Indocyanine Green for Leakage Control in Isolated Limb Perfusion
Abstract
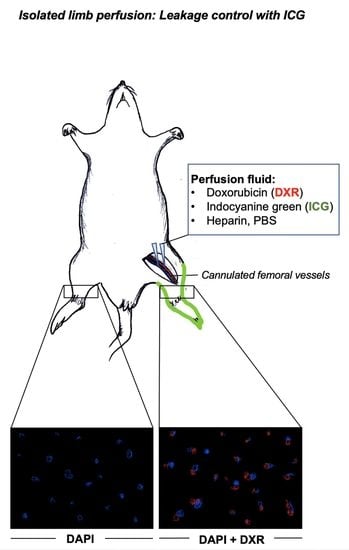
:1. Introduction
2. Materials and Methods
Fluorescence Microscopy
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vodanovich, D.A.; Choong, P.F.M. Soft-tissue Sarcomas. Indian J. Orthop. 2018, 52, 35–44. [Google Scholar] [CrossRef]
- Skubitz, K.M.; D’Adamo, D.R. Sarcoma. Mayo Clin. Proc. 2007, 82, 1409–1432. [Google Scholar] [CrossRef]
- Hoang, N.T.; Acevedo, L.A.; Mann, M.J.; Tolani, B. A review of soft-tissue sarcomas: Translation of biological advances into treatment measures. Cancer Manag. Res. 2018, 10, 1089–1114. [Google Scholar] [CrossRef] [Green Version]
- Faries, M.B.; Morton, D.L. Surgery and sentinel lymph node biopsy. Semin. Oncol. 2007, 34, 498–508. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaushal, A.; Citrin, D. The role of radiation therapy in the management of sarcomas. Surg. Clin. N. Am. 2008, 88, 629–646. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Allam, O.; Park, K.E.; Chandler, L.; Mozaffari, M.A.; Ahmad, M.; Lu, X.; Alperovich, M. The impact of radiation on lymphedema: A review of the literature. Gland Surg. 2020, 9, 596–602. [Google Scholar] [CrossRef]
- Togami, S.; Kawamura, T.; Fukuda, M.; Yanazume, S.; Kamio, M.; Kobayashi, H. Risk factors for lymphatic complications following lymphadenectomy in patients with cervical cancer. Jpn. J. Clin. Oncol. 2018, 48, 1036–1040. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, H.; Ni, J.; Huang, J. Molecular mechanisms of chemoresistance in osteosarcoma (Review). Oncol. Lett. 2014, 7, 1352–1362. [Google Scholar] [CrossRef] [Green Version]
- Eggermont, A.M.; Koops, H.S.; Klausner, J.M.; Kroon, B.B.; Schlag, P.M.; Liénard, D.; van Geel, A.N.; Hoekstra, H.J.; Meller, I.; Nieweg, O.E.; et al. Isolated limb perfusion with tumor necrosis factor and melphalan for limb salvage in 186 patients with locally advanced soft tissue extremity sarcomas. The cumulative multicenter European experience. Ann. Surg. 1996, 224, 756–764. [Google Scholar] [CrossRef]
- Moreno-Ramirez, D.; de la Cruz-Merino, L.; Ferrandiz, L.; Villegas-Portero, R.; Nieto-Garcia, A. Isolated limb perfusion for malignant melanoma: Systematic review on effectiveness and safety. Oncologist 2010, 15, 416–427. [Google Scholar] [CrossRef] [Green Version]
- Duprat Neto, J.P.; Oliveira, F.; Bertolli, E.; Molina, A.S.; Nishinari, K.; Facure, L.; Fregnani, J.H. Isolated limb perfusion with hyperthermia and chemotherapy: Predictive factors for regional toxicity. Clinics 2012, 67, 237–241. [Google Scholar] [CrossRef]
- Teras, J.; Carr, M.J.; Zager, J.S.; Kroon, H.M. Molecular Aspects of the Isolated Limb Infusion Procedure. Biomedicines 2021, 9, 163. [Google Scholar] [CrossRef]
- Kroon, H.M.; Thompson, J.F. Isolated limb infusion: A review. J. Surg. Oncol. 2009, 100, 169–177. [Google Scholar] [CrossRef]
- Brady, M.S.; Brown, K.; Patel, A.; Fisher, C.; Marx, W. A phase II trial of isolated limb infusion with melphalan and dactinomycin for regional melanoma and soft tissue sarcoma of the extremity. Ann. Surg. Oncol. 2006, 13, 1123–1129. [Google Scholar] [CrossRef] [PubMed]
- Martin-Tellez, K.S.; van Houdt, W.J.; van Coevorden, F.; Colombo, C.; Fiore, M. Isolated limb perfusion for soft tissue sarcoma: Current practices and future directions. A survey of experts and a review of literature. Cancer Treat. Rev. 2020, 88, 102058. [Google Scholar] [CrossRef]
- Sprenger, H.J.; Markwardt, J.; Schlag, P.M. Quantitative radionuclide leakage control during isolated limb perfusion. Nuklearmedizin 1994, 33, 248–253. [Google Scholar] [PubMed]
- Paulsen, I.F.; Chakera, A.H.; Schmidt, G.; Drejøe, J.; Klyver, H.; Oturai, P.S.; Hesse, B.; Drzewiecki, K.; Mortensen, J. Radionuclide leakage monitoring during hyperthermic isolated limb perfusion for treatment of local melanoma metastasis in an extremity. Clin. Physiol. Funct. Imaging 2015, 35, 301–305. [Google Scholar] [CrossRef] [PubMed]
- Rastrelli, M.; Campana, L.G.; Valpione, S.; Tropea, S.; Zanon, A.; Rossi, C.R. Hyperthermic isolated limb perfusion in locally advanced limb soft tissue sarcoma: A 24-year single-centre experience. Int. J. Hyperth. 2016, 32, 165–172. [Google Scholar] [CrossRef] [Green Version]
- Barnaby, F.; Boeker, E. Is technetium-99 (Tc-99) radiologically significant? Med. Confl. Surviv. 1999, 15, 57–70. [Google Scholar] [CrossRef]
- Santos-Oliveira, R.; Machado, M. Pitfalls with radiopharmaceuticals. Am. J. Med. Sci. 2011, 342, 50–53. [Google Scholar] [CrossRef] [PubMed]
- Johnson-Arbor, K.; Dubey, R. Doxorubicin. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2020. [Google Scholar]
- Hegazy, M.A.; Kotb, S.Z.; Sakr, H.; El Dosoky, E.; Amer, T.; Hegazi, R.A.; Farouk, O. Preoperative isolated limb infusion of Doxorubicin and external irradiation for limb-threatening soft tissue sarcomas. Ann. Surg. Oncol. 2007, 14, 568–576. [Google Scholar] [CrossRef]
- Friedmann, D.; Wunder, J.S.; Ferguson, P.; O’Sullivan, B.; Roberge, D.; Catton, C.; Freeman, C.; Saran, N.; Turcotte, R.E. Incidence and Severity of Lymphoedema following Limb Salvage of Extremity Soft Tissue Sarcoma. Sarcoma 2011, 2011, 289673. [Google Scholar] [CrossRef] [Green Version]
- Boughey, J.C.; Hoskin, T.L.; Cheville, A.L.; Miller, J.; Loprinzi, M.D.; Thomsen, K.M.; Maloney, S.; Baddour, L.M.; Degnim, A.C. Risk factors associated with breast lymphedema. Ann. Surg. Oncol. 2014, 21, 1202–1208. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- DiSipio, T.; Rye, S.; Newman, B.; Hayes, S. Incidence of unilateral arm lymphoedema after breast cancer: A systematic review and meta-analysis. Lancet Oncol. 2013, 14, 500–515. [Google Scholar] [CrossRef]
- McLaughlin, S.A.; Bagaria, S.; Gibson, T.; Arnold, M.; Diehl, N.; Crook, J.; Parker, A.; Nguyen, J. Trends in risk reduction practices for the prevention of lymphedema in the first 12 months after breast cancer surgery. J. Am. Coll. Surg. 2013, 216, 380–389. [Google Scholar] [CrossRef] [PubMed]
- Wu, P.; Elswick, S.M.; Arkhavan, A.; Molinar, V.E.; Mohan, A.T.; Curiel, D.; Sim, F.H.; Martinez-Jorge, J.; Saint-Cyr, M. Risk Factors for Lymphedema after Thigh Sarcoma Resection and Reconstruction. Plast. Reconstr. Surg. Glob. Open 2020, 8, e2912. [Google Scholar] [CrossRef]
- Tiong, S.S.; Dickie, C.; Haas, R.L.; O’Sullivan, B. The role of radiotherapy in the management of localized soft tissue sarcomas. Cancer Biol. Med. 2016, 13, 373–383. [Google Scholar] [CrossRef] [Green Version]
- Scaglioni, M.F.; Fontein, D.B.Y.; Arvanitakis, M.; Giovanoli, P. Systematic review of lymphovenous anastomosis (LVA) for the treatment of lymphedema. Microsurgery 2017, 37, 947–953. [Google Scholar] [CrossRef]
- Heise, U.; Minet-Sommer, S. The Borggreve rotation-plasty. A surgical method in therapy of malignant bone tumors and functional results. Z. Orthop. Ihre Grenzgeb. 1993, 131, 452–460. [Google Scholar] [CrossRef]
- Borggreve, J. Kniegelenkersatz durch das in der Beinlängsachse um 180 Grad gedrehte Fußgelenk. Arch. Für Orthopädische Und Unf.-Chir. 1930, 28, 175–178. [Google Scholar] [CrossRef]
- Winkelmann, W.W. Clavicula pro humero—a new surgical method for malignant tumors of the proximal humerus. Z. Orthop. Ihre Grenzgeb. 1992, 130, 197–201. [Google Scholar] [CrossRef] [PubMed]
- Askari, R.; Umer, M. Our experience with Van Nes Rotationplasty for locally advanced lower extremity tumours. JPMA J. Pak. Med. Assoc. 2014, 64, S139–S143. [Google Scholar] [PubMed]
- Lossignol, D. A little help from steroids in oncology. J. Transl. Int. Med. 2016, 4, 52–54. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Metcalf, D. The colony-stimulating factors and cancer. Nat. Rev. Cancer 2010, 10, 425–434. [Google Scholar] [CrossRef] [Green Version]
- Wray, C.J.; Benjamin, R.S.; Hunt, K.K.; Cormier, J.N.; Ross, M.I.; Feig, B.W. Isolated limb perfusion for unresectable extremity sarcoma: Results of 2 single-institution phase 2 trials. Cancer 2011, 117, 3235–3241. [Google Scholar] [CrossRef]
- Eroğlu, A.; Ozcan, H.; Eryavuz, Y.; Kocağlu, H.; Demirci, S.; Aytac, S.K. Deep venous thrombosis of the extremity diagnosed by color Doppler ultrasonography after isolated limb perfusion. Tumori 2001, 87, 187–190. [Google Scholar] [CrossRef]
- Wieberdink, J.; Benckhuysen, C.; Braat, R.P.; van Slooten, E.A.; Olthuis, G.A. Dosimetry in isolation perfusion of the limbs by assessment of perfused tissue volume and grading of toxic tissue reactions. Eur. J. Cancer Clin. Oncol. 1982, 18, 905–910. [Google Scholar] [CrossRef]
- Reinhart, M.B.; Huntington, C.R.; Blair, L.J.; Heniford, B.T.; Augenstein, V.A. Indocyanine Green: Historical Context, Current Applications, and Future Considerations. Surg. Innov. 2016, 23, 166–175. [Google Scholar] [CrossRef] [PubMed]
- Alander, J.T.; Kaartinen, I.; Laakso, A.; Pätilä, T.; Spillmann, T.; Tuchin, V.V.; Venermo, M.; Välisuo, P. A review of indocyanine green fluorescent imaging in surgery. Int. J. Biomed. Imaging 2012, 2012, 940585. [Google Scholar] [CrossRef]
- Burnier, P.; Niddam, J.; Bosc, R.; Hersant, B.; Meningaud, J.P. Indocyanine green applications in plastic surgery: A review of the literature. J. Plast. Reconstr. Aesthet. Surg. 2017, 70, 814–827. [Google Scholar] [CrossRef]
- Liu, D.Z.; Mathes, D.W.; Zenn, M.R.; Neligan, P.C. The application of indocyanine green fluorescence angiography in plastic surgery. J. Reconstr. Microsurg. 2011, 27, 355–364. [Google Scholar] [CrossRef] [PubMed]
- Ludolph, I.; Horch, R.E.; Arkudas, A.; Schmitz, M. Enhancing Safety in Reconstructive Microsurgery Using Intraoperative Indocyanine Green Angiography. Front. Surg. 2019, 6, 39. [Google Scholar] [CrossRef] [Green Version]
- Schaafsma, B.E.; Mieog, J.S.; Hutteman, M.; van der Vorst, J.R.; Kuppen, P.J.; Löwik, C.W.; Frangioni, J.V.; van de Velde, C.J.; Vahrmeijer, A.L. The clinical use of indocyanine green as a near-infrared fluorescent contrast agent for image-guided oncologic surgery. J. Surg. Oncol. 2011, 104, 323–332. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Predina, J.D.; Keating, J.; Newton, A.; Corbett, C.; Xia, L.; Shin, M.; Frenzel Sulyok, L.; Deshpande, C.; Litzky, L.; Nie, S.; et al. A clinical trial of intraoperative near-infrared imaging to assess tumor extent and identify residual disease during anterior mediastinal tumor resection. Cancer 2019, 125, 807–817. [Google Scholar] [CrossRef] [PubMed]
- Mahjoub, A.; Morales-Restrepo, A.; Fourman, M.S.; Mandell, J.B.; Feiqi, L.; Hankins, M.L.; Watters, R.J.; Weiss, K.R. Tumor Resection Guided by Intraoperative Indocyanine Green Dye Fluorescence Angiography Results in Negative Surgical Margins and Decreased Local Recurrence in an Orthotopic Mouse Model of Osteosarcoma. Ann. Surg. Oncol. 2019, 26, 894–898. [Google Scholar] [CrossRef] [Green Version]
- Kwon, J.G.; Choi, Y.J.; Kim, S.C.; Hong, J.P.; Jeong, W.S.; Oh, T.S. A technique for safe deep facial tissue dissection: Indocyanine green-assisted intraoperative real-time visualization of the vasa nervorum of facial nerve with a near-infrared camera. J. Craniomaxillofac. Surg. 2019, 47, 1819–1826. [Google Scholar] [CrossRef]
- Vahrmeijer, A.L.; Hutteman, M.; van der Vorst, J.R.; van de Velde, C.J.; Frangioni, J.V. Image-guided cancer surgery using near-infrared fluorescence. Nat. Rev. Clin. Oncol. 2013, 10, 507–518. [Google Scholar] [CrossRef] [Green Version]
- Okazaki, M.; Date, H.; Inokawa, H.; Okutani, D.; Aokage, K.; Nagahiro, I.; Aoe, M.; Sano, Y.; Shimizu, N. Optimal time for post-mortem heparinization in canine lung transplantation with non-heart-beating donors. J. Heart Lung Transpl. 2006, 25, 454–460. [Google Scholar] [CrossRef] [Green Version]
- Marchioro, T.L.; Huntley, R.T.; Waddell, W.R.; Starzl, T.E. Extracorporeal perfusion for obtaining postmortem homografts. Surgery 1963, 54, 900–911. [Google Scholar]
- Liu, H.L. Microvascular anastomosis of submillimeter vessels-a training model in rats. J. Hand Microsurg. 2013, 5, 14–17. [Google Scholar] [CrossRef] [Green Version]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zucal, I.; Geis, S.; Prantl, L.; Haerteis, S.; Aung, T. Indocyanine Green for Leakage Control in Isolated Limb Perfusion. J. Pers. Med. 2021, 11, 1152. https://doi.org/10.3390/jpm11111152
Zucal I, Geis S, Prantl L, Haerteis S, Aung T. Indocyanine Green for Leakage Control in Isolated Limb Perfusion. Journal of Personalized Medicine. 2021; 11(11):1152. https://doi.org/10.3390/jpm11111152
Chicago/Turabian StyleZucal, Isabel, Sebastian Geis, Lukas Prantl, Silke Haerteis, and Thiha Aung. 2021. "Indocyanine Green for Leakage Control in Isolated Limb Perfusion" Journal of Personalized Medicine 11, no. 11: 1152. https://doi.org/10.3390/jpm11111152
APA StyleZucal, I., Geis, S., Prantl, L., Haerteis, S., & Aung, T. (2021). Indocyanine Green for Leakage Control in Isolated Limb Perfusion. Journal of Personalized Medicine, 11(11), 1152. https://doi.org/10.3390/jpm11111152