Non-Invasive Assessment of Left Ventricle Ejection Fraction: Where Do We Stand?
Abstract
:1. Introduction
- Transthoracic echocardiography (TTE);
- Cardiovascular Magnetic Resonance (CMR);
- Cardiac computed tomography (CT); and
- Nuclear cardiology imaging modalities: radionuclide multiple-gated acquisition (MUGA) ventriculography, or ECG-gated single photon emission computed tomography (SPECT).
2. Imaging Modalities for Non-Invasive Assessment of LVEF
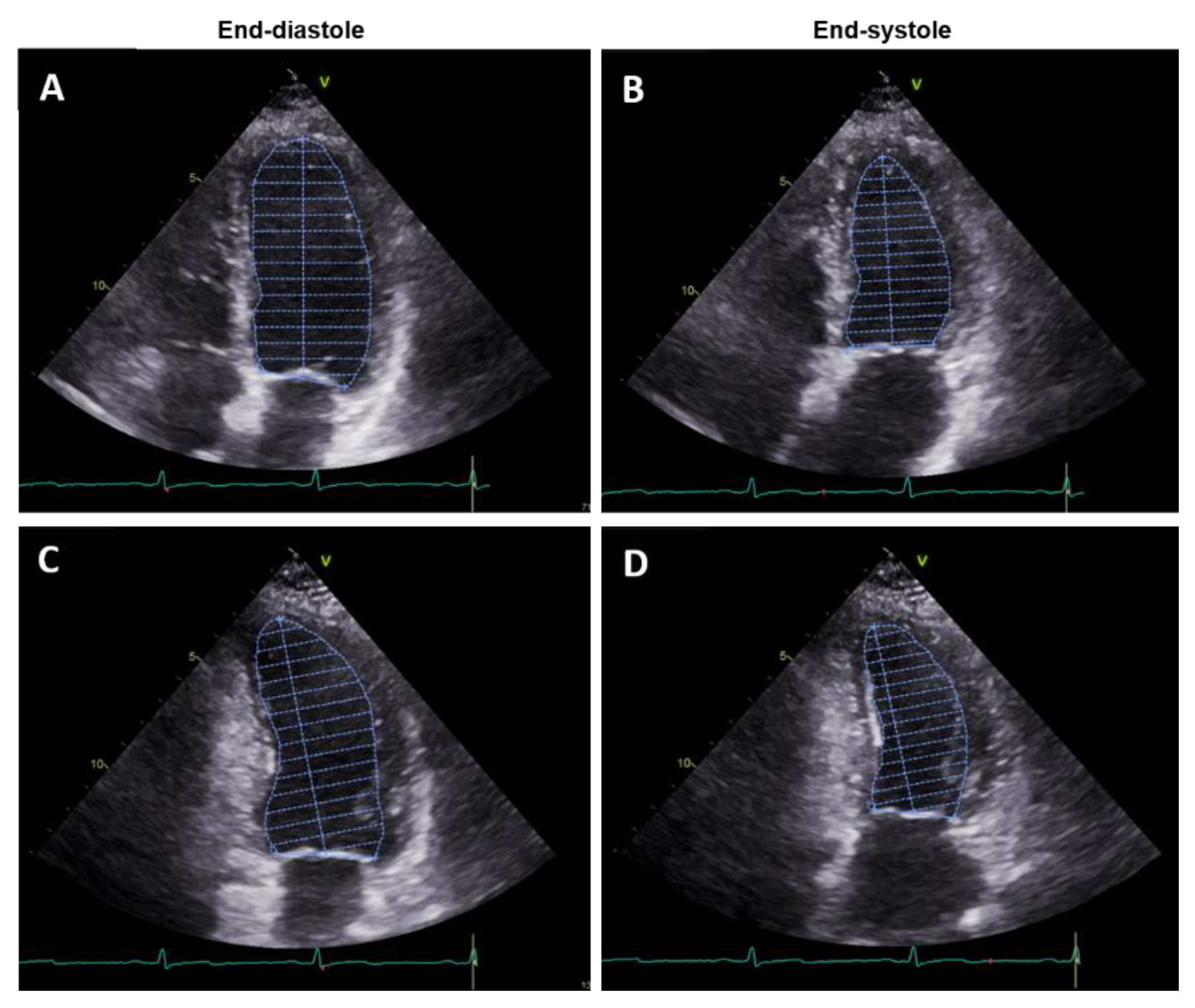
2.1. Transthoracic Echocardiography
3D-Transthoracic Echocardiography (3D TTE)
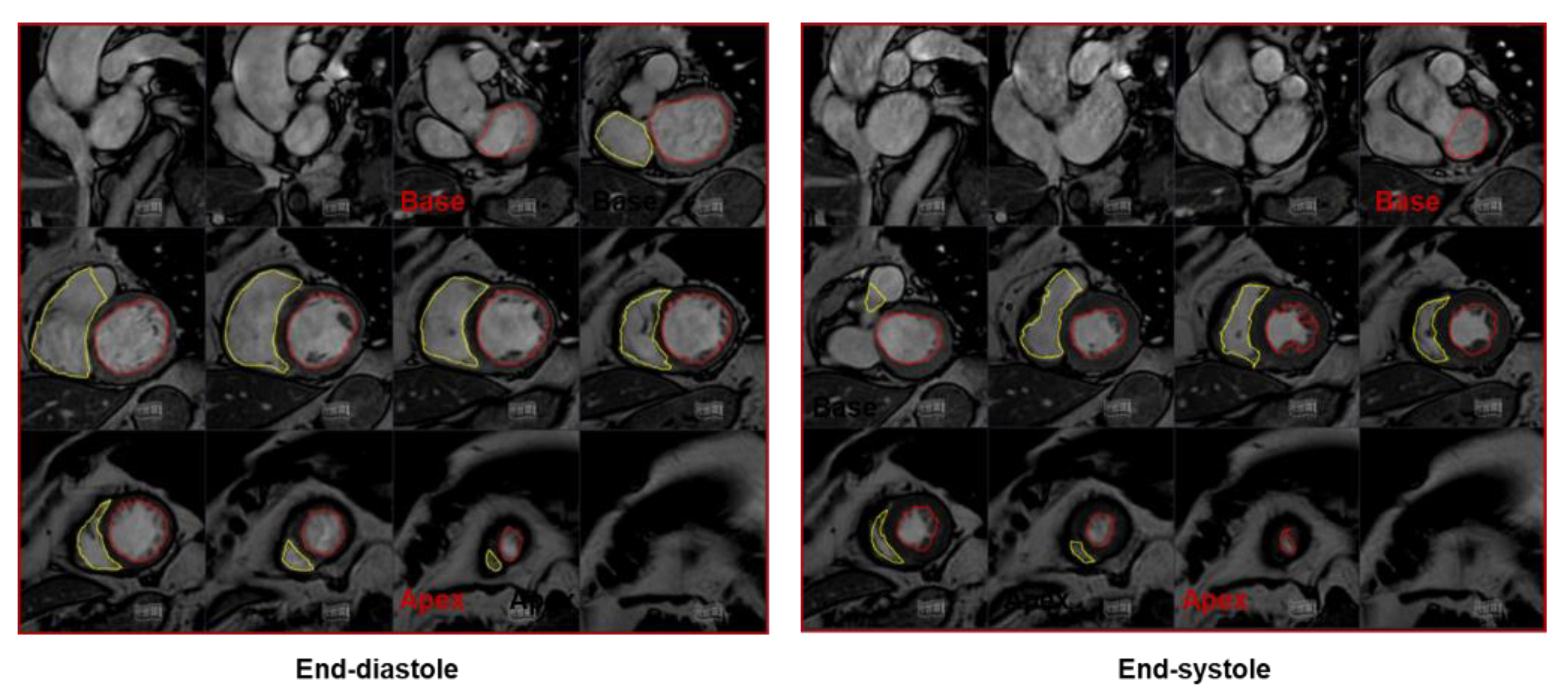
2.2. Cardiac Magnetic Resonance
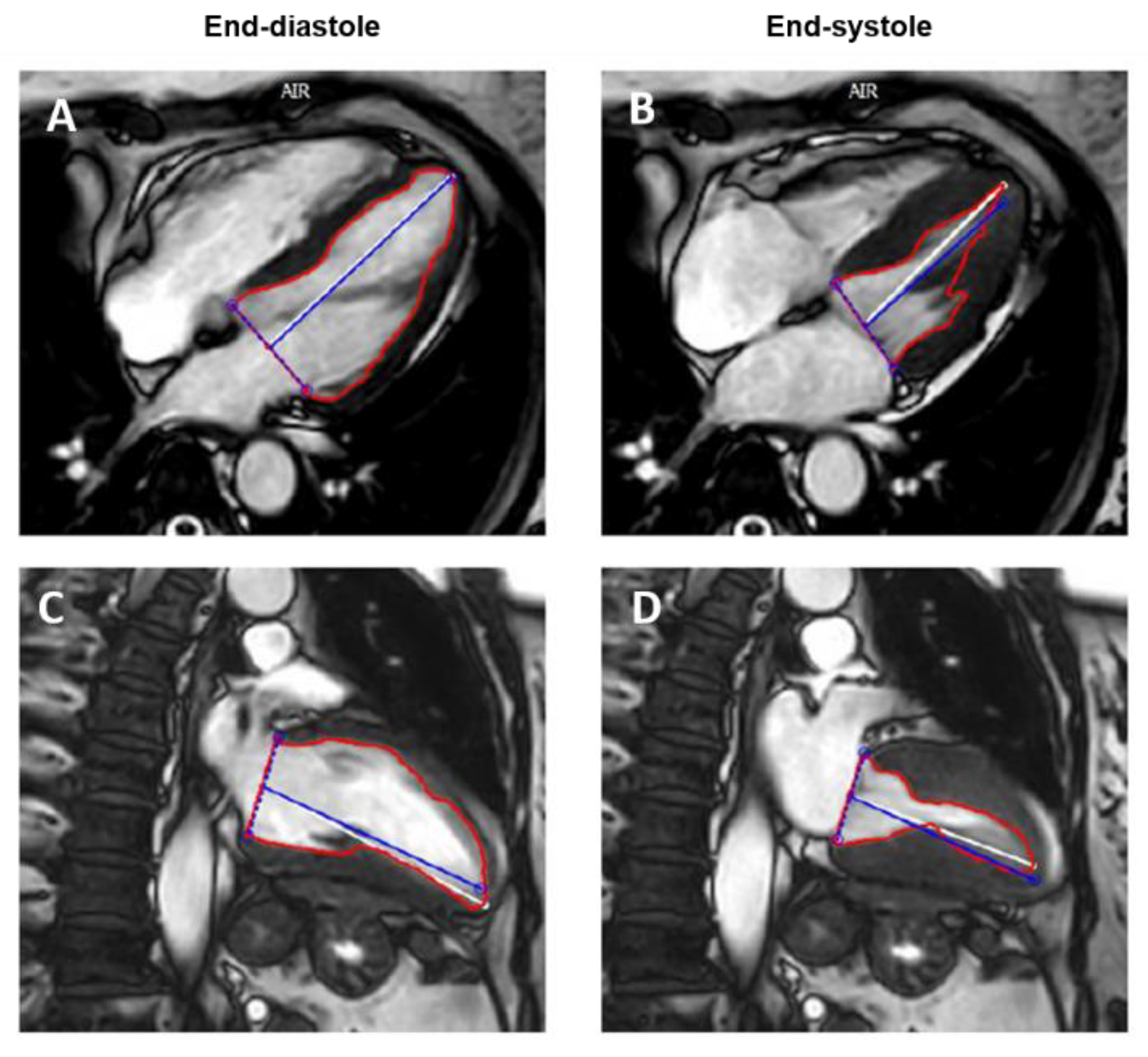
2.3. Computed Tomography
2.4. Nuclear Cardiology
3. Assessment of LV Systolic Function beyond LVEF: Myocardial Strain
4. Multimodality Imaging for LVEF Assessment
Funding
Conflicts of Interest
References
- Ponikowski, P.; Voors, A.A.; Anker, S.D.; Bueno, H.; Cleland, J.G.F.; Coats, A.J.S.; Falk, V.; González-Juanatey, J.R.; Harjola, V.P.; Jankowska, E.A.; et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur. Heart J. 2016, 37, 2129–2200. [Google Scholar] [CrossRef]
- Baumgartner, H.; Falk, V.; Bax, J.J.; De Bonis, M.; Hamm, C.; Holm, P.J.; Iung, B.; Lancellotti, P.; Lansac, E.; Muñoz, D.R.; et al. 2017 ESC/EACTS Guidelines for the management of valvular heart disease. Eur. Heart J. 2017, 38, 2739–2791. [Google Scholar] [CrossRef] [PubMed]
- Zamorano, J.L.; Lancellotti, P.; Rodriguez Muñoz, D.; Aboyans, V.; Asteggiano, R.; Galderisi, M.; Habib, G.; Lenihan, D.J.; Lip, G.Y.H.; Lyon, A.R.; et al. 2016 ESC Position Paper on cancer treatments and cardiovascular toxicity developed under the auspices of the ESC Committee for Practice Guidelines. Eur. Heart J. 2016, 37, 2768–2801. [Google Scholar] [CrossRef]
- Aimo, A.; Januzzi, J.L.; Vergaro, G.; Petersen, C.; Pasanisi, E.M.; Molinaro, S.; Passino, C.; Emdin, M. Left ventricular ejection fraction for risk stratification in chronic systolic heart failure. Int. J. Cardiol. 2018, 273, 136–140. [Google Scholar] [CrossRef]
- Halliday, B.P.; Senior, R.; Pennell, D.J. Assessing left ventricular systolic function: From ejection fraction to strain analysis. Eur. Heart J. 2021, 42, 789–797. [Google Scholar] [CrossRef] [PubMed]
- Lang, R.M.; Badano, L.P.; Mor-Avi, V.; Afilalo, J.; Armstrong, A.; Ernande, L.; Flachskampf, F.A.; Foster, E.; Goldstein, S.A.; Kuznetsova, T.; et al. Recommendations for Cardiac Chamber Quantification by Echocardiography in Adults: An Update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J. Am. Soc. Echocardiogr. 2015, 28, 1–39.e14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kosaraju, A.; Goyal, A.; Grigorova, Y.; Makaryus, A.N. Left Ventricular Ejection Fraction; StatPearls Publishing: Treasure Island, FL, USA, 2020. [Google Scholar]
- Hoffmann, R.; Barletta, G.; Von Bardeleben, S.; Vanoverschelde, J.L.; Kasprzak, J.; Greis, C.; Becher, H. Analysis of left ventricular volumes and function: A multicenter comparison of cardiac magnetic resonance imaging, cine ventriculography, and unenhanced and contrast-enhanced two-dimensional and three-dimensional echocardiography. J. Am. Soc. Echocardiogr. 2014, 27, 292–301. [Google Scholar] [CrossRef]
- Larsson, M.K.; Da Silva, C.; Gunyeli, E.; Akebat, A.; Ilami, B.; Szummer, K.; Winter, R.; Bjällmark, A. The potential clinical value of contrast- enhanced echocardiography beyond current recommendations. Cardiovasc. Ultrasound 2016, 14, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Modonesi, E.; Balbi, M.; Bezante, G.P. Limitations and potential clinical application on contrast echocardio-graphy. Curr. Cardiol. Rev. 2010, 6, 24–30. [Google Scholar] [CrossRef]
- Rosenberg, R.D.; Patil, P.V. Multimodality imaging of the left ventricle: Choosing soundly. J. Nucl. Cardiol. 2019, 26, 1865–1868. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marwick, T.H. Ejection Fraction Pros and Cons: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2018, 72, 2360–2379. [Google Scholar] [CrossRef]
- Galderisi, M.; Cosyns, B.; Edvardsen, T.; Cardim, N.; Delgado, V.; Di Salvo, G.; Donal, E.; Sade, L.E.; Ernande, L.; Garbi, M.; et al. Standardization of Adult Transthoracic Echocardiography Reporting in Agreement With Recent Chamber Quantification, Diastolic Function, and Heart Valve Disease Recommendations: An Expert Consensus Document of the European Association of Cardiovascular Imaging. Eur. Heart J. Cardiovasc. Imaging 2017, 18, 1301–1310. [Google Scholar] [PubMed] [Green Version]
- Wood, P.W.; Choy, J.B.; Nanda, N.C.; Becher, H. Left ventricular ejection fraction and volumes: It depends on the imaging method. Echocardiography 2014, 31, 87–100. [Google Scholar] [CrossRef] [Green Version]
- Grothues, F.; Smith, G.C.; Moon, J.C.C.; Bellenger, N.G.; Collins, P.; Klein, H.U.; Pennell, D.J. Comparison of Inter-study Reproducibility of Cardiovascular Magnetic Resonance With Two-Dimensional Echocardiography in Nor-mal Subjects and in Patients With Heart Failure or Left Ventricular Hypertrophy. Am J. Cardiol. 2002, 90, 29–34. [Google Scholar] [CrossRef]
- Maceira, A.M.; Prasad, S.K.; Khan, M.; Pennell, D.J. Normalized left ventricular systolic and diastolic function by steady state free precession cardiovascular magnetic resonance. J. Cardiovasc. Magn. Reson. 2006, 8, 417–426. [Google Scholar] [CrossRef] [PubMed]
- Pennell, D.J. Ventricular Volume and Mass by CMR. J. Cardiovasc. Magn. Reson. 2002, 4, 507–513. [Google Scholar] [CrossRef]
- Childs, H.; Ma, L.; Ma, M.; Clarke, J.; Cocker, M.; Green, J.; Strohm, O.; Friedrich, M.G. Comparison of long and short axis quantification of left ventricular volume parameters by cardiovascular magnetic resonance, with ex-vivo validation. J. Cardiovasc. Magn. Reson. 2011, 13, 40. [Google Scholar] [CrossRef] [Green Version]
- Bellenger, N.G.; Francis, J.M.; Davies, C.L.; Coats, A.J.; Pennell, D.J. Establishment and Performance of a Magnetic Resonance Cardiac Function Clinic. J. Cardiovasc. Magn. Reson. 2000, 2, 15–22. [Google Scholar] [CrossRef]
- Kopp, A.F.; Schroeder, S.; Kuettner, A.; Heuschmid, M.; Georg, C.; Ohnesorge, B.; Kuzo, R.; Claussen, C.D. Coronary arteries: Retrospectively ECG-gated multi-detector row CT angiography with selective optimization of the image reconstruction window. Radiology 2001, 221, 683–688. [Google Scholar] [CrossRef]
- Achenbach, S.; Giesler, T.; Ropers, D.; Ulzheimer, S.; Derlien, H.; Schulte, C.; Wenkel, E.; Moshage, W.; Bautz, W.; Daniel, W.G.; et al. Detection of coronary artery stenoses by contrast-enhanced, retrospectively electrocardiographically-gated, multislice spiral computed tomography. Circulation 2001, 103, 2535–2538. [Google Scholar] [CrossRef] [Green Version]
- Hedgire, S.; Ghoshhajra, B.; Kalra, M. Dose optimization in cardiac CT. Phys. Med. 2017, 41, 97–103. [Google Scholar] [CrossRef]
- Mahnken, A.H.; Hohl, C.; Suess, C.; Bruder, H.; Mühlenbruch, G.; Das, M.; Günther, R.W.; Wildberger, J. Influence of Heart Rate and Temporal Resolution on Left-Ventricular Volumes in Cardiac Multislice Spiral Computed Tomography. Investig. Radiol. 2006, 41, 429–435. [Google Scholar] [CrossRef]
- Mahnken, A.H.; Henzler, D.; Klotz, E.; Hennemuth, A.; Wildberger, J.E.; Günther, R.W. Determination of cardiac output with multislice spiral computed tomography: A validation study. Investig. Radiol. 2004, 39, 451–454. [Google Scholar] [CrossRef] [PubMed]
- Mahnken, A.H.; Bruder, H.; Suess, C.; Mühlenbruch, G.; Bruners, P.; Hohl, C.; Guenther, R.W.; Wildberger, J.E. Dual-source computed tomography for assessing cardiac function: A phantom study. Investig. Radiol. 2007, 42, 491–498. [Google Scholar] [CrossRef] [PubMed]
- Belge, B.; Coche, E.; Pasquet, A.; Vanoverschelde, J.L.J.; Gerber, B.L. Accurate estimation of global and regional cardiac function by retrospectively gated multidetector row computed tomography: Comparison with cine magnetic resonance imaging. Eur. Radiol. 2006, 16, 1424–1433. [Google Scholar] [CrossRef]
- Greupner, J.; Zimmermann, E.; Grohmann, A.; Dübel, H.P.; Althoff, T.; Borges, A.C.; Rutsch, W.; Schlattmann, P.; Hamm, B.; Dewey, M. Head-to-head comparison of left ventricular function assessment with 64-row computed tomography, biplane left cineventriculography, and both 2- and 3-dimensional transthoracic echocardiography: Comparison with magnetic resonance imaging as the reference standard. J. Am. Coll. Cardiol. 2012, 59, 1897–1907. [Google Scholar] [CrossRef] [Green Version]
- Cury, R.C.; Nieman, K.; Shapiro, M.D.; Butler, J.; Nomura, C.H.; Ferencik, M.; Hoffmann, U.; Abbara, S.; Jassal, D.S.; Yasuda, T.; et al. Comprehensive assessment of myocardial perfusion defects, regional wall motion, and left ventricular function by using 64-section multidetector CT. Radiology 2008, 248, 466–475. [Google Scholar] [CrossRef] [Green Version]
- Matsuo, S.; Nakajima, K.; Kinuya, S. Clinical use of nuclear cardiology in the assessment of heart failure. World J. Cardiol. 2010, 2, 344–356. [Google Scholar] [CrossRef]
- Fathala, A. Comparison of cardiac magnetic resonance with gated SPECT for evaluation of left ventricular function and volumes in patients with severe and multiple myocardial perfusion defects. J. Saudi Heart Assoc. 2010, 22, 203–208. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, H.; Nijjar, P.S.; Misialek, J.R.; Blaes, A.; Derrico, N.P.; Kazmirczak, F.; Klem, I.; Farzaneh-Far, A.; Shenoy, C. Accuracy of left ventricular ejection fraction by contemporary multiple gated acquisition scanning in patients with cancer: Comparison with cardiovascular magnetic resonance. J. Cardiovasc. Magn. Reson. 2017, 19, 34. [Google Scholar] [CrossRef] [Green Version]
- Scatteia, A.; Baritussio, A.; Bucciarelli-Ducci, C. Strain imaging using cardiac magnetic resonance. Heart Fail. Rev. 2017, 22, 465–476. [Google Scholar] [CrossRef]
- Altiok, E.; Tiemann, S.; Becker, M.; Koos, R.; Zwicker, C.; Schroeder, J.; Kraemer, N.; Schoth, F.; Adam, D.; Friedman, Z.; et al. Myocardial deformation imaging by two-dimensional speckle-tracking echocardiography for prediction of global and segmental functional changes after acute myocardial infarction: A comparison with late gadolinium enhancement cardiac magnetic resonance. J. Am. Soc. Echocardiogr. 2014, 27, 249–257. [Google Scholar] [CrossRef]
- Claus, P.; Omar, A.M.S.; Pedrizzetti, G.; Sengupta, P.P.; Nagel, E. Tissue Tracking Technology for Assessing Cardiac Mechanics: Principles, Normal Values, and Clinical Applications. JACC Cardiovasc. Imaging 2015, 8, 1444–1460. [Google Scholar] [CrossRef] [Green Version]
- Pedrizzetti, G.; Claus, P.; Kilner, P.J.; Nagel, E. Principles of cardiovascular magnetic resonance feature tracking and echocardiographic speckle tracking for informed clinical use. J. Cardiovasc. Magn. Reson. 2016, 18, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Potter, E.; Marwick, T.H. Assessment of Left Ventricular Function by Echocardiography: The Case for Routinely Adding Global Longitudinal Strain to Ejection Fraction. JACC Cardiovasc. Imaging 2018, 11, 260–274. [Google Scholar] [CrossRef]
- Cameli, M.; Mondillo, S.; Solari, M.; Righini, F.M.; Andrei, V.; Contaldi, C.; De Marco, E.; Di Mauro, M.; Esposito, R.; Gallina, S.; et al. Echocardiographic assessment of left ventricular systolic function: From ejection fraction to torsion. Heart Fail. Rev. 2016, 21, 77–94. [Google Scholar] [CrossRef] [PubMed]
- Plana, J.C.; Thavendiranathan, P.; Bucciarelli-Ducci, C.; Lancellotti, P. Multi-Modality Imaging in the Assessment of Cardiovascular Toxicity in the Cancer Patient. JACC Cardiovasc. Imaging 2018, 11, 1173–1186. [Google Scholar] [CrossRef] [PubMed]
- Čelutkienė, J.; Plymen, C.M.; Flachskampf, F.A.; De Boer, R.A.; Grapsa, J.; Manka, R.; Anderson, L.; Garbi, M.; Barberis, V.; Filardi, P.P.; et al. Innovative imaging methods in heart failure: A shifting paradigm in cardiac assessment. Position statement on behalf of the Heart Failure Association of the European Society of Cardiology. Eur. J. Heart Fail. 2018, 20, 1615–1633. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, E.; Alessio, A. What are the basic concepts of temporal, contrast, and spatial resolution in cardiac CT? J. Car-Diovasc. Comput. Tomogr. 2009, 3, 403–408. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dewey, M.; Siebes, M.; Kachelrieß, M.; Kofoed, K.F.; Maurovich-Horvat, P.; Nikolaou, K.; Bai, W.; Kofler, A.; Manka, R.; Kozerke, S.; et al. Clinical quantitative cardiac imaging for the assessment of myocardial ischaemia. Nat. Rev. Cardiol. 2020, 17, 427–450. [Google Scholar] [CrossRef] [Green Version]
- AlJaroudi, W.; Chen, J.; Jaber, W.A.; Lloyd, S.G.; Cerqueira, M.D.; Marwick, T. Nonechocardiographic imaging in evaluation for cardiac resynchronization therapy. Circ. Cardiovasc. Imaging 2011, 4, 334–343. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heusch, A.; Koch, J.A.; Krogmann, O.N.; Korbmacher, B.; Bourgeois, M. Volumetric analysis of the right and left ventricle in a porcine heart model: Comparison of three-dimensional echocardiography, magnetic resonance imaging and angiocardiography. Eur. J. Ultrasound 1999, 9, 245–255. [Google Scholar] [CrossRef]
- Bristow, M.R.; Kao, D.P.; Breathett, K.K.; Altman, N.L.; Gorcsan, J.; Gill, E.A.; Lowes, B.D.; Gilbert, E.M.; Quaife, R.A.; Mann, D.L. Structural and Functional Phenotyping of the Failing Heart: Is the Left Ventricular Ejection Fraction Obsolete? JACC Heart Fail. 2017, 5, 772–781. [Google Scholar] [CrossRef] [PubMed]
- Squeri, A.; Censi, S.; Reverberi, C.; Gaibazzi, N.; Baldelli, M.; Binno, S.M.; Properzi, E.; Bosi, S. Three-dimensional echocardiography in various types of heart disease: A comparison study of magnetic resonance imaging and 64-slice computed tomography in a real-world population. J. Echocardiogr. 2017, 15, 18–26. [Google Scholar] [CrossRef]
- Bellenger, N.G.; Burgess, M.I.; Ray, S.G.; Lahiri, A.; Coats, A.J.S.; Cleland, J.G.F.; Pennell, D.J. Comparison of left ventricular ejection fraction and volumes in heart failure by echocardiography, radionuclide ventriculography and cardiovascular magnetic resonance. Are they interchangeable? Eur. Heart J. 2000, 21, 1387–1396. [Google Scholar] [CrossRef] [Green Version]
- Knuuti, J.; Wijns, W.; Achenbach, S.; Agewall, S.; Barbato, E.; Bax, J.J.; Capodanno, D.; Cuisset, T.; Deaton, C.; Dickstein, K.; et al. 2019 ESC guidelines for the diagnosis and management of chronic coronary syndromes. Eur. Heart J. 2020, 41, 407–477. [Google Scholar] [CrossRef] [PubMed]





| Imaging Modality | Advantages | Disadvantages |
|---|---|---|
| Trans-Thoracic Echocardiography (TTE) |
|
|
| Contrast-TTE |
|
|
| 3D-TTE |
|
|
| Cardiac Magnetic Resonance (CMR) |
|
|
| Computed Tomography (CT) |
|
|
| Nuclear Cardiology Imaging |
|
|
| Imaging Technique | In-Plane Spatial Resolution (mm) | Temporal Resolution (ms) |
|---|---|---|
| TTE | 0.5–2 | 15–30 |
| CMR | 1–2 | 20–50 |
| CT | <1 | 60–165 |
| SPECT | 4–15 | 15–45 |
| Imaging Technique | Correlation Coefficient (r2) versus CMR |
|---|---|
| TTE | 0.67 |
| Contrast TTE | 0.75 |
| 3D TTE | 0.86 |
| CT | 0.52 |
| SPECT | 0.67 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Scatteia, A.; Silverio, A.; Padalino, R.; De Stefano, F.; America, R.; Cappelletti, A.M.; Dalla Vecchia, L.A.; Guarini, P.; Donatelli, F.; Caiazza, F.; et al. Non-Invasive Assessment of Left Ventricle Ejection Fraction: Where Do We Stand? J. Pers. Med. 2021, 11, 1153. https://doi.org/10.3390/jpm11111153
Scatteia A, Silverio A, Padalino R, De Stefano F, America R, Cappelletti AM, Dalla Vecchia LA, Guarini P, Donatelli F, Caiazza F, et al. Non-Invasive Assessment of Left Ventricle Ejection Fraction: Where Do We Stand? Journal of Personalized Medicine. 2021; 11(11):1153. https://doi.org/10.3390/jpm11111153
Chicago/Turabian StyleScatteia, Alessandra, Angelo Silverio, Roberto Padalino, Francesco De Stefano, Raffaella America, Alberto Maria Cappelletti, Laura Adelaide Dalla Vecchia, Pasquale Guarini, Francesco Donatelli, Francesco Caiazza, and et al. 2021. "Non-Invasive Assessment of Left Ventricle Ejection Fraction: Where Do We Stand?" Journal of Personalized Medicine 11, no. 11: 1153. https://doi.org/10.3390/jpm11111153
APA StyleScatteia, A., Silverio, A., Padalino, R., De Stefano, F., America, R., Cappelletti, A. M., Dalla Vecchia, L. A., Guarini, P., Donatelli, F., Caiazza, F., & Dellegrottaglie, S. (2021). Non-Invasive Assessment of Left Ventricle Ejection Fraction: Where Do We Stand? Journal of Personalized Medicine, 11(11), 1153. https://doi.org/10.3390/jpm11111153







