 2013. [Google Scholar]
- Baio, J.; Wiggins, L.; Christensen, D.L.; Maenner, M.J.; Shaw, K.A.; Washington, A.; Patrick, M.; DiRienzo, M.; Pettygrove, S.; Andrews, J.G.; et al. Prevalence of Autism Spectrum Disorder Among Children Aged 8 Years-Autism and Developmental Disabilities Monitoring Network, 11 Sites, United States, 2016. Morb. Mortal. Wkly. Rep. Surveill. Summ. 2020, 69, 1–12. [Google Scholar] [CrossRef]
- Ambros, V. The functions of animal microRNAs. Nature 2004, 431, 350–355. [Google Scholar] [CrossRef]
- Bartel, D.P. Metazoan MicroRNAs. Cell 2018, 173, 20–51. [Google Scholar] [CrossRef] [Green Version]
- Bartel, D.P. MicroRNAs: Target recognition and regulatory functions. Cell 2009, 136, 215–233. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, W. MicroRNAs: Biomarkers, Diagnostics, and Therapeutics. Methods Mol. Biol. 2017, 1617, 57–67. [Google Scholar] [CrossRef] [PubMed]
- Garofalo, M.; Condorelli, G.L.; Croce, C.M.; Condorelli, G. MicroRNAs as regulators of death receptors signaling. Cell Death Differ. 2010, 17, 200–208. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ivey, K.N.; Srivastava, D. MicroRNAs as regulators of differentiation and cell fate decisions. Cell Stem Cell 2010, 7, 36–41. [Google Scholar] [CrossRef] [Green Version]
- Chandan, K.; Gupta, M.; Sarwat, M. Role of Host and Pathogen-Derived MicroRNAs in Immune Regulation During Infectious and Inflammatory Diseases. Front. Immunol. 2019, 10, 3081. [Google Scholar] [CrossRef] [Green Version]
- Hicks, S.D.; Ignacio, C.; Gentile, K.; Middleton, F.A. Salivary miRNA profiles identify children with autism spectrum disorder, correlate with adaptive behavior, and implicate ASD candidate genes involved in neurodevelopment. BMC Pediatrics 2016, 16, 52. [Google Scholar] [CrossRef] [Green Version]
- Wu, X.; Li, W.; Zheng, Y. Recent Progress on Relevant microRNAs in Autism Spectrum Disorders. Int. J. Mol. Sci. 2020, 21, 5904. [Google Scholar] [CrossRef]
- Vaishnavi, V.; Manikandan, M.; Tiwary, B.K.; Munirajan, A.K. Insights on the functional impact of microRNAs present in autism-associated copy number variants. PLoS ONE 2013, 8, e56781. [Google Scholar] [CrossRef] [Green Version]
- Marrale, M.; Albanese, N.N.; Cali, F.; Romano, V. Assessing the impact of copy number variants on miRNA genes in autism by Monte Carlo simulation. PLoS ONE 2014, 9, e90947. [Google Scholar] [CrossRef] [PubMed]
- Abu-Elneel, K.; Liu, T.; Gazzaniga, F.S.; Nishimura, Y.; Wall, D.P.; Geschwind, D.H.; Lao, K.; Kosik, K.S. Heterogeneous dysregulation of microRNAs across the autism spectrum. Neurogenetics 2008, 9, 153–161. [Google Scholar] [CrossRef]
- Rose, S.; Bennuri, S.C.; Wynne, R.; Melnyk, S.; James, S.J.; Frye, R.E. Mitochondrial and redox abnormalities in autism lymphoblastoid cells: A sibling control study. FASEB J. 2017, 31, 904–909. [Google Scholar] [CrossRef] [Green Version]
- Li, H.Y.; He, H.C.; Song, J.F.; Du, Y.F.; Guan, M.; Wu, C.Y. Bone marrow-derived mesenchymal stem cells repair severe acute pancreatitis by secreting miR-181a-5p to target PTEN/Akt/TGF-beta1 signaling. Cell. Signal. 2020, 66, 109436. [Google Scholar] [CrossRef] [PubMed]
- Grove, J.; Ripke, S.; Als, T.D.; Mattheisen, M.; Walters, R.K.; Won, H.; Pallesen, J.; Agerbo, E.; Andreassen, O.A.; Anney, R.; et al. Identification of common genetic risk variants for autism spectrum disorder. Nat. Genet. 2019, 51, 431–444. [Google Scholar] [CrossRef] [Green Version]
- Satterstrom, F.K.; Kosmicki, J.A.; Wang, J.; Breen, M.S.; De Rubeis, S.; An, J.Y.; Peng, M.; Collins, R.; Grove, J.; Klei, L.; et al. Large-Scale Exome Sequencing Study Implicates Both Developmental and Functional Changes in the Neurobiology of Autism. Cell 2020, 180, 568–584.e523. [Google Scholar] [CrossRef]
- Werling, D.M.; Brand, H.; An, J.Y.; Stone, M.R.; Zhu, L.; Glessner, J.T.; Collins, R.L.; Dong, S.; Layer, R.M.; Markenscoff-Papadimitriou, E.; et al. An analytical framework for whole-genome sequence association studies and its implications for autism spectrum disorder. Nat. Genet. 2018, 50, 727–736. [Google Scholar] [CrossRef]
- Geaghan, M.; Cairns, M.J. MicroRNA and Posttranscriptional Dysregulation in Psychiatry. Biol. Psychiatry 2015, 78, 231–239. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghahramani Seno, M.M.; Hu, P.; Gwadry, F.G.; Pinto, D.; Marshall, C.R.; Casallo, G.; Scherer, S.W. Gene and miRNA expression profiles in autism spectrum disorders. Brain Res. 2011, 1380, 85–97. [Google Scholar] [CrossRef]
- Talebizadeh, Z.; Butler, M.G.; Theodoro, M.F. Feasibility and relevance of examining lymphoblastoid cell lines to study role of microRNAs in autism. Autism Res. 2008, 1, 240–250. [Google Scholar] [CrossRef] [PubMed]
- Sarachana, T.; Zhou, R.; Chen, G.; Manji, H.K.; Hu, V.W. Investigation of post-transcriptional gene regulatory networks associated with autism spectrum disorders by microRNA expression profiling of lymphoblastoid cell lines. Genome Med. 2010, 2, 23. [Google Scholar] [CrossRef] [Green Version]
- Mundalil Vasu, M.; Anitha, A.; Thanseem, I.; Suzuki, K.; Yamada, K.; Takahashi, T.; Wakuda, T.; Iwata, K.; Tsujii, M.; Sugiyama, T.; et al. Serum microRNA profiles in children with autism. Mol. Autism 2014, 5, 40. [Google Scholar] [CrossRef] [Green Version]
- Ludwig, N.; Leidinger, P.; Becker, K.; Backes, C.; Fehlmann, T.; Pallasch, C.; Rheinheimer, S.; Meder, B.; Stahler, C.; Meese, E.; et al. Distribution of miRNA expression across human tissues. Nucleic Acids Res. 2016, 44, 3865–3877. [Google Scholar] [CrossRef]
- Indrieri, A.; Carrella, S.; Carotenuto, P.; Banfi, S.; Franco, B. The Pervasive Role of the miR-181 Family in Development, Neurodegeneration, and Cancer. Int. J. Mol. Sci. 2020, 21, 2092. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ouyang, Y.-B.; Xu, L.; Yue, S.; Liu, S.; Giffard, R.G. Neuroprotection by astrocytes in brain ischemia: Importance of microRNAs. Neurosci. Lett. 2014, 565, 53–58. [Google Scholar] [CrossRef] [Green Version]
- Di, Y.; Lei, Y.; Yu, F.; Changfeng, F.; Song, W.; Xuming, M. MicroRNAs expression and function in cerebral ischemia reperfusion injury. J. Mol. Neurosci. 2014, 53, 242–250. [Google Scholar] [CrossRef]
- Ma, Q.; Zhao, H.; Tao, Z.; Wang, R.; Liu, P.; Han, Z.; Ma, S.; Luo, Y.; Jia, J. MicroRNA-181c Exacerbates Brain Injury in Acute Ischemic Stroke. Aging Dis. 2016, 7, 705–714. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, L.J.; Ouyang, Y.B.; Xiong, X.; Stary, C.M.; Giffard, R.G. Post-stroke treatment with miR-181 antagomir reduces injury and improves long-term behavioral recovery in mice after focal cerebral ischemia. Exp. Neurol. 2015, 264, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Yuan, L.; Fan, L.; Li, Q.; Cui, W.; Wang, X.; Zhang, Z. Inhibition of miR-181b-5p protects cardiomyocytes against ischemia/reperfusion injury by targeting AKT3 and PI3KR3. J. Cell. Biochem. 2019, 120, 19647–19659. [Google Scholar] [CrossRef]
- Ouyang, Y.B.; Lu, Y.; Yue, S.; Giffard, R.G. miR-181 targets multiple Bcl-2 family members and influences apoptosis and mitochondrial function in astrocytes. Mitochondrion 2012, 12, 213–219. [Google Scholar] [CrossRef] [Green Version]
- Hutchison, E.R.; Kawamoto, E.M.; Taub, D.D.; Lal, A.; Abdelmohsen, K.; Zhang, Y.; Wood, W.H., 3rd; Lehrmann, E.; Camandola, S.; Becker, K.G.; et al. Evidence for miR-181 involvement in neuroinflammatory responses of astrocytes. Glia 2013, 61, 1018–1028. [Google Scholar] [CrossRef]
- Rodriguez-Ortiz, C.J.; Baglietto-Vargas, D.; Martinez-Coria, H.; LaFerla, F.M.; Kitazawa, M. Upregulation of miR-181 decreases c-Fos and SIRT-1 in the hippocampus of 3xTg-AD mice. J. Alzheimers Dis. 2014, 42, 1229–1238. [Google Scholar] [CrossRef] [PubMed]
- Williams, A.; Henao-Mejia, J.; Harman, C.C.; Flavell, R.A. miR-181 and metabolic regulation in the immune system. Cold Spring Harb. Symp. Quant. Biol. 2013, 78, 223–230. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, C.Z.; Li, L.; Lodish, H.F.; Bartel, D.P. MicroRNAs modulate hematopoietic lineage differentiation. Science 2004, 303, 83–86. [Google Scholar] [CrossRef] [Green Version]
- Owen, H.C.; Torrance, H.D.; Jones, T.F.; Pearse, R.M.; Hinds, C.J.; Brohi, K.; O’Dwyer, M.J. Epigenetic regulatory pathways involving microRNAs may modulate the host immune response following major trauma. J. Trauma Acute Care Surg. 2015, 79, 766–772. [Google Scholar] [CrossRef]
- Voynova, E.N.; Skinner, J.; Bolland, S. Expansion of an atypical NK cell subset in mouse models of systemic lupus erythematosus. J. Immunol. 2015, 194, 1503–1513. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harville, T.; Rhodes-Clark, B.; Bennuri, S.C.; Delhey, L.; Slattery, J.; Tippett, M.; Wynne, R.; Rose, S.; Kahler, S.; Frye, R.E. Inheritance of HLA-Cw7 Associated With Autism Spectrum Disorder (ASD). Front. Psychiatry 2019, 10, 612. [Google Scholar] [CrossRef]
- Tekirdag, K.A.; Korkmaz, G.; Ozturk, D.G.; Agami, R.; Gozuacik, D. MIR181A regulates starvation- and rapamycin-induced autophagy through targeting of ATG5. Autophagy 2013, 9, 374–385. [Google Scholar] [CrossRef] [Green Version]
- Ren, X.P.; Wu, J.; Wang, X.; Sartor, M.A.; Jones, K.; Qian, J.; Nicolaou, P.; Pritchard, T.J.; Fan, G.C. MicroRNA-320 is involved in the regulation of cardiac ischemia/reperfusion injury by targeting heat-shock protein 20. Circulation 2009, 119, 2357–2366. [Google Scholar] [CrossRef] [Green Version]
- Costa, C.; Indovina, P.; Mattioli, E.; Forte, I.M.; Iannuzzi, C.A.; Luzzi, L.; Bellan, C.; De Summa, S.; Bucci, E.; Di Marzo, D.; et al. P53-regulated miR-320a targets PDL1 and is downregulated in malignant mesothelioma. Cell Death Dis. 2020, 11, 748. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.; Fu, L.; Li, J.; Li, J.; Zhao, Y.; Luan, Y.; Liu, A.; Liu, H.; Li, X.; Zhao, S.; et al. Transcriptome Analysis of Potential miRNA Involved in Adipogenic Differentiation of C2C12 Myoblasts. Lipids 2018, 53, 375–386. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Wang, F.L.; Wang, H.B.; Dong, N.; Zhu, X.M.; Wu, Y.; Wang, Y.T.; Yao, Y.M. TNF-alpha mRNA is negatively regulated by microRNA-181a-5p in maturation of dendritic cells induced by high mobility group box-1 protein. Sci. Rep. 2017, 7, 12239. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khandelwal, A.; Sharma, U.; Barwal, T.S.; Seam, R.K.; Gupta, M.; Rana, M.K.; Vasquez, K.M.; Jain, A. Circulating miR-320a Acts as a Tumor Suppressor and Prognostic Factor in Non-small Cell Lung Cancer. Front. Oncol. 2021, 11, 645475. [Google Scholar] [CrossRef]
- Sharma, A.; Mehan, S. Targeting PI3K-AKT/mTOR signaling in the prevention of autism. Neurochem. Int. 2021, 147, 105067. [Google Scholar] [CrossRef]
- Wang, Q.G.; Cheng, B.C.; He, Y.Z.; Li, L.J.; Ling, Y.; Luo, G.; Wang, L.; Liang, S.; Zhang, Y. miR-320a in serum exosomes promotes myocardial fibroblast proliferation via regulating the PIK3CA/Akt/mTOR signaling pathway in HEH2 cells. Exp. Med. 2021, 22, 873. [Google Scholar] [CrossRef]
- Hell, J.W. CaMKII: Claiming center stage in postsynaptic function and organization. Neuron 2014, 81, 249–265. [Google Scholar] [CrossRef] [Green Version]
- Fan, X.; Jin, W.Y.; Wang, Y.T. The NMDA receptor complex: A multifunctional machine at the glutamatergic synapse. Front. Cell. Neurosci. 2014, 8, 160. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Matt, L.; Patriarchi, T.; Malik, Z.A.; Chowdhury, D.; Park, D.K.; Renieri, A.; Ames, J.B.; Hell, J.W. Capping of the N-terminus of PSD-95 by calmodulin triggers its postsynaptic release. EMBO J. 2014, 33, 1341–1353. [Google Scholar] [CrossRef] [Green Version]
- Marsili, L.; Suppa, A.; Di Stasio, F.; Belvisi, D.; Upadhyay, N.; Berardelli, I.; Pasquini, M.; Petrucci, S.; Ginevrino, M.; Fabbrini, G.; et al. BDNF and LTP-/LTD-like plasticity of the primary motor cortex in Gilles de la Tourette syndrome. Exp. Brain Res. 2017, 235, 841–850. [Google Scholar] [CrossRef] [PubMed]
- Laoutidis, Z.G.; Lekka, G.E.; Kioulos, K.T. Glutamatergic Agents as Add-On Medication for the Treatment of Obsessive-Compulsive Disorder: A Systematic Review and Meta-Analysis. J. Clin. Psychiatry 2016, 77, e1576–e1583. [Google Scholar] [CrossRef]
- Kury, S.; van Woerden, G.M.; Besnard, T.; Proietti Onori, M.; Latypova, X.; Towne, M.C.; Cho, M.T.; Prescott, T.E.; Ploeg, M.A.; Sanders, S.; et al. De Novo Mutations in Protein Kinase Genes CAMK2A and CAMK2B Cause Intellectual Disability. Am. J. Hum. Genet. 2017, 101, 768–788. [Google Scholar] [CrossRef] [PubMed]
- Stephenson, J.R.; Wang, X.; Perfitt, T.L.; Parrish, W.P.; Shonesy, B.C.; Marks, C.R.; Mortlock, D.P.; Nakagawa, T.; Sutcliffe, J.S.; Colbran, R.J. A Novel Human CAMK2A Mutation Disrupts Dendritic Morphology and Synaptic Transmission, and Causes ASD-Related Behaviors. J. Neurosci. 2017, 37, 2216–2233. [Google Scholar] [CrossRef] [Green Version]
| Unrelated Controls | Sibling Controls | Autistic Disorder | |||
|---|---|---|---|---|---|
| ID | Age | ID | Age | ID | Age |
| GM09659 | 4 | AU1393305 | 4 | AU1393306 | 3 |
| GM11599 | 9 | AU038803 | 9 | AU038804 | 8 |
| GM15862 | 11 | AU0939302 | 8 | AU0939303 | 11 |
| GM10153 | 10 | AU1267303 | 7 | AU1267302 | 10 |
| GM16007 | 12 | AU1348302 | 13 | AU1348303 | 12 |
| GM09621 | 8 | AU1344303 | 4 | AU1344302 | 7 |
| AU1280304 | 1 | AU1280302 | 7 | ||
| AU1215305 | 2 | AU1215301 | 12 | ||
| AU008405 | 9 | AU008404 | 13 | ||
| GM11626 | 13 | AU1165303 | 12 | AU1165302 | 13 |
| Average (SD) | 9.6 (3.0) | 6.9 (4.1) | 9.6 (3.3) | ||
| ASD vs. Controls | ASD vs. Sibling | ||||
|---|---|---|---|---|---|
| miRNA ID | log2(FC) | −log10(pVal) | miRNA ID | log2(FC) | −log10(pVal) |
| PC-5p-35875_59 | −2.7 | 1.521688009 | hsa-miR-451a_R-1 | −5.9 | 0.5123528 |
| hsa-mir-18b-p3 | −2.5 | 1.686252607 | PC-5p-8577_335 | −2.8 | 1.8479091 |
| hsa-miR-451a_R-1 | −2.5 | 2.213487364 | hsa-miR-96-5p_R-2 | −2.7 | 0.6983189 |
| hsa-miR-92a-2-5p_R+1 | −1.4 | 1.766163822 | hsa-miR-4485-3p_L+3R+2 | −2.6 | 1.7373332 |
| PC-3p-16340_153 | −1.3 | 2.341434396 | hsa-miR-99b-5p | −2.2 | 1.5697133 |
| hsa-let-7i-3p_R-2 | −1.3 | 2.490787931 | hsa-miR-4521_R+3 | −1.5 | 3.8991403 |
| hsa-miR-4437_L+2 | −1.2 | 1.895455942 | hsa-let-7e-5p | −1.4 | 1.2824785 |
| hsa-mir-5100-p3_1ss17TC | −1.1 | 2.794312062 | hsa-miR-125a-5p_R-1 | −1.3 | 0.7832214 |
| PC-3p-12325_216 | −1.1 | 2.088661411 | hsa-miR-766-5p_R-1 | −1.2 | 3.1763432 |
| hsa-miR-181a-5p | −1.1 | 1.945138848 | hsa-miR-1270 | −1.1 | 1.5145443 |
| hsa-miR-363-3p_R+1 | −1.1 | 1.795244892 | hsa-miR-106a-5p | −1.1 | 1.3240308 |
| hsa-miR-10a-5p_R-1 | −1.1 | 1.790370904 | hsa-miR-320a | −1.0 | 2.5399924 |
| hsa-miR-20b-5p | −1.0 | 1.352069587 | hsa-miR-1246_L-1R+1 | −1.0 | 1.5790633 |
| hsa-miR-1271-5p | 1.2 | 1.801259553 | hsa-miR-5701_1ss2TG | 1.2 | 1.560449 |
| hsa-miR-151a-5p | 1.6 | 1.504469843 | hsa-miR-150-5p | 1.7 | 1.4184257 |
| Pathway | p-Value | Genes Involved |
|---|---|---|
| Up-regulated Pathways | ||
| PI3K-Akt signaling pathway | 0.002 | SGK3, OSMR, FGF14, COL3A1, HGF, C8ORF44-SGK3, ATF2, LAMA2, VEGFA, SOS2, PIK3AP1, EIF4E2, AKT2 |
| ErbB signaling pathway | 0.006 | EREG, ERBB4, SOS2, MAPK9, CAMK2A, AKT2 |
| Neurotrophin signaling pathway | 0.02 | RPS6KA6, SOS2, MAPK9, CAMK2A, PRKCD, AKT2 |
| HIF-1 signaling pathway | 0.04 | PFKFB3, VEGFA, CAMK2A, EIF4E2, AKT2 |
| Adrenergic signaling: cardiomyocytes | 0.05 | ACTC1, ADRB1, ATP1A2, CAMK2A, AKT2, ATF2 |
| Down-regulated Pathways | ||
| Transcriptional misregulation in cancer | <0.0001 | SUPT3H, FLT1, UTY, GZMB, AFF1, ATM, ATF1, MLF1, CCR7, PBX1, ETV6, PBX3, MLLT3, HIST1H3I |
| T cell receptor signaling pathway | <0.01 | PIK3CG, TNFα, RASGRP1, NFATC2, NFATC3, AKT3, TEC, IL2 |
| Chagas disease (American trypanosomiasis) | <0.01 | PIK3CG, TNFα, GNAI1, FADD, TRAF6, PLCB1, AKT3, IL2 |
| Sphingolipid signaling pathway | <.015 | PIK3CG, TNFα, SPTLC1, GNAI1, ACER2, PLCB1, AKT3, ASAH2 |
| PI3K-Akt signaling pathway | 0.018 | PIK3CG, FLT1, KITLG, RPS6KB1, TCL1B, COL4A6, HSP90B1, YWHAH, PRLR, ITGA5, COL6A2, THBS3, AKT3, SPP1, IL2 |
| Inflammatory mediator regulation of TRP channels | 0.02 | PIK3CG, HRH1, ADCY2, CALM3, PLA2G6, PLCB1, ALOX12 |
| Estrogen signaling pathway | 0.02 | PIK3CG, HSP90B1, ADCY2, GNAI1, CALM3, PLCB1, AKT3 |
| Glucagon signaling pathway | 0.02 | GCG, ADCY2, ACACA, CALM3, PLCB1, AKT3, PYGB |
| cGMP-PKG signaling pathway | 0.03 | PIK3CG, ADCY2, GNAI1, CALM3, NFATC2, PLCB1, NFATC3, AKT3, VDAC1 |
| Cytokine–cytokine receptor interaction | 0.03 | IL17A, TNFRSF11B, CCR7, TNFα, FLT1, PRLR, CXCL16, IL25, KITLG, BMPR1A, IL2 |
| Graft-versus-host disease | 0.035 | TNFα, GZMB, HLA-DOA, IL2 |
| Apoptosis | 0.04 | PIK3CG, TNFα, FADD, AKT3, ATM |
| Allograft rejection | <0.05 | TNFα, GZMB, HLA-DOA, IL2 |
| HTLV-I infection | 0.05 | PIK3CG, TNFα, ADCY2, HLA-DOA, NFATC2, NFATC3, AKT3, ATF1, ATM, VDAC1, IL2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Frye, R.E.; Rose, S.; McCullough, S.; Bennuri, S.C.; Porter-Gill, P.A.; Dweep, H.; Gill, P.S. MicroRNA Expression Profiles in Autism Spectrum Disorder: Role for miR-181 in Immunomodulation. J. Pers. Med. 2021, 11, 922. https://doi.org/10.3390/jpm11090922
Frye RE, Rose S, McCullough S, Bennuri SC, Porter-Gill PA, Dweep H, Gill PS. MicroRNA Expression Profiles in Autism Spectrum Disorder: Role for miR-181 in Immunomodulation. Journal of Personalized Medicine. 2021; 11(9):922. https://doi.org/10.3390/jpm11090922
Chicago/Turabian StyleFrye, Richard E., Shannon Rose, Sandra McCullough, Sirish C. Bennuri, Patricia A. Porter-Gill, Harsh Dweep, and Pritmohinder S. Gill. 2021. "MicroRNA Expression Profiles in Autism Spectrum Disorder: Role for miR-181 in Immunomodulation" Journal of Personalized Medicine 11, no. 9: 922. https://doi.org/10.3390/jpm11090922
APA StyleFrye, R. E., Rose, S., McCullough, S., Bennuri, S. C., Porter-Gill, P. A., Dweep, H., & Gill, P. S. (2021). MicroRNA Expression Profiles in Autism Spectrum Disorder: Role for miR-181 in Immunomodulation. Journal of Personalized Medicine, 11(9), 922. https://doi.org/10.3390/jpm11090922








