Lifestyle Variables Such as Daily Internet Use, as Promising Protective Factors against Cognitive Impairment in Patients with Subjective Memory Complaints. Preliminary Results
Abstract
:1. Introduction
2. Materials and Methods
2.1. Bibliographic Review
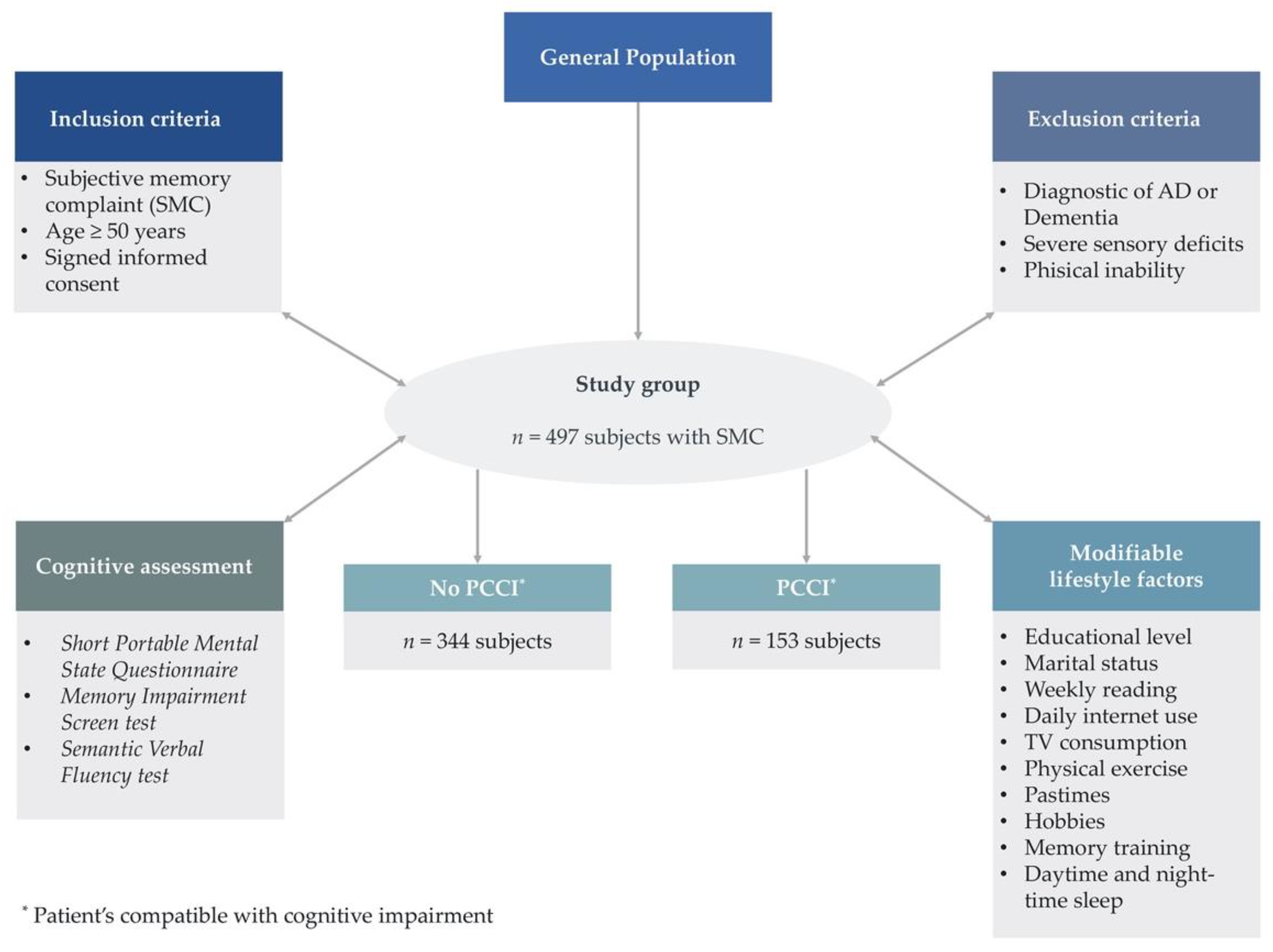
2.2. Patient Recruitment
2.3. Cognitive Impairment Assessment
2.3.1. Memory Impairment Screen
2.3.2. Short Portable Mental State Questionnaire (Spanish Version)
2.3.3. Semantic Verbal Fluency
2.4. Data Collection
2.5. Sample Size Calculation
2.6. Statistical Treatment of the Data
2.7. Ethical Approval
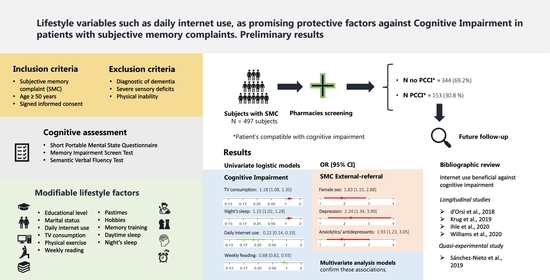
3. Results
3.1. Bibliographic Review
3.2. Demographic Characteristics of Individuals with a Subjective Memory Complaint
3.3. Patient Scores on the Cognitive Tests
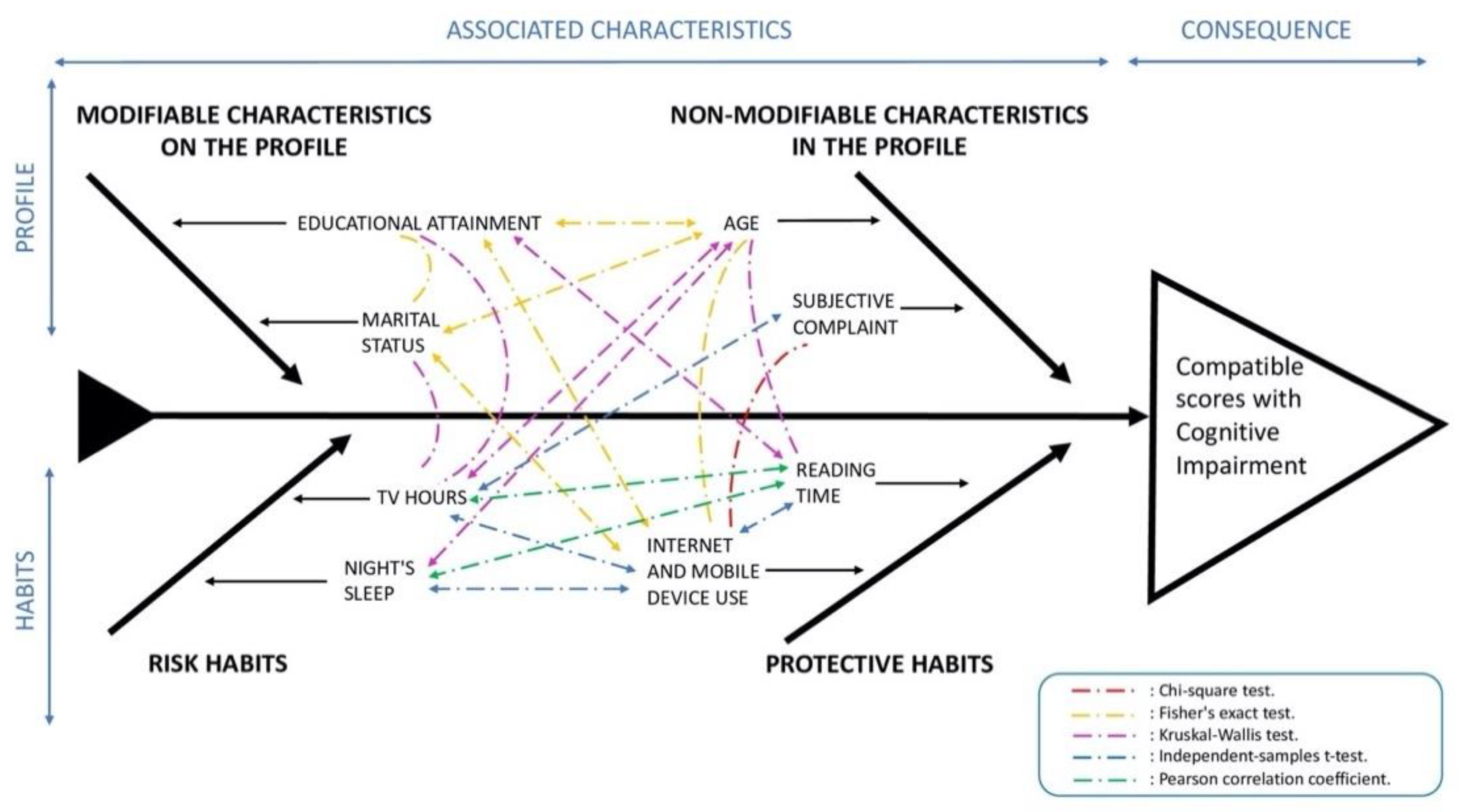
3.4. Qualitative Variables
3.5. Quantitative Variables
3.6. Multivariate Logistic Regression Models of the Patient Profile and Each of the Significant Modifiable Life Habits
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Livingston, G.; Huntley, J.; Sommerlad, A.; Ames, D.; Ballard, C.; Banerjee, S.; Brayne, C.; Burns, A.; Cohen-Mansfield, J.; Cooper, C.; et al. Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. Lancet 2020, 396, 413–446. [Google Scholar] [CrossRef]
- Alacreu, M.; Pardo, J.; Azorín, M.; Climent, M.T.; Gasull, V.; Moreno, L. Importance of Increasing Modifiable Risk Factors Knowledge on Alzheimer’s Disease Among Community Pharmacists and General Practitioners in Spain. Front. Pharmacol. 2019, 10, 860. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jack Jr, C.R.; Bennett, D.A.; Blennow, K.; Carrillo, M.C.; Dunn, B.; Haeberlein, S.B.; Holtzman, D.M.; Jagust, W.; Jessen, F.; Karlawish, J.; et al. NIA-AA Research Framework: Toward a biological definition of Alzheimer’s disease. Alzheimer’s Dement. 2018, 14, 535–562. [Google Scholar] [CrossRef]
- Aisen, P.S.; Cummings, J.; Jack, C.R.; Morris, J.C.; Sperling, R.; Frölich, L.; Jones, R.W.; Dowsett, S.A.; Matthews, B.R.; Raskin, J.; et al. On the path to 2025: Understanding the Alzheimer’s disease continuum. Alzheimer’s Res. Ther. 2017, 9, 60. [Google Scholar] [CrossRef] [PubMed]
- Jessen, F.; Amariglio, R.E.; Buckley, R.F.; van der Flier, W.M.; Han, Y.; Molinuevo, J.L.; Rabin, L.; Rentz, D.M.; Rodriguez-Gomez, O.; Saykin, A.J.; et al. The characterisation of subjective cognitive decline. Lancet Neurol. 2020, 19, 271–278. [Google Scholar] [CrossRef]
- van Wanrooij, L.L.; Richard, E.; Jongstra, S.; van Charante, E.P.M.; van Gool, W.A. Associations of subjective memory complaints and simple memory task scores with future dementia in the primary care setting. Ann. Fam. Med. 2019, 17, 412–418. [Google Scholar] [CrossRef]
- Mitchell, A.J.; Beaumont, H.; Ferguson, D.; Yadegarfar, M.; Stubbs, B. Risk of dementia and mild cognitive impairment in older people with subjective memory complaints: Meta-analysis. Acta Psychiatr. Scand. 2014, 130, 439–451. [Google Scholar] [CrossRef]
- Petersen, R.C.; Smith, G.E.; Waring, S.C.; Ivnik, R.J.; Tangalos, E.G.; Kokmen, E. Mild cognitive impairment: Clinical characterization and outcome. Arch. Neurol. 1999, 56, 303–308. [Google Scholar] [CrossRef]
- Lara, E.; Koyanagi, A.; Olaya, B.; Lobo, A.; Miret, M.; Tyrovolas, S.; Ayuso-Mateos, J.L.; Haro, J.M. Mild cognitive impairment in a Spanish representative sample: Prevalence and associated factors. Int. J. Geriatr. Psychiatry 2016, 31, 858–867. [Google Scholar] [CrossRef]
- Dubois, B.; Feldman, H.H.; Jacova, C.; Hampel, H.; Molinuevo, J.L.; Blennow, K.; Dekosky, S.T.; Gauthier, S.; Selkoe, D.; Bateman, R.; et al. Advancing research diagnostic criteria for Alzheimer’s disease: The IWG-2 criteria. Lancet Neurol. 2014, 13, 614–629. [Google Scholar] [CrossRef]
- Stern, Y. Cognitive reserve in ageing and Alzheimer’s disease. Lancet Neurol. 2012, 11, 1006–1012. [Google Scholar] [CrossRef] [Green Version]
- Hertzog, C.; Kramer, A.F.; Wilson, R.S.; Lindenberger, U. Enrichment Effects on Adult Cognitive Development: Can the Functional Capacity of Older Adults Be Preserved and Enhanced? Psychol. Sci. Public Interest 2008, 9, 1–65. [Google Scholar] [CrossRef] [Green Version]
- Scarmeas, N.; Stern, Y. Cognitive reserve: Implications for diagnosis and prevention of Alzheimer’s disease. Curr. Neurol. Neurosci. Rep. 2004, 4, 374–380. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jones, R.N.; Manly, J.; Glymour, M.M.; Rentz, D.M.; Jefferson, A.L.; Stern, Y. Conceptual and measurement challenges in research on cognitive reserve. J. Int. Neuropsychol. Soc. 2011, 17, 593–601. [Google Scholar] [CrossRef] [Green Version]
- Evans, I.E.M.; Martyr, A.; Collins, R.; Brayne, C.; Clare, L. Social Isolation and Cognitive Function in Later Life: A Systematic Review and Meta-Analysis. J. Alzheimer’s Dis. 2019, 70, S119–S144. [Google Scholar] [CrossRef] [Green Version]
- Gindrat, A.-D.; Chytiris, M.; Balerna, M.; Rouiller, E.M.; Ghosh, A. Use-dependent cortical processing from fingertips in touchscreen phone users. Curr. Biol. 2015, 25, 109–116. [Google Scholar] [CrossRef] [Green Version]
- Firth, J.; Torous, J.; Stubbs, B.; Firth, J.A.; Steiner, G.Z.; Smith, L.; Alvarez-Jimenez, M.; Gleeson, J.; Vancampfort, D.; Armitage, C.J.; et al. The “online brain”: How the Internet may be changing our cognition. World Psychiatry 2019, 18, 119–129. [Google Scholar] [CrossRef] [Green Version]
- Climent, M.T.; Pardo, J.; Muñoz-Almaraz, F.J.; Guerrero, M.D.; Moreno, L. Decision tree for early detection of cognitive impairment by community pharmacists. Front. Pharmacol. 2018, 9, 1232. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muñoz-Almaraz, F.J.; Climent, M.T.; Guerrero, M.D.; Moreno, L.; Pardo, J. A machine learning approach to design an efficient selective screening of mild cognitive impairment. J. Vis. Exp. 2020, 155, e59649. [Google Scholar] [CrossRef]
- Böhm, P.; Peña-Casanova, J.; Gramunt, N.; Manero, R.M.; Terrón, C.; Quiñones-Úbeda, S. Spanish version of the Memory Impairment Screen (MIS): Normative data and discriminant validity. Neurologia 2005, 20, 402–411. [Google Scholar]
- Martínez de la Iglesia, J.; Dueñas, R.; Onís, M.C.; Aguado, C.; Albert, C.; Luque, R. Spanish language adaptation and validation of the Pfeiffer’s questionnaire (SPMSQ) to detect cognitive deterioration in people over 65 years of age. Med. Clin. 2001, 117, 129–134. [Google Scholar] [CrossRef]
- Price, S.E.; Kinsella, G.J.; Ong, B.; Storey, E.; Mullaly, E.; Phillips, M.; Pangnadasa-Fox, L.; Perre, D. Semantic verbal fluency strategies in amnestic mild cognitive impairment. Neuropsychology 2012, 26, 490–497. [Google Scholar] [CrossRef]
- Pérez-Díaz, A.G.L.; Calero, M.D.; Navarro-González, E. Predicción del deterioro cognitivo en ancianos mediante el análisis del rendimiento en fluidez verbal y en atención sostenida. Rev. Neurol. 2013, 56, 1–7. [Google Scholar] [CrossRef]
- Ramos, H.; Pardo, J.; Sánchez, R.; Puchades, E.; Pérez-Tur, J.; Navarro, A.; Moreno, L. Pharmacist-Physician Interprofessional Collaboration to Promote Early Detection of Cognitive Impairment: Increasing Diagnosis Rate. Front. Pharmacol. 2021, 12, 579489. [Google Scholar] [CrossRef]
- Climent Catalá, M.T.; Moreno Royo, L.; Gasull Molinera, V.; Sánchez Roy, R.; Pérez Tur, J. Proyecto CRIDECO: Cribado de deterioro cognitivo en farmacia comunitaria a partir de la queja subjetiva de memoria. Farm. Comunitarios 2019, 10, 20–26. [Google Scholar] [CrossRef]
- d’Orsi, E.; Xavier, A.J.; Rafnsson, S.B.; Steptoe, A.; Hogervorst, E.; Orrell, M. Is use of the internet in midlife associated with lower dementia incidence? Results from the English Longitudinal Study of Ageing. Aging Ment. Health 2018, 22, 1525–1533. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Nieto, J.M.; Martínez-Maldonado, M.D.L.L.; Montero-López Lena, M.; Mendoza-Núñez, V.M. Effect of a mental stimulation program of computer and internet learning on cognitive functions and wellbeing in older community-dwelling mexicans. Brain Sci. 2019, 9, 151. [Google Scholar] [CrossRef] [Green Version]
- Krug, R.D.R.; D’Orsi, E.; Xavier, A.J. Association between use of internet and the cognitive function in older adults, populational longitudinal study EpiFloripa Idoso. Rev. Bras. Epidemiol. 2019, 22, e190012. [Google Scholar] [CrossRef] [PubMed]
- Ihle, A.; Bavelier, D.; Maurer, J.; Oris, M.; Kliegel, M. Internet use in old age predicts smaller cognitive decline only in men. Sci. Rep. 2020, 10, 8969. [Google Scholar] [CrossRef] [PubMed]
- Williams, B.D.; Pendleton, N.; Chandola, T. Cognitively stimulating activities and risk of probable dementia or cognitive impairment in the English Longitudinal Study of Ageing. SSM—Popul. Health 2020, 12, 100656. [Google Scholar] [CrossRef]
- Mintzer, J.; Donovan, K.A.; Kindy, A.Z.; Lock, S.L.; Chura, L.R.; Barracca, N. Lifestyle Choices and Brain Health. Front. Med. 2019, 6, 204. [Google Scholar] [CrossRef]
- Gabelle, A.; Gutierrez, L.-A.; Jaussent, I.; Navucet, S.; Grasselli, C.; Bennys, K.; Marelli, C.; David, R.; Andrieu, S.; Berr, C.; et al. Excessive sleepiness and longer nighttime in bed increase the risk of cognitive decline in frail elderly subjects: The MAPT-sleep study. Front. Aging Neurosci. 2017, 9, 312. [Google Scholar] [CrossRef] [PubMed]
- Fancourt, D.; Steptoe, A. Television viewing and cognitive decline in older age: Findings from the English Longitudinal Study of Ageing. Sci. Rep. 2019, 9, 2851. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jung, M.S.; Chung, E. Television viewing and cognitive dysfunction of korean older adults. Healthcare 2020, 8, 547. [Google Scholar] [CrossRef]
- Verghese, J.; Lipton, R.B.; Katz, M.J.; Hall, C.B.; Derby, C.A.; Kuslansky, G.; Ambrose, A.F.; Sliwinski, M.; Buschke, H. Leisure activities and the risk of dementia in the elderly. N. Engl. J. Med. 2003, 348, 2508–2516. [Google Scholar] [CrossRef] [Green Version]
- Chang, Y.-H.; Wu, I.-C.; Hsiung, C.A. Reading activity prevents long-term decline in cognitive function in older people: Evidence from a 14-year longitudinal study. Int. Psychogeriatr. 2021, 33, 63–74. [Google Scholar] [CrossRef] [PubMed]
- Berns, G.S.; Blaine, K.; Prietula, M.J.; Pye, B.E. Short- and long-term effects of a novel on connectivity in the brain. Brain Connect. 2013, 3, 590–600. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takeuchi, H.; Taki, Y.; Hashizume, H.; Asano, K.; Asano, M.; Sassa, Y.; Yokota, S.; Kotozaki, Y.; Nouchi, R.; Kawashima, R. The impact of television viewing on brain structures: Cross-sectional and longitudinal analyses. Cereb. Cortex 2015, 25, 1188–1197. [Google Scholar] [CrossRef] [Green Version]
- Shin, Y.-B.; Kim, H.; Kim, S.J.; Kim, J.J. A neural mechanism of the relationship between impulsivity and emotion dysregulation in patients with Internet gaming disorder. Addict. Biol. 2021, 26, e12916. [Google Scholar] [CrossRef]
- Chamberlain, S.R.; Redden, S.A.; Leppink, E.; Grant, J.E. Problematic internet use in gamblers: Impact on clinical and cognitive measures. CNS Spectr. 2017, 22, 495–503. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Qin, Y.; Li, H.; Yao, D.; Sun, B.; Gong, J.; Dai, Y.; Wen, C.; Zhang, L.; Zhang, C.; et al. Identifying Internet Addiction and Evaluating the Efficacy of Treatment Based on Functional Connectivity Density: A Machine Learning Study. Front. Neurosci. 2021, 15, 665578. [Google Scholar] [CrossRef]
- Takeuchi, H.; Taki, Y.; Asano, K.; Asano, M.; Sassa, Y.; Yokota, S.; Kotozaki, Y.; Nouchi, R.; Kawashima, R. Impact of frequency of internet use on development of brain structures and verbal intelligence: Longitudinal analyses. Hum. Brain Mapp. 2018, 39, 4471–4479. [Google Scholar] [CrossRef] [Green Version]
- Wolfsgruber, S.; Polcher, A.; Koppara, A.; Kleineidam, L.; Frölich, L.; Peters, O.; Hüll, M.; Rüther, E.; Wiltfang, J.; Maier, W.; et al. Cerebrospinal Fluid Biomarkers and Clinical Progression in Patients with Subjective Cognitive Decline and Mild Cognitive Impairment. J. Alzheimer’s Dis. 2017, 58, 939–950. [Google Scholar] [CrossRef] [PubMed]
- Slot, R.E.R.; Sikkes, S.A.M.; Berkhof, J.; Brodaty, H.; Buckley, R.; Cavedo, E.; Dardiotis, E.; Guillo-Benarous, F.; Hampel, H.; Kochan, N.A.; et al. Subjective cognitive decline and rates of incident Alzheimer’s disease and non–Alzheimer’s disease dementia. Alzheimer’s Dement. 2019, 15, 465–476. [Google Scholar] [CrossRef] [PubMed]
- Linnemann, C.; Lang, U.E. Pathways Connecting Late-Life Depression and Dementia. Front. Pharmacol. 2020, 11, 279. [Google Scholar] [CrossRef]
- Cereseto, M.; Reinés, A.; Ferrero, A.; Sifonios, L.; Rubio, M.; Wikinski, S. Chronic treatment with high doses of corticosterone decreases cytoskeletal proteins in the rat hippocampus. Eur. J. Neurosci. 2006, 24, 3354–3364. [Google Scholar] [CrossRef] [PubMed]
- Mascherek, A.; Werkle, N.; Göritz, A.S.; Kühn, S.; Moritz, S. Lifestyle Variables Do Not Predict Subjective Memory Performance Over and Above Depression and Anxiety. Front. Psychol. 2020, 11, 484. [Google Scholar] [CrossRef]
- Sundström, A.; Westerlund, O.; Kotyrlo, E. Marital status and risk of dementia: A nationwide population-based prospective study from Sweden. BMJ Open 2016, 6, e008565. [Google Scholar] [CrossRef] [Green Version]
- Sommerlad, A.; Ruegger, J.; Singh-Manoux, A.; Lewis, G.; Livingston, G. Marriage and risk of dementia: Systematic review and meta-analysis of observational studies. J. Neurol. Neurosurg. Psychiatry 2018, 89, 231–238. [Google Scholar] [CrossRef]
- Sjöberg, L.; Fratiglioni, L.; Lövdén, M.; Wang, H.X. Low Mood and Risk of Dementia: The Role of Marital Status and Living Situation. Am. J. Geriatr. Psychiatry 2020, 28, 33–44. [Google Scholar] [CrossRef]
- Martorana, A.; Assogna, M.; De Lucia, V.; Motta, C.; Bonomi, C.G.; Bernocchi, F.; Di Donna, M.G.; Koch, G. Cognitive reserve and Alzheimer’s biological continuum: Clues for prediction and prevention of dementia. Minerva Med. 2021, 112, 441–447. [Google Scholar] [CrossRef] [PubMed]
- Ramos, H.; Moreno, L.; Gil, M.; García-Lluch, G.; Sendra-Lillo, J.; Alacreu, M. Pharmacists’ Knowledge of Factors Associated with Dementia: The A-to-Z Dementia Knowledge List. Int. J. Environ. Res. Public Health 2021, 18, 9934. [Google Scholar] [CrossRef] [PubMed]
- Jia, F.; Li, Y.; Li, M.; Cao, F. Subjective Cognitive Decline, Cognitive Reserve Indicators, and the Incidence of Dementia. J. Am. Med. Dir. Assoc. 2021, 22, 1449–1455. [Google Scholar] [CrossRef] [PubMed]
| Inclusion Criteria | Exclusion Criteria |
|---|---|
| - Published in the last 5 years (2017–2021) | - Duplicated manuscripts |
| - Published in PubMed or Web of Science before November 2021 | - Manuscripts not related to CI |
| - Population over 50 years old | - Screening using the title and abstract |
| - Language: English | - Manuscripts not specifically mentioning internet use |
| - Key words: “internet use” and “cognitive impairment” or “dementia” | - Manuscripts about molecular or non-commercialized drugs |
| Study Type | Country (N) | Follow-Up | Sample Age | Relationship to Cognitive Impairment | Citation |
|---|---|---|---|---|---|
| Longitudinal | England; N = 8238 participants | 10 years | >50 years | Internet use in individuals over 50 years of age was significantly associated with a 43–58% reduction in the risk of dementia. | d’Orsi et al., 2018 [26] |
| Quasi-experimental | Mexico; N = 27 participants | 10 weeks | >60 years | Subjects who participated in the computer-based mental stimulation and internet learning program significantly improved their episodic memory and visuospatial processing compared to the control group. | Sánchez-Nieto et al., 2019 [27] |
| Longitudinal | Brazil; N = 1197 participants | 4 years | >60 years | Significant association between continued internet use and cognitive status, with greater likelihood of cognitive gain and less cognitive decline. | Krug et al., 2019 [28] |
| Longitudinal | Switzerland; N = 897 participants | 6 years | >65 years | Frequent internet use was associated with less subsequent cognitive decline. This effect was observed mainly in men. | Ihle et al., 2020 [29] |
| Longitudinal | England; N = 2530–3937 participants | 8 years | >50 years | Internet use was associated with lower risk of cognitive impairment in the models used. | Williams et al., 2020 [30] |
| Variable | Group | n (% Column) | SMC External Referral | SMC Self-Referral | p-Value | OR [95% CI] | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| n | % Row | % Column | n | % Row | % Column | |||||
| Sex | Male | 131 (26.6) | 39 | 29.8 | 36.4 | 92 | 70.2 | 23.9 | < 0.05 a | 1 |
| Female | 361 (73.4) | 68 | 18.8 | 63.6 | 293 | 81.2 | 76.1 | 1.83 [1.15, 2.88] ** | ||
| Depression | No | 341 (69.3) | 87 | 25.5 | 81.3 | 254 | 74.5 | 66.0 | < 0.05 a | 1 |
| Yes | 151 (30.7) | 20 | 13.2 | 18.7 | 131 | 86.8 | 34.0 | 2.24 [1.34, 3.90] ** | ||
| Anxiolytics/Antidepressants | No | 260 (54.5 | 70 | 26.9 | 66.7 | 190 | 73.1 | 50.9 | < 0.05 a | 1 |
| Yes | 218 (45.6) | 35 | 16.1 | 33.3 | 183 | 83.9 | 49.1 | 1.93 [1.23, 3.05] ** | ||
| Test | Total n (% Column) | No PCCI n (% Row) | PCCI n (% Row) | Total n (% Row) | |
|---|---|---|---|---|---|
| SPMSQ | Normal | 400 (80.4) | 344 (86) | 56 (14) | 400 (100) |
| Slightly impaired | 74 (14.9) | 0 (0) | 74 (100) | 74 (100) | |
| Moderately impaired | 19 (3.8) | 0 (0) | 19 (100) | 19 (100) | |
| Severely impaired | 4 (0.8) | 0 (0) | 4 (100) | 4 (100) | |
| MIS Questionnaire | Normal | 412 (82.9) | 344 (83.5) | 68 (16.5) | 412 (100) |
| Impaired | 85 (17.1) | 0 (0) | 85 (100) | 85 (100) | |
| Verbal fluency Test | Normal | 423 (85.1) | 344 (81.3) | 79 (18.7) | 423 (100) |
| Impaired | 74 (14.9) | 0 (0) | 74 (100) | 74 (100) | |
| N positive test | Zero | 344 (69.2) | 344 (100) | 0 (0) | 344 (100) |
| One | 76 (15.3) | 0 (0) | 76 (100) | 76 (100) | |
| Two | 51 (10.3) | 0 (0) | 51 (100) | 51 (100) | |
| Three | 26 (5.2) | 0 (0) | 26 (100) | 26 (100) | |
| Total | 497 (100) | 344 (69.2) | 153 (30.8) | 497 (100) | |
| Variable | Group | n (% Column) | No PCCI | PCCI | p-Value | OR [95% CI] | |||
|---|---|---|---|---|---|---|---|---|---|
| n | % Row | n | % Row | ||||||
| Non-Modifiable Characteristics | Sex | Female | 364 (73.2) | 250 | 68.7 | 114 | 31.3 | 0.742 a | |
| Male | 133 (26.8) | 94 | 70.7 | 39 | 29.3 | ||||
| Age | 50–59 | 74 (14.9) | 65 | 87.8 | 9 | 12.2 | <0.001 b | 1 | |
| 60–69 | 155 (31.2) | 130 | 83.9 | 25 | 16.1 | 1.39 [0.63, 3.30] | |||
| 70–79 | 191 (38.4) | 117 | 61.3 | 74 | 38.7 | 4.57 [2.24, 10.33] *** | |||
| ≥80 | 75 (15.1) | 30 | 40.0 | 45 | 60.0 | 10.83 [4.88, 26.34] *** | |||
| Family history of dementia | No | 315 (63.4) | 210 | 66.7 | 105 | 33.3 | 0.130 a | ||
| Yes | 181 (36.4) | 133 | 73.5 | 48 | 26.5 | ||||
| SMC | External referral | 107 (21.5) | 87 | 81.3 | 20 | 18.7 | 0.001 a | 1 | |
| Self-referral | 385 (77.5) | 252 | 65.5 | 133 | 34.5 | 2.30 [1.35, 3.90] ** | |||
| Modifiable Characteristics | Educational level | Preprimary | 123 (24.7) | 50 | 40.7 | 73 | 59.3 | 3.93 [2.46, 6.35] *** | |
| Primary | 203 (40.8) | 148 | 72.9 | 55 | 27.1 | 1 | |||
| Secondary | 111 (22.3) | 92 | 82.9 | 19 | 17.1 | 0.56 [0.30, 0.98] ** | |||
| Tertiary | 57 (11.5) | 52 | 91.2 | 5 | 8.8 | 0.26 [0.09, 0.63] ** | |||
| Marital status | Married | 345 (69.4) | 250 | 72.5 | 95 | 27.5 | <0.001 b | 1 | |
| Separate | 29 (5.8) | 28 | 96.6 | 1 | 3.4 | 0.09 [0.005, 0.45] ** | |||
| Single | 24 (4.8) | 14 | 58.3 | 10 | 41.7 | 1.88 [0.79, 4.35] | |||
| Widowed | 99 (19.9) | 52 | 52.5 | 47 | 47.5 | 2.38 [1.5, 3.77] *** | |||
| Depression | No | 345 (69.4) | 247 | 71.6 | 98 | 28.4 | 0.092 a | ||
| Yes | 152 (30.6) | 97 | 63.8 | 55 | 36.2 | ||||
| Daily internet use | No | 205 (41.2) | 107 | 52.2 | 98 | 47.8 | <0.001 a | 1 | |
| Yes | 270 (54.3) | 225 | 83.3 | 45 | 16.7 | 0.22 [0.14, 0.33] *** | |||
| Total | 497 (100) | 344 | 69.2 | 153 | 30.8 | ||||
| Variable | No PCCI | PCCI | p-Value | OR [95% CI] | ||||
|---|---|---|---|---|---|---|---|---|
| n (%) | Mean | SD | n (%) | Mean | SD | |||
| Daytime sleep | 343 (69) | 0.41 | 0.58 | 153 (30.8) | 0.47 | 0.7 | 0.325 c | |
| Night’s sleep | 343 (69) | 6.64 | 1.59 | 153 (30.8) | 7.03 | 1.9 | 0.018 c | 1.15 [1.02, 1.28] ** |
| Hobbies | 344 (69.2) | 2.33 | 5.79 | 153 (30.8) | 1.62 | 4.5 | 0.180 c | |
| Physical exercise | 344 (69.2) | 3.75 | 4.46 | 153 (30.8) | 3.3 | 4.3 | 0.293 c | |
| Memory training | 344 (69.2) | 0.29 | 0.78 | 153 (30.8) | 0.18 | 0.6 | 0.101 c | |
| Weekly reading | 344 (69.2) | 3.92 | 5.94 | 153 (30.8) | 1.64 | 3.5 | <0.001 c | 0.88 [0.82, 0.93] *** |
| Pastimes | 344 (69.2) | 0.48 | 1.14 | 153 (30.8) | 0.62 | 1.7 | 0.274 c | |
| Tv consumption | 344 (69.2) | 2.6 | 1.87 | 153 (30.8) | 3.29 | 2.1 | <0.001 c | 1.18 [1.08, 1.30] *** |
| Variable | Profile | Profile + Night-Time Sleep | Profile + Reading | Profile + TV | Profile + Internet | |||
|---|---|---|---|---|---|---|---|---|
| OR [95% CI] | OR [95% CI] | OR [95% CI] | OR [95% CI] | OR [95% CI] | ||||
| Profile | Age | 50–59 | 1 | 1 | 1 | 1 | 1 | |
| 60–69 | 1.17 [0.50, 2.96] | 1.20 [0.51, 3.02] | 1.22 [0.52, 3.09] | 1.15 [0.49, 2.92] | 1.04 [0.43, 2.66] | |||
| 70–79 | 2.68 [1.22, 6.49] ** | 2.67 [1.21, 6.48] ** | 2.81 [1.26, 6.87] ** | 2.55 [1.14, 6.20] ** | 2.08 [0.89, 5.26] | |||
| ≥80 | 4.62 [1.88, 12.24] ** | 4.51 [1.83, 11.97] ** | 5.23 [2.08, 14.16] *** | 4.26 [1.71, 11.41] ** | 3.31 [1.24, 9.43] ** | |||
| SMC | External referral | 1 | 1 | 1 | 1 | 1 | ||
| Self-referral | 2.10 [1.17, 3.91] ** | 2.10 [1.17, 3.92] ** | 1.98 [1.09, 3.72] ** | 2.04 [1.13, 3.82] ** | 1.94 [1.05, 3.69] ** | |||
| Educational level | Preprimary | 2.86 [1.17, 4.81] *** | 2.89 [1.73, 4.87] *** | 2.47 [1.46, 4.19] *** | 2.98 [1.77, 5.03] *** | 2.63 [1.53, 4.55] *** | ||
| Primary | 1 | 1 | 1 | 1 | 1 | |||
| Secondary | 0.62 [0.32, 1.15] | 0.64 [0.34, 1.20] | 0.70 [0.36, 1.31] | 0.66 [0.34, 1.23] | 0.75 [0.38, 1.43] | |||
| Tertiary | 0.26 [0.08, 0.70] ** | 0.27 [0.08, 0.71] ** | 0.29 [0.08, 0.83] ** | 0.29 [0.09, 0.80] ** | 0.31 [0.09, 0.86] ** | |||
| Marital status | Married | 1 | 1 | 1 | 1 | 1 | ||
| Separate | 0.l8 [0.01, 0.93] | 0.18 [0.01, 0.92] | 0.17 [0.01, 0.85] * | 0.16, [0.01, 0.82] * | 0.20 [0.01, 1.01] | |||
| Single | 4.33 [1.56, 12.19] ** | 4.16 [1.49, 11.74] ** | 5.17 [1.79, 15.25] ** | 4.45 [1.58, 12.61] ** | 4.63 [1.57, 13.74] ** | |||
| Widowed | 1.43 [0.84, 2.41] | 1.42 [0.84, 2.41] | 1.48 [0.87, 2.52] | 1.40 [0.82, 2.36] | 1.47 [0.85, 2.53] | |||
| Habits | Night-time sleep | 1.07 [0.94, 1.21] | ||||||
| Reading | 0.90 [0.84, 0.96] ** | |||||||
| TV | 1.13 [1.01, 1.27] ** | |||||||
| Internet | No | 1 | ||||||
| Yes | 0.56 [0.33, 0.95] ** | |||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ramos, H.; Alacreu, M.; Guerrero, M.D.; Sánchez, R.; Moreno, L. Lifestyle Variables Such as Daily Internet Use, as Promising Protective Factors against Cognitive Impairment in Patients with Subjective Memory Complaints. Preliminary Results. J. Pers. Med. 2021, 11, 1366. https://doi.org/10.3390/jpm11121366
Ramos H, Alacreu M, Guerrero MD, Sánchez R, Moreno L. Lifestyle Variables Such as Daily Internet Use, as Promising Protective Factors against Cognitive Impairment in Patients with Subjective Memory Complaints. Preliminary Results. Journal of Personalized Medicine. 2021; 11(12):1366. https://doi.org/10.3390/jpm11121366
Chicago/Turabian StyleRamos, Hernán, Mónica Alacreu, María Dolores Guerrero, Rafael Sánchez, and Lucrecia Moreno. 2021. "Lifestyle Variables Such as Daily Internet Use, as Promising Protective Factors against Cognitive Impairment in Patients with Subjective Memory Complaints. Preliminary Results" Journal of Personalized Medicine 11, no. 12: 1366. https://doi.org/10.3390/jpm11121366
APA StyleRamos, H., Alacreu, M., Guerrero, M. D., Sánchez, R., & Moreno, L. (2021). Lifestyle Variables Such as Daily Internet Use, as Promising Protective Factors against Cognitive Impairment in Patients with Subjective Memory Complaints. Preliminary Results. Journal of Personalized Medicine, 11(12), 1366. https://doi.org/10.3390/jpm11121366