Dysregulated Notch Signaling in the Airway Epithelium of Children with Wheeze
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Study Participants and Cell Types
2.3. Treatment of pAEC with γ-Secretase Inhibitor (GSI) & Blocking Antibodies
2.4. Vector Construction
2.5. Vector Propagation
2.6. Generation of Stable Notch2-GFP-Expressing pAEC Cultures
2.7. RNA Extraction and Gene Expression Analysis
2.8. In-Cell™ Western Assay
2.9. Immunocytochemistry and Microscopy
2.10. Cell Proliferation Assay
2.11. Wound Repair Assays
2.12. Interrogation of Publicly Available Bulk and Single-Cell Transcriptomic Datasets
2.13. Statistics
3. Results
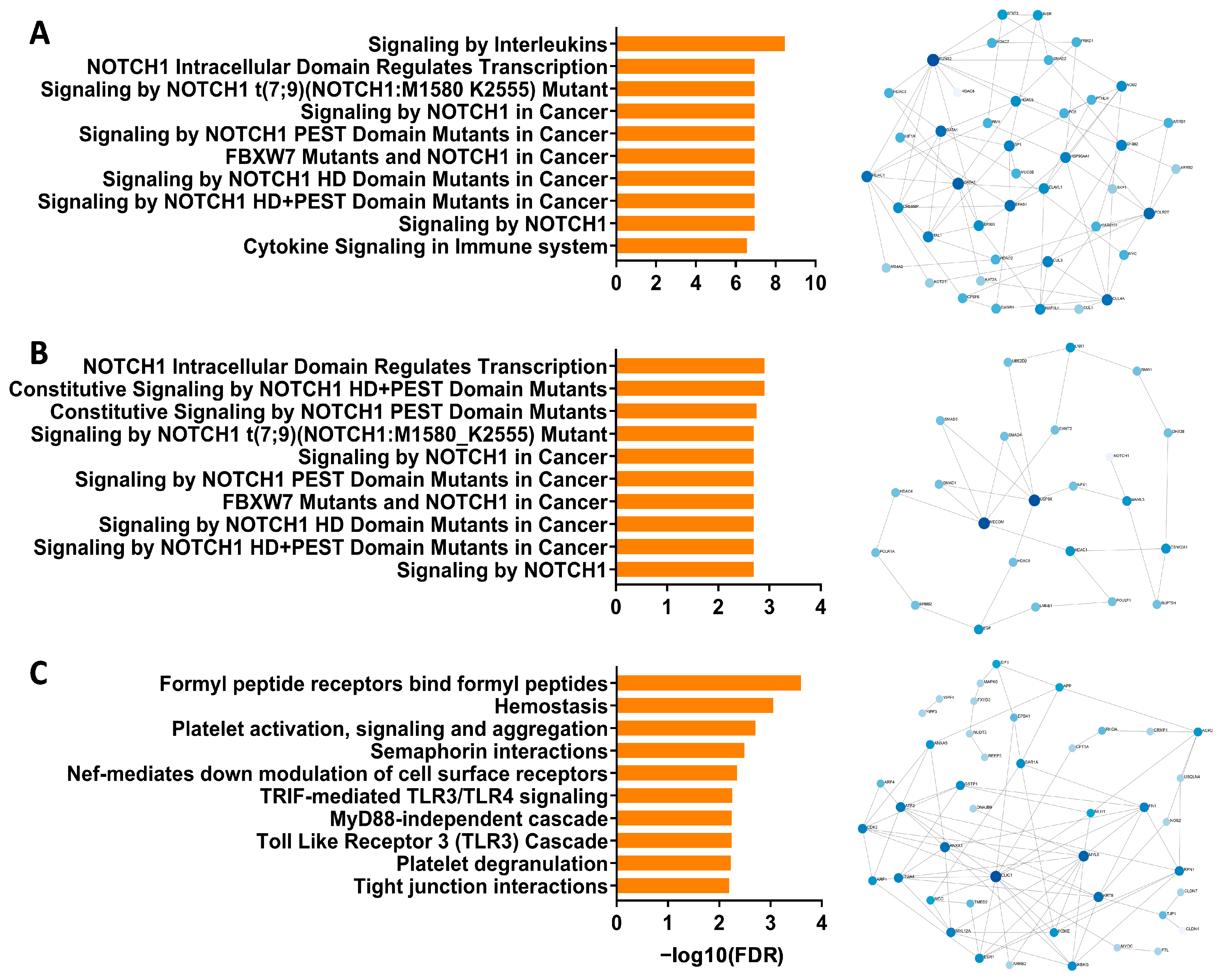
3.1. Identification of Notch Signaling Pathway in Pediatric Respiratory Wheeze Cohorts
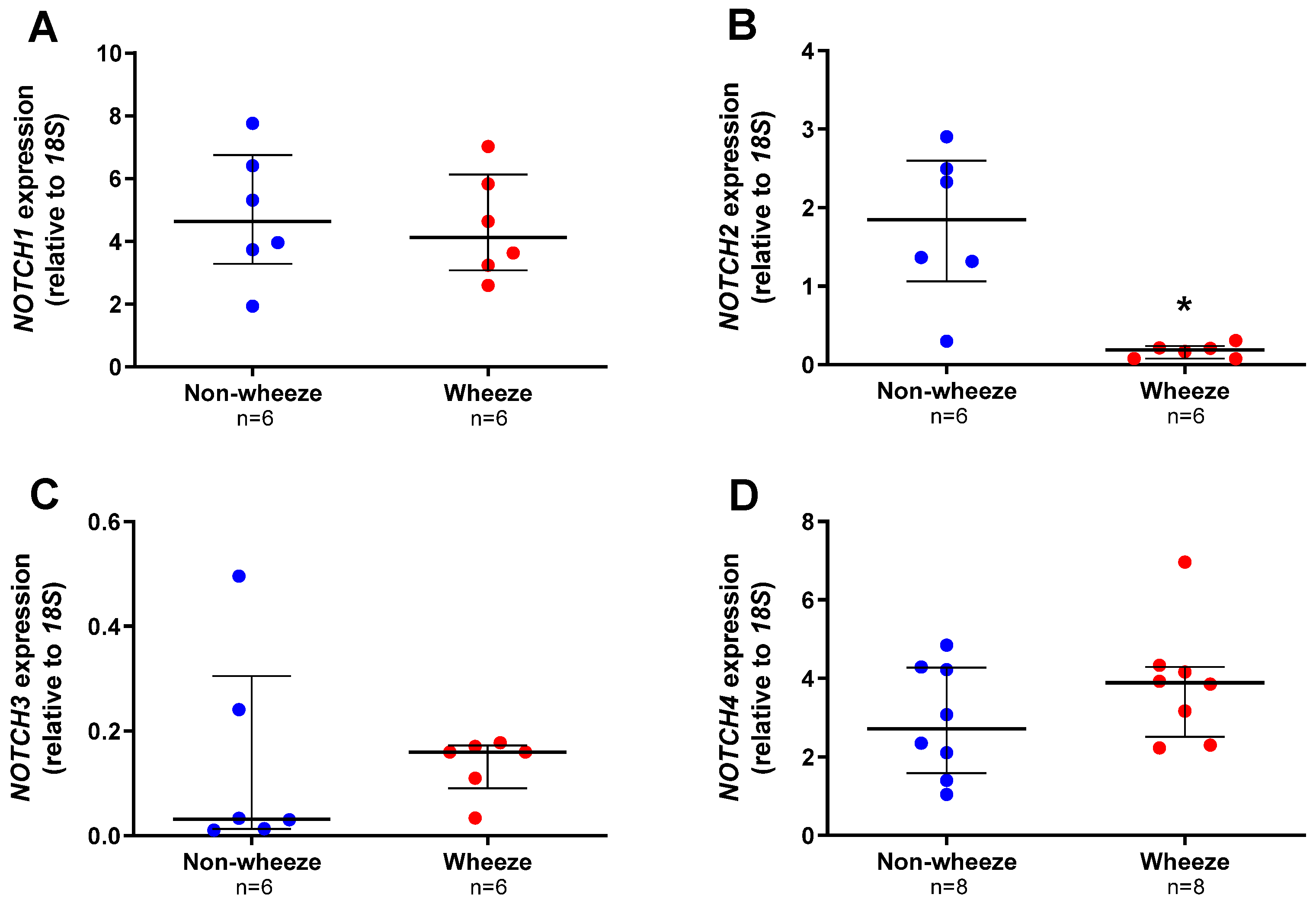
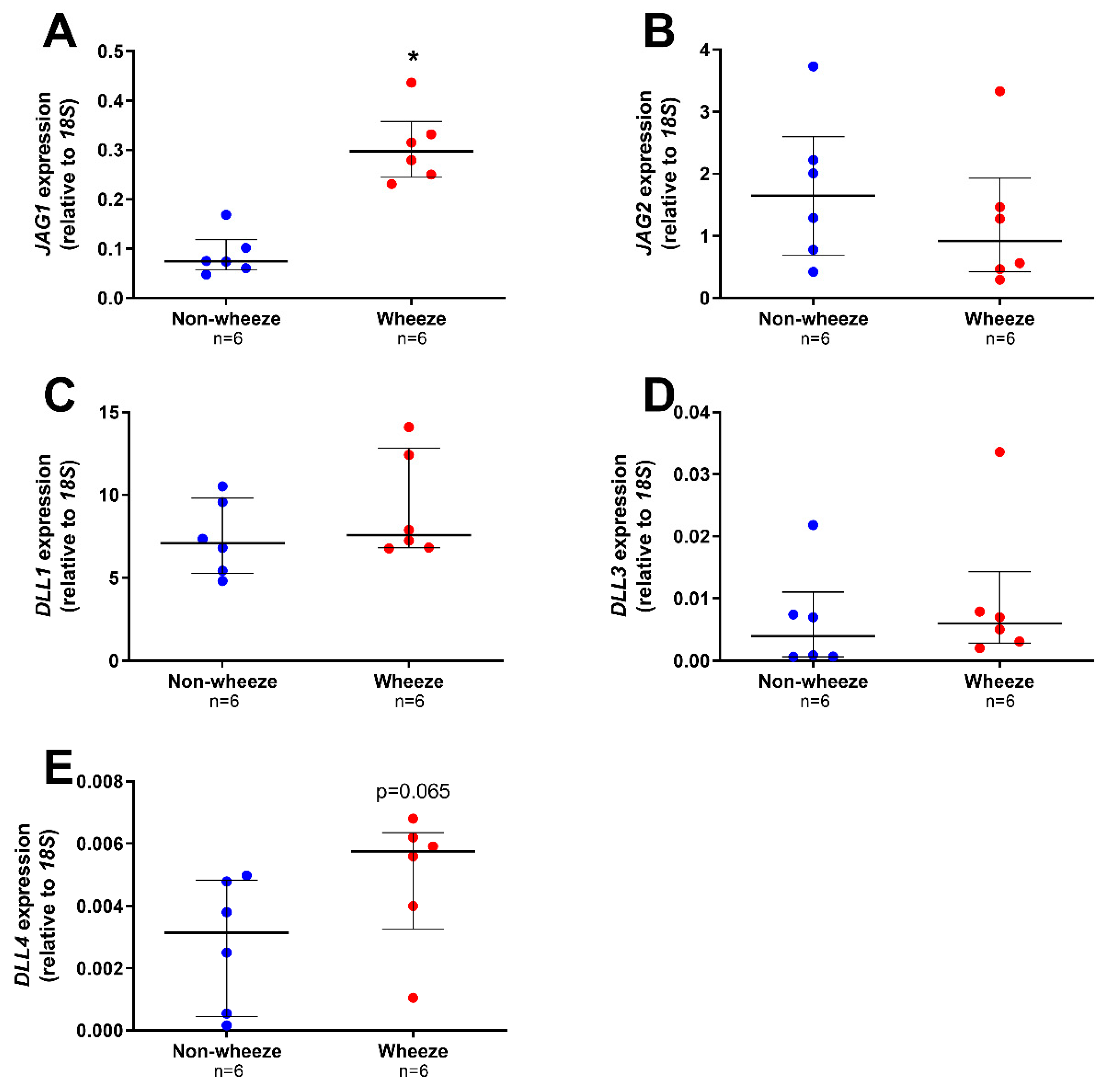
3.2. Altered Expression Profiles of Notch Receptors and Ligands in Ex Vivo pAEC from Children
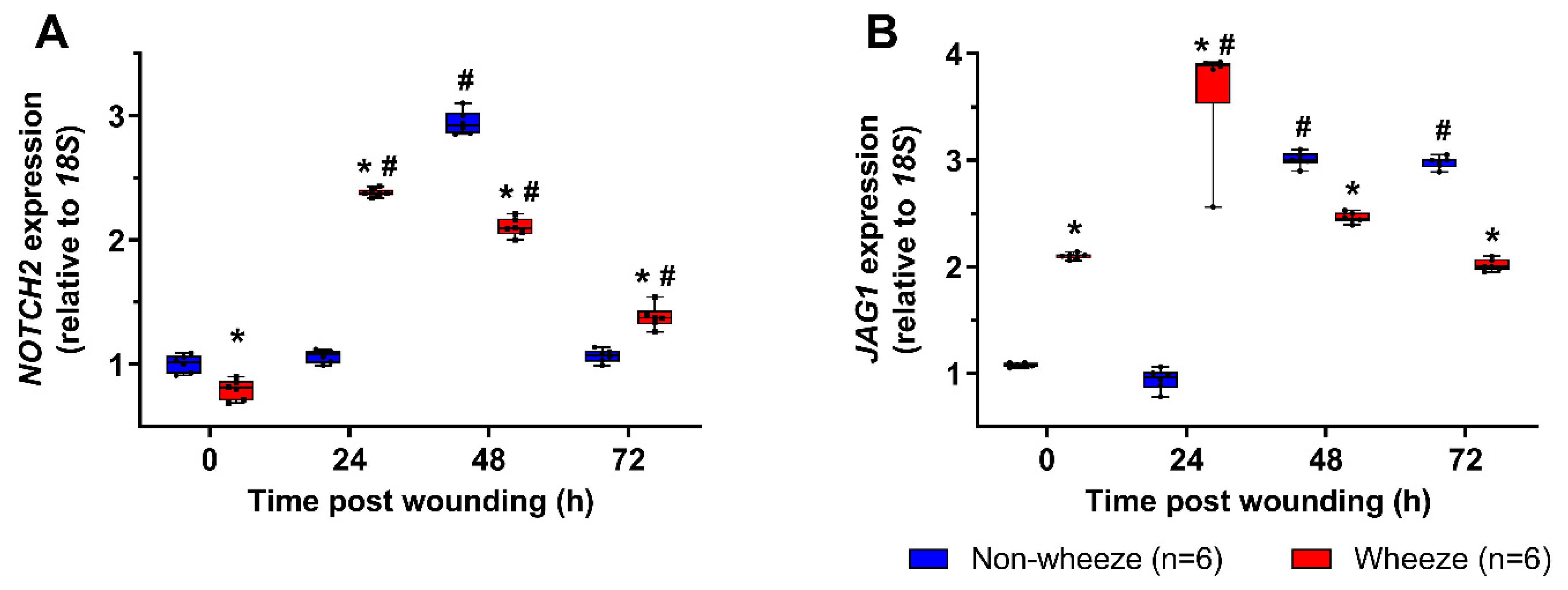
3.3. Dysregulated Expression of NOTCH2 and JAG1 during In Vitro Wound Repair in pAEC Cultures from Children
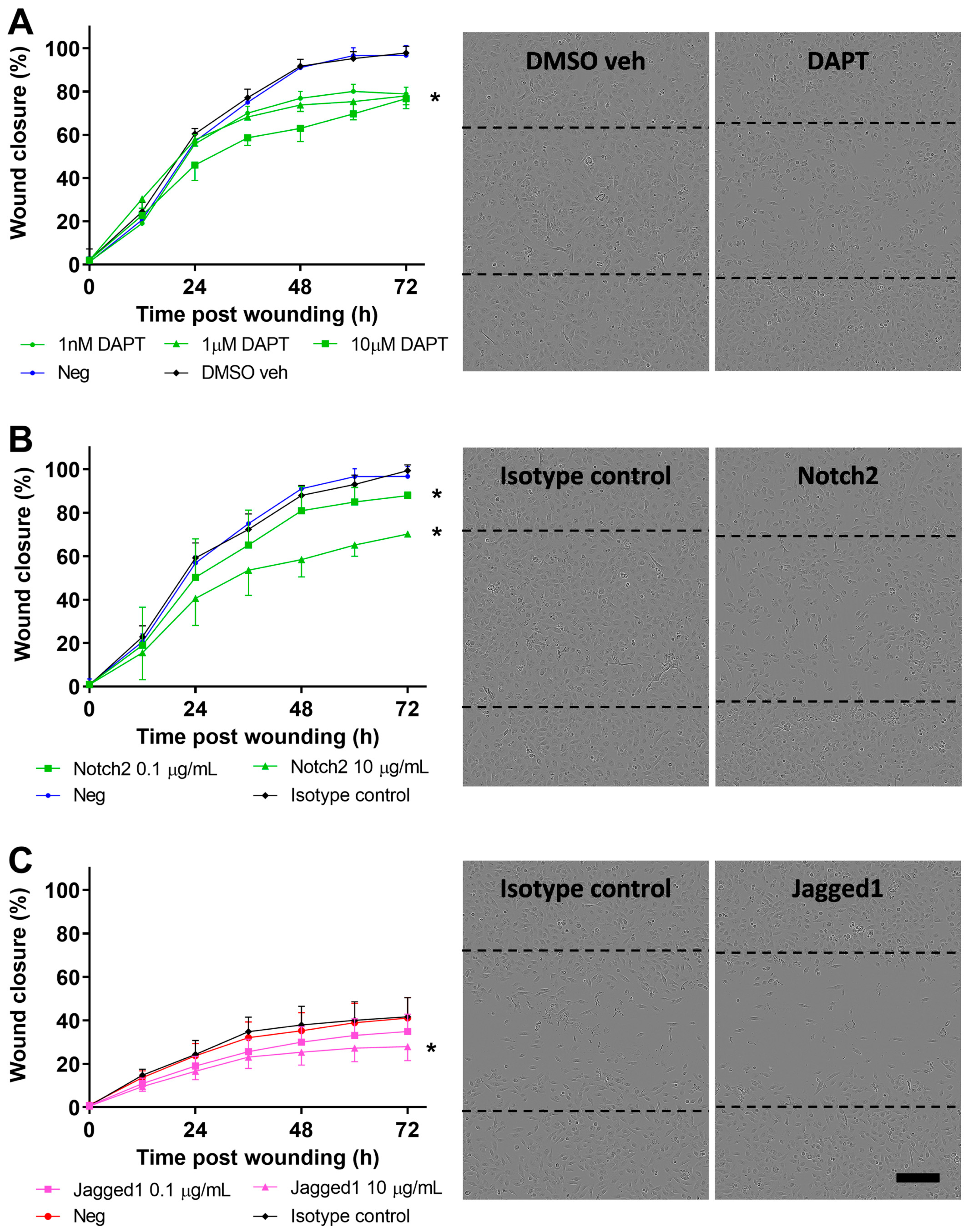
3.4. Inhibition of Notch Signaling Abrogates pAEC Wound Repair In Vitro
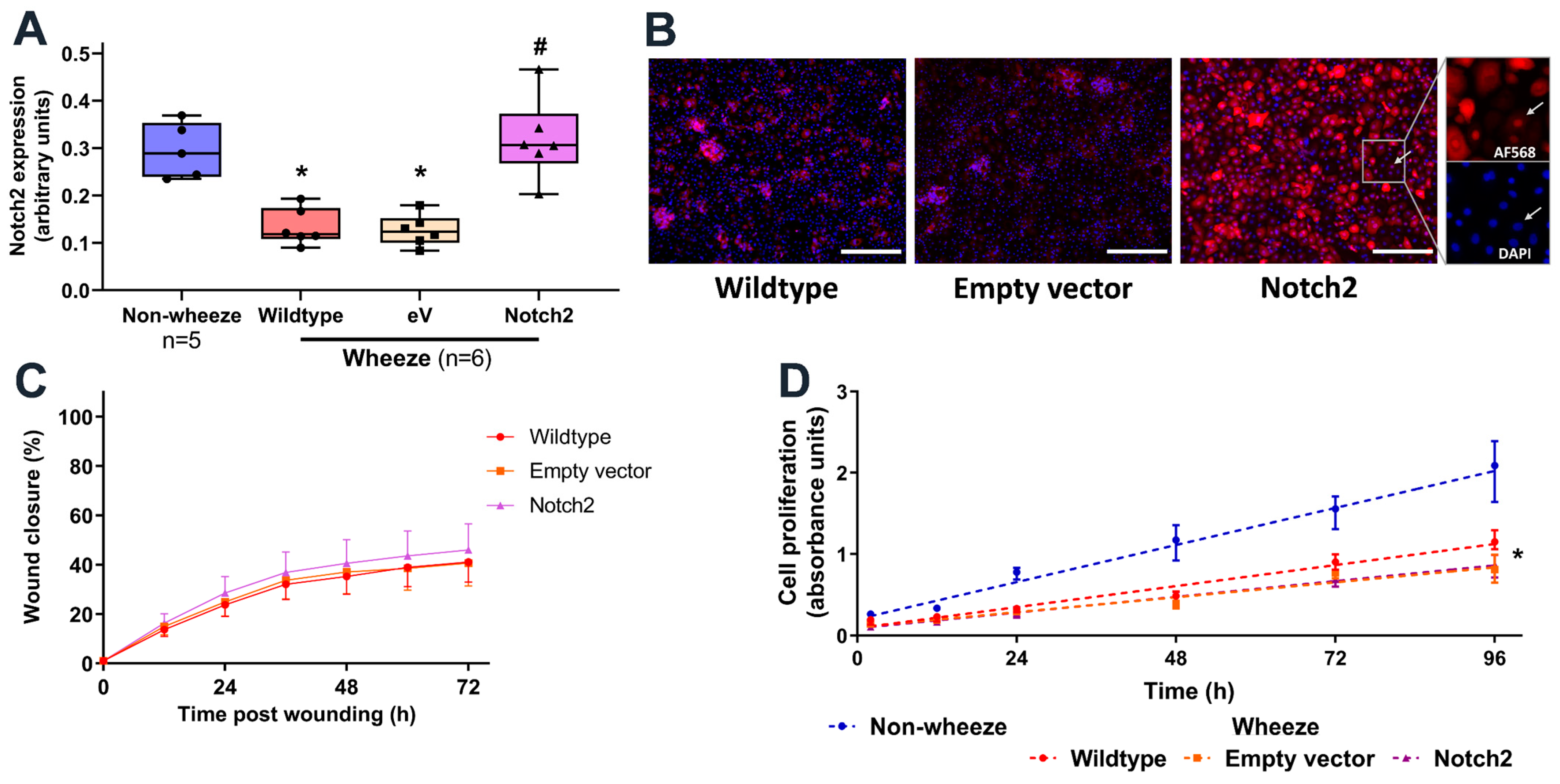
3.5. Overexpression of Notch2 Has no Effect on the Reparative Capacity of pAEC from Children with Wheeze
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
References
- Gautam, Y.; Afanador, Y.; Ghandikota, S.; Mersha, T.B. Comprehensive functional annotation of susceptibility variants associated with asthma. Hum. Genet. 2020, 139, 1037–1053. [Google Scholar] [CrossRef]
- Li, X.; Hastie, A.T.; Hawkins, G.A.; Moore, W.C.; Ampleford, E.J.; Milosevic, J.; Li, H.; Busse, W.W.; Erzurum, S.C.; Kaminski, N.; et al. eQTL of bronchial epithelial cells and bronchial alveolar lavage deciphers GWAS-identified asthma genes. Allergy 2015, 70, 1309–1318. [Google Scholar] [CrossRef] [PubMed]
- Moffatt, M.F.; Gut, I.G.; Demenais, F.; Strachan, D.P.; Bouzigon, E.; Heath, S.; von Mutius, E.; Farrall, M.; Lathrop, M.; Cookson, W.; et al. A large-scale, consortium-based genomewide association study of asthma. N. Engl. J. Med. 2010, 363, 1211–1221. [Google Scholar] [CrossRef] [Green Version]
- Ling, K.M.; Sutanto, E.N.; Iosifidis, T.; Kicic-Starcevich, E.; Looi, K.; Garratt, L.W.; Martinovich, K.M.; Lannigan, F.J.; Knight, D.A.; Stick, S.M.; et al. Reduced transforming growth factor beta1 (TGF-beta1) in the repair of airway epithelial cells of children with asthma. Respirology 2016, 21, 1219–1226. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barbato, A.; Turato, G.; Baraldo, S.; Bazzan, E.; Calabrese, F.; Panizzolo, C.; Zanin, M.E.; Zuin, R.; Maestrelli, P.; Fabbri, L.M.; et al. Epithelial damage and angiogenesis in the airways of children with asthma. Am. J. Respir. Crit. Care Med. 2006, 174, 975–981. [Google Scholar] [CrossRef]
- Saglani, S.; Molyneux, C.; Gong, H.; Rogers, A.; Malmstrom, K.; Pelkonen, A.; Makela, M.; Adelroth, E.; Bush, A.; Payne, D.N.; et al. Ultrastructure of the reticular basement membrane in asthmatic adults, children and infants. Eur. Respir. J. 2006, 28, 505–512. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kicic, A.; Sutanto, E.N.; Stevens, P.T.; Knight, D.A.; Stick, S.M. Intrinsic biochemical and functional differences in bronchial epithelial cells of children with asthma. Am. J. Respir. Crit. Care Med. 2006, 174, 1110–1118. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Laitinen, L.A.; Heino, M.; Laitinen, A.; Kava, T.; Haahtela, T. Damage of the airway epithelium and bronchial reactivity in patients with asthma. Am. Rev. Respir. Dis. 1985, 131, 599–606. [Google Scholar] [CrossRef]
- Looi, K.; Buckley, A.G.; Rigby, P.J.; Garratt, L.W.; Iosifidis, T.; Zosky, G.R.; Larcombe, A.N.; Lannigan, F.J.; Ling, K.M.; Martinovich, K.M.; et al. Effects of human rhinovirus on epithelial barrier integrity and function in children with asthma. Clin. Exper. Allergy 2018, 48, 513–524. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stevens, P.T.; Kicic, A.; Sutanto, E.N.; Knight, D.A.; Stick, S.M. Dysregulated repair in asthmatic paediatric airway epithelial cells: The role of plasminogen activator inhibitor-1. Clin. Exper. Allergy 2008, 38, 1901–1910. [Google Scholar] [CrossRef]
- Iosifidis, T.; Sutanto, E.N.; Buckley, A.G.; Coleman, L.; Gill, E.E.; Lee, A.H.; Ling, K.M.; Hillas, J.; Looi, K.; Garratt, L.W.; et al. Aberrant cell migration contributes to defective airway epithelial repair in childhood wheeze. JCI Insight 2020, 5, e133125. [Google Scholar] [CrossRef] [Green Version]
- McErlean, P.; Berdnikovs, S.; Favoreto, S., Jr.; Shen, J.; Biyasheva, A.; Barbeau, R.; Eisley, C.; Barczak, A.; Ward, T.; Schleimer, R.P.; et al. Asthmatics with exacerbation during acute respiratory illness exhibit unique transcriptional signatures within the nasal mucosa. Genome Med. 2014, 6, 1. [Google Scholar] [CrossRef] [Green Version]
- Redington, A.E.; Madden, J.; Frew, A.J.; Djukanovic, R.; Roche, W.R.; Holgate, S.T.; Howarth, P.H. Transforming growth factor-beta 1 in asthma. Measurement in bronchoalveolar lavage fluid. Am. J. Respir. Crit. Care Med. 1997, 156, 642–647. [Google Scholar] [CrossRef]
- Humbles, A.A.; Lloyd, C.M.; McMillan, S.J.; Friend, D.S.; Xanthou, G.; McKenna, E.E.; Ghiran, S.; Gerard, N.P.; Yu, C.; Orkin, S.H.; et al. A critical role for eosinophils in allergic airways remodeling. Science 2004, 305, 1776–1779. [Google Scholar] [CrossRef] [PubMed]
- Vignola, A.M.; Paganin, F.; Capieu, L.; Scichilone, N.; Bellia, M.; Maakel, L.; Bellia, V.; Godard, P.; Bousquet, J.; Chanez, P. Airway remodelling assessed by sputum and high-resolution computed tomography in asthma and COPD. Eur. Respir. J. 2004, 24, 910–917. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hodkinson, P.S.; Elliott, P.A.; Lad, Y.; McHugh, B.J.; MacKinnon, A.C.; Haslett, C.; Sethi, T. Mammalian NOTCH-1 activates beta1 integrins via the small GTPase R-Ras. J. Biol. Chem. 2007, 282, 28991–29001. [Google Scholar] [CrossRef] [Green Version]
- Ma, A.; Zhao, B.; Boulton, M.; Albon, J. A role for Notch signaling in corneal wound healing. Wound Repair Regen. 2011, 19, 98–106. [Google Scholar] [CrossRef] [Green Version]
- Riahi, R.; Sun, J.; Wang, S.; Long, M.; Zhang, D.D.; Wong, P.K. Notch1-Dll4 signalling and mechanical force regulate leader cell formation during collective cell migration. Nat. Commun. 2015, 6, 6556. [Google Scholar] [CrossRef] [Green Version]
- Carrer, M.; Crosby, J.R.; Sun, G.; Zhao, C.; Damle, S.S.; Kuntz, S.G.; Monia, B.P.; Hart, C.E.; Grossman, T.R. Antisense Oligonucleotides Targeting Jagged 1 Reduce House Dust Mite-induced Goblet Cell Metaplasia in the Adult Murine Lung. Am. J. Respir. Cell Mol. Biol. 2020, 63, 46–56. [Google Scholar] [CrossRef]
- Reid, A.T.; Nichol, K.S.; Chander Veerati, P.; Moheimani, F.; Kicic, A.; Stick, S.M.; Bartlett, N.W.; Grainge, C.L.; Wark, P.A.B.; Hansbro, P.M.; et al. Blocking Notch3 Signaling Abolishes MUC5AC Production in Airway Epithelial Cells from Individuals with Asthma. Am. J. Respir. Cell Mol. Biol. 2020, 62, 513–523. [Google Scholar] [CrossRef] [PubMed]
- Kicic, A.; Hallstrand, T.S.; Sutanto, E.N.; Stevens, P.T.; Kobor, M.S.; Taplin, C.; Pare, P.D.; Beyer, R.P.; Stick, S.M.; Knight, D.A. Decreased fibronectin production significantly contributes to dysregulated repair of asthmatic epithelium. Am. J. Respir. Crit. Care Med. 2010, 181, 889–898. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lai, E.C. Notch signaling: Control of cell communication and cell fate. Development 2004, 131, 965–973. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ranganathan, P.; Weaver, K.L.; Capobianco, A.J. Notch signalling in solid tumours: A little bit of everything but not all the time. Nat. Rev. Cancer 2011, 11, 338–351. [Google Scholar] [CrossRef]
- Radtke, F.; Wilson, A.; MacDonald, H.R. Notch signaling in hematopoiesis and lymphopoiesis: Lessons from Drosophila. Bioessays 2005, 27, 1117–1128. [Google Scholar] [CrossRef]
- Tanigaki, K.; Honjo, T. Regulation of lymphocyte development by Notch signaling. Nat. Immunol. 2007, 8, 451–456. [Google Scholar] [CrossRef] [PubMed]
- Lane, C. The use of non-bronchoscopic brushings to study the paediatric airway. Respir. Res. 2005, 6, 53. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martinovich, K.M.; Iosifidis, T.; Buckley, A.G.; Looi, K.; Ling, K.M.; Sutanto, E.N.; Kicic-Starcevich, E.; Garratt, L.W.; Shaw, N.C.; Montgomery, S.; et al. Conditionally reprogrammed primary airway epithelial cells maintain morphology, lineage and disease specific functional characteristics. Sci. Rep. 2017, 7, 17971. [Google Scholar] [CrossRef]
- Liu, X.; Ory, V.; Chapman, S.; Yuan, H.; Albanese, C.; Kallakury, B.; Timofeeva, O.A.; Nealon, C.; Dakic, A.; Simic, V.; et al. ROCK inhibitor and feeder cells induce the conditional reprogramming of epithelial cells. Am. J. Pathol. 2012, 180, 599–607. [Google Scholar] [CrossRef] [Green Version]
- Garratt, L.W.; Sutanto, E.N.; Ling, K.M.; Looi, K.; Iosifidis, T.; Martinovich, K.M.; Shaw, N.C.; Buckley, A.G.; Kicic-Starcevich, E.; Lannigan, F.J.; et al. Alpha 1-antitrypsin Mitigates the Inhibition of Airway Epithelial Cell Repair by Neutrophil Elastase. Am. J. Respir. Cell Mol. Biol. 2015. [Google Scholar] [CrossRef]
- Kicic, A.; de Jong, E.; Ling, K.M.; Nichol, K.; Anderson, D.; Wark, P.A.B.; Knight, D.A.; Bosco, A.; Stick, S.M.; Kicic, A.; et al. Assessing the unified airway hypothesis in children via transcriptional profiling of the airway epithelium. J. Allergy Clin. Immunol. 2020, 145, 1562–1573. [Google Scholar] [CrossRef]
- Altman, M.C.; Calatroni, A.; Ramratnam, S.; Jackson, D.J.; Presnell, S.; Rosasco, M.G.; Gergen, P.J.; Bacharier, L.B.; O’Connor, G.T.; Sandel, M.T.; et al. Endotype of allergic asthma with airway obstruction in urban children. J. Allergy Clin. Immunol. 2021, 148, 1198–1209. [Google Scholar] [CrossRef] [PubMed]
- Zhou, G.; Soufan, O.; Ewald, J.; Hancock, R.E.W.; Basu, N.; Xia, J. NetworkAnalyst 3.0: A visual analytics platform for comprehensive gene expression profiling and meta-analysis. Nucleic Acids Res. 2019, 47, W234–W241. [Google Scholar] [CrossRef] [Green Version]
- Plasschaert, L.W.; Zilionis, R.; Choo-Wing, R.; Savova, V.; Knehr, J.; Roma, G.; Klein, A.M.; Jaffe, A.B. A single-cell atlas of the airway epithelium reveals the CFTR-rich pulmonary ionocyte. Nature 2018, 560, 377–381. [Google Scholar] [CrossRef] [PubMed]
- Weinreb, C.; Wolock, S.; Klein, A.M. SPRING: A kinetic interface for visualizing high dimensional single-cell expression data. Bioinformatics 2018, 34, 1246–1248. [Google Scholar] [CrossRef]
- Konishi, S.; Gotoh, S.; Tateishi, K.; Yamamoto, Y.; Korogi, Y.; Nagasaki, T.; Matsumoto, H.; Muro, S.; Hirai, T.; Ito, I.; et al. Directed induction of functional multi-ciliated cells in proximal airway epithelial spheroids from human pluripotent stem cells. Stem Cell Rep. 2016, 6, 18–25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rock, J.R.; Gao, X.; Xue, Y.; Randell, S.H.; Kong, Y.Y.; Hogan, B.L. Notch-dependent differentiation of adult airway basal stem cells. Cell Stem Cell 2011, 8, 639–648. [Google Scholar] [CrossRef] [Green Version]
- Campos, L.S.; Decker, L.; Taylor, V.; Skarnes, W. Notch, epidermal growth factor receptor, and beta1-integrin pathways are coordinated in neural stem cells. J. Biol. Chem. 2006, 281, 5300–5309. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, N.; Rollin, J.; Masse, I.; Lamartine, J.; Gidrol, X. p63 regulates human keratinocyte proliferation via MYC-regulated gene network and differentiation commitment through cell adhesion-related gene network. J. Biol. Chem. 2012, 287, 5627–5638. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Movahedan, A.; Majdi, M.; Afsharkhamseh, N.; Sagha, H.M.; Saadat, N.S.; Shalileh, K.; Milani, B.Y.; Ying, H.; Djalilian, A.R. Notch inhibition during corneal epithelial wound healing promotes migration. Investig. Ophthalmol. Vis. Sci. 2012, 53, 7476–7483. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- KleinJan, A. Airway inflammation in asthma: Key players beyond the Th2 pathway. Curr. Opin. Pulm. Med. 2016, 22, 46–52. [Google Scholar] [CrossRef]
- Lafkas, D.; Shelton, A.; Chiu, C.; de Leon Boenig, G.; Chen, Y.; Stawicki, S.S.; Siltanen, C.; Reichelt, M.; Zhou, M.; Wu, X.; et al. Therapeutic antibodies reveal Notch control of transdifferentiation in the adult lung. Nature 2015, 528, 127–131. [Google Scholar] [CrossRef]
- Zhang, W.; Zhang, X.; Sheng, A.; Weng, C.; Zhu, T.; Zhao, W.; Li, C. Gamma-Secretase Inhibitor alleviates acute airway inflammation of allergic asthma in mice by downregulating Th17 cell differentiation. Mediat. Inflamm. 2015, 2015, 258168. [Google Scholar] [CrossRef] [Green Version]
- Zhou, M.; Cui, Z.L.; Guo, X.J.; Ren, L.P.; Yang, M.; Fan, Z.W.; Han, R.C.; Xu, W.G. Blockade of Notch signalling by Gamma-Secretase Inhibitor in lung T cells of asthmatic mice affects T cell differentiation and pulmonary inflammation. Inflammation 2015, 38, 1281–1288. [Google Scholar] [CrossRef] [PubMed]
- Ou-Yang, H.F.; Wu, C.G.; Qu, S.Y.; Li, Z.K. Notch signaling downregulates MUC5AC expression in airway epithelial cells through Hes1-dependent mechanisms. Respiration 2013, 86, 341–346. [Google Scholar] [CrossRef]
- Kang, J.H.; Kim, B.S.; Uhm, T.G.; Lee, S.H.; Lee, G.R.; Park, C.S.; Chung, I.Y. Gamma-secretase inhibitor reduces allergic pulmonary inflammation by modulating Th1 and Th2 responses. Am. J. Respir. Crit. Care Med. 2009, 179, 875–882. [Google Scholar] [CrossRef] [PubMed]
- Deford, P.; Brown, K.; Richards, R.L.; King, A.; Newburn, K.; Westover, K.; Albig, A.R. MAGP2 controls Notch via interactions with RGD binding integrins: Identification of a novel ECM-integrin-Notch signaling axis. Exp. Cell Res. 2016, 341, 84–91. [Google Scholar] [CrossRef] [Green Version]
- Leong, K.G.; Hu, X.; Li, L.; Noseda, M.; Larrivee, B.; Hull, C.; Hood, L.; Wong, F.; Karsan, A. Activated Notch4 inhibits angiogenesis: Role of beta 1-integrin activation. Mol. Cell. Biol. 2002, 22, 2830–2841. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shimizu, K.; Chiba, S.; Hosoya, N.; Kumano, K.; Saito, T.; Kurokawa, M.; Kanda, Y.; Hamada, Y.; Hirai, H. Binding of Delta1, Jagged1, and Jagged2 to Notch2 rapidly induces cleavage, nuclear translocation, and hyperphosphorylation of Notch2. Mol. Cell. Biol. 2000, 20, 6913–6922. [Google Scholar] [CrossRef] [Green Version]
- Bray, S.J. Notch signalling: A simple pathway becomes complex. Nat. Rev. Mol. Cell Biol. 2006, 7, 678–689. [Google Scholar] [CrossRef]
- Izrailit, J.; Jaiswal, A.; Zheng, W.; Moran, M.F.; Reedijk, M. Cellular stress induces TRB3/USP9x-dependent Notch activation in cancer. Oncogene 2017, 36, 1048–1057. [Google Scholar] [CrossRef]
- Li, Y.; Cheng, C.N.; Verdun, V.A.; Wingert, R.A. Zebrafish nephrogenesis is regulated by interactions between retinoic acid, mecom, and Notch signaling. Dev. Biol. 2014, 386, 111–122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shu, Y.; Wang, Y.; Lv, W.Q.; Peng, D.Y.; Li, J.; Zhang, H.; Jiang, G.J.; Yang, B.J.; Liu, S.; Zhang, J.; et al. ARRB1-Promoted NOTCH1 Degradation Is Suppressed by OncomiR miR-223 in T-cell Acute Lymphoblastic Leukemia. Cancer Res. 2020, 80, 988–998. [Google Scholar] [CrossRef]
- Baldi, A.; De Falco, M.; De Luca, L.; Cottone, G.; Paggi, M.G.; Nickoloff, B.J.; Miele, L.; De Luca, A. Characterization of tissue specific expression of Notch-1 in human tissues. Biol. Cell 2004, 96, 303–311. [Google Scholar] [CrossRef] [PubMed]
- Sade, H.; Krishna, S.; Sarin, A. The anti-apoptotic effect of Notch-1 requires p56lck-dependent, Akt/PKB-mediated signaling in T cells. J. Biol. Chem. 2004, 279, 2937–2944. [Google Scholar] [CrossRef] [Green Version]
- Gutierrez, A.; Look, A.T. NOTCH and PI3K-AKT pathways intertwined. Cancer Cell 2007, 12, 411–413. [Google Scholar] [CrossRef] [Green Version]
- Warner, S.M.B.; Hackett, T.L.; Shaheen, F.; Hallstrand, T.S.; Kicic, A.; Stick, S.M.; Knight, D.A. Transcription factor p63 regulates key genes and wound repair in human airway epithelial basal cells. Am. J. Respir. Cell Mol. 2013, 49, 978–988. [Google Scholar] [CrossRef] [Green Version]
- Liu, C.; Xiang, Y.; Liu, H.; Li, Y.; Tan, Y.; Zhu, X.; Zeng, D.; Li, M.; Zhang, L.; Qin, X. Integrin beta4 was downregulated on the airway epithelia of asthma patients. Acta Biochim. Biophys Sin. 2010, 42, 538–547. [Google Scholar] [CrossRef] [Green Version]
- Pardo-Saganta, A.; Law, B.M.; Tata, P.R.; Villoria, J.; Saez, B.; Mou, H.; Zhao, R.; Rajagopal, J. Injury induces direct lineage segregation of functionally distinct airway basal stem/progenitor cell subpopulations. Cell Stem Cell 2015, 16, 184–197. [Google Scholar] [CrossRef] [Green Version]
- Tsao, P.N.; Vasconcelos, M.; Izvolsky, K.I.; Qian, J.; Lu, J.; Cardoso, W.V. Notch signaling controls the balance of ciliated and secretory cell fates in developing airways. Development 2009, 136, 2297–2307. [Google Scholar] [CrossRef] [Green Version]
- Kaur, D.; Gomez, E.; Doe, C.; Berair, R.; Woodman, L.; Saunders, R.; Hollins, F.; Rose, F.R.; Amrani, Y.; May, R.; et al. IL-33 drives airway hyper-responsiveness through IL-13-mediated mast cell: Airway smooth muscle crosstalk. Allergy 2015, 70, 556–567. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Z.; Li, Y.; Kong, D.; Ahmad, A.; Banerjee, S.; Sarkar, F.H. Cross-talk between miRNA and Notch signaling pathways in tumor development and progression. Cancer Lett. 2010, 292, 141–148. [Google Scholar] [CrossRef] [PubMed] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iosifidis, T.; Sutanto, E.N.; Montgomery, S.T.; Agudelo-Romero, P.; Looi, K.; Ling, K.-M.; Shaw, N.C.; Garratt, L.W.; Hillas, J.; Martinovich, K.M.; et al. Dysregulated Notch Signaling in the Airway Epithelium of Children with Wheeze. J. Pers. Med. 2021, 11, 1323. https://doi.org/10.3390/jpm11121323
Iosifidis T, Sutanto EN, Montgomery ST, Agudelo-Romero P, Looi K, Ling K-M, Shaw NC, Garratt LW, Hillas J, Martinovich KM, et al. Dysregulated Notch Signaling in the Airway Epithelium of Children with Wheeze. Journal of Personalized Medicine. 2021; 11(12):1323. https://doi.org/10.3390/jpm11121323
Chicago/Turabian StyleIosifidis, Thomas, Erika N. Sutanto, Samuel T. Montgomery, Patricia Agudelo-Romero, Kevin Looi, Kak-Ming Ling, Nicole C. Shaw, Luke W. Garratt, Jessica Hillas, Kelly M. Martinovich, and et al. 2021. "Dysregulated Notch Signaling in the Airway Epithelium of Children with Wheeze" Journal of Personalized Medicine 11, no. 12: 1323. https://doi.org/10.3390/jpm11121323
APA StyleIosifidis, T., Sutanto, E. N., Montgomery, S. T., Agudelo-Romero, P., Looi, K., Ling, K.-M., Shaw, N. C., Garratt, L. W., Hillas, J., Martinovich, K. M., Kicic-Starcevich, E., Vijayasekaran, S., Lannigan, F. J., Rigby, P. J., Knight, D. A., Stick, S. M., & Kicic, A. (2021). Dysregulated Notch Signaling in the Airway Epithelium of Children with Wheeze. Journal of Personalized Medicine, 11(12), 1323. https://doi.org/10.3390/jpm11121323






