Corticoreticular Pathway in Post-Stroke Spasticity: A Diffusion Tensor Imaging Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Population
2.2. Study Population
2.3. Data Collection and Variables
2.4. Assessment of Spasticity
2.5. MR Data Acquisition
2.6. DTI Data Processing
2.7. Statistical Analysis
3. Results
3.1. General Characteristics
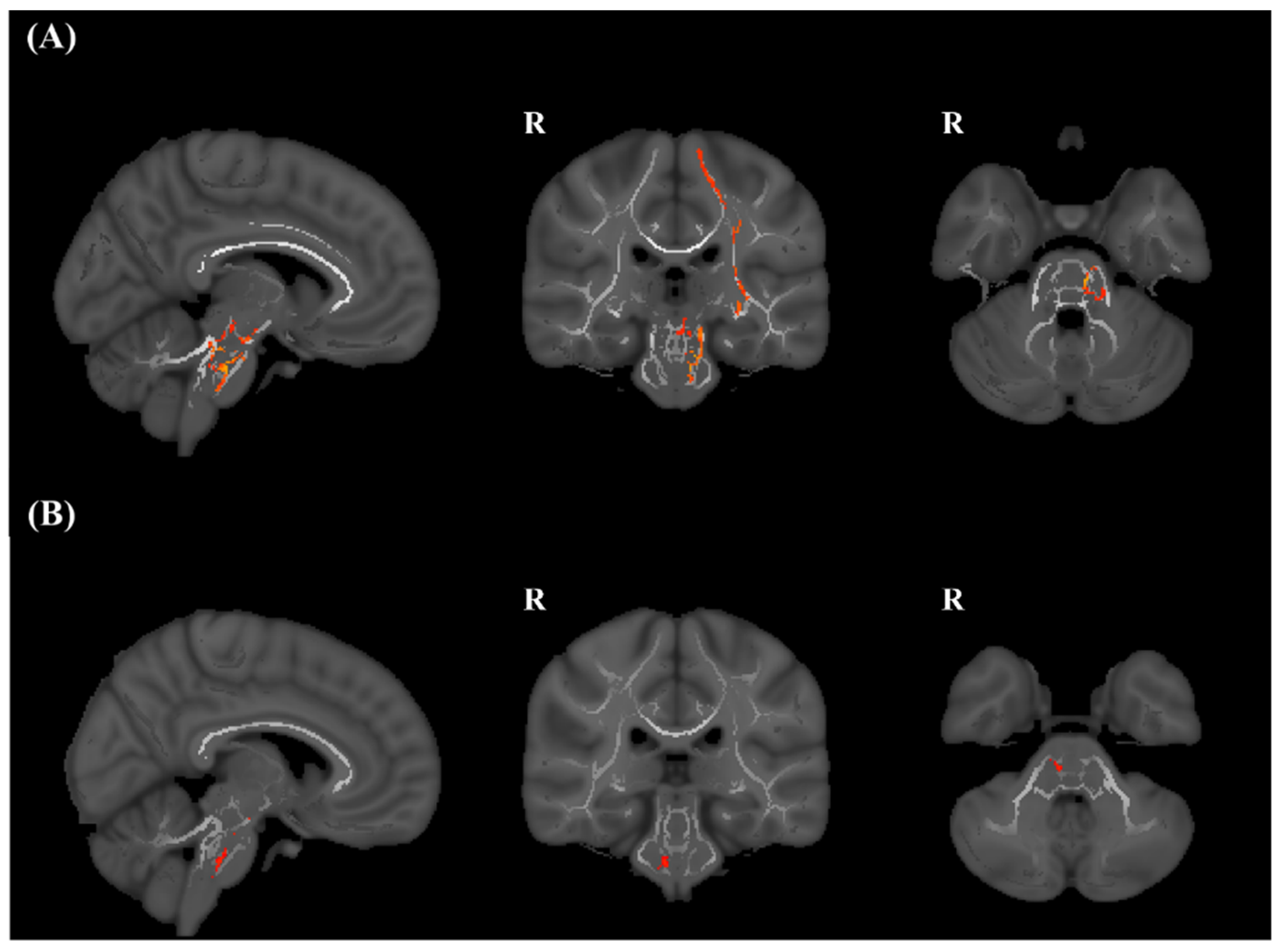
3.2. TBSS
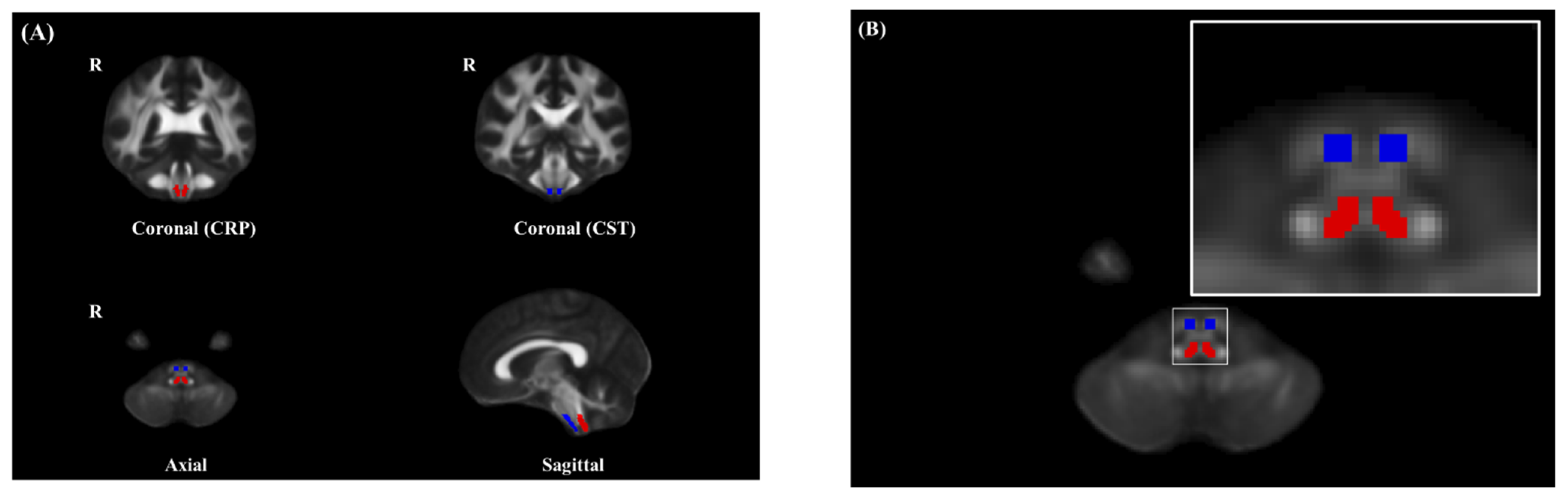
3.3. ROI Analysis of FA of the CST in the Brain Stem
3.4. ROI Analysis of FA of the CRP in the Brain Stem
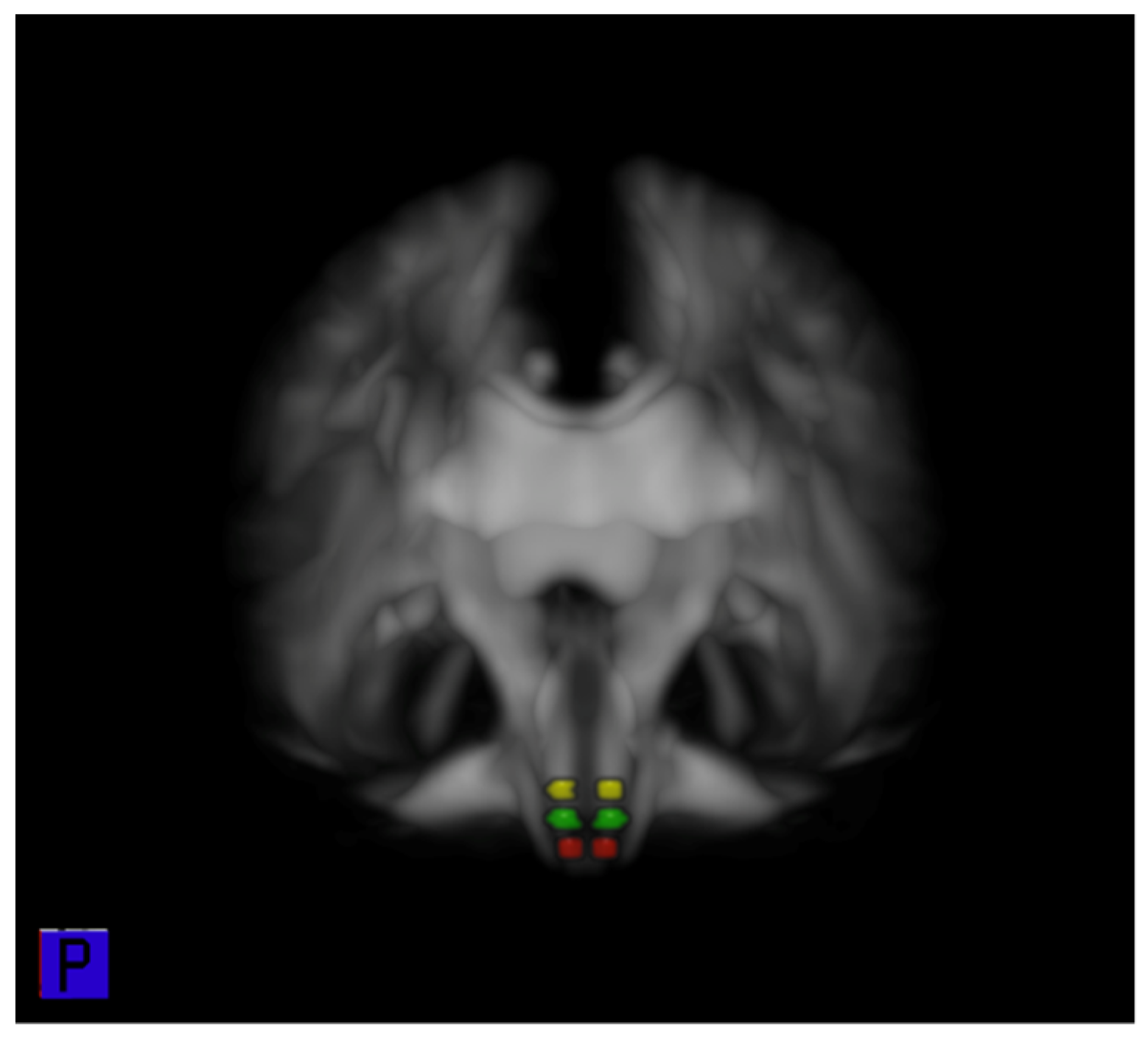
3.5. ROI Analysis of FA of the CRP in the Pons, Pontomedullary Junction, and Medulla
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Benjamin, E.J.; Muntner, P.; Alonso, A.; Bittencourt, M.S.; Callaway, C.W.; Carson, A.P.; Chamberlain, A.M.; Chang, A.R.; Cheng, S.; Das, S.R.; et al. Heart Disease and Stroke Statistics—2019 Update: A Report From the American Heart Association. Circulation 2019, 139, e56–e528. [Google Scholar] [CrossRef]
- O’Dell, M.W.; Lin, C.-C.D.; Harrison, V. Stroke Rehabilitation: Strategies to Enhance Motor Recovery. Annu. Rev. Med. 2009, 60, 55–68. [Google Scholar] [CrossRef] [PubMed]
- Lance, J. Symposium synopsis. In Spasticity: Disordered Motor Control Chicago; Felman, R.G., Young, R.R., Koella, W.P., Eds.; Year Book Medical Publishers: Maryland, MO, USA, 1980; Volume 494. [Google Scholar]
- Wissel, J.; Manack, A.; Brainin, M. Toward an epidemiology of poststroke spasticity. Neurology 2013, 80, S13–S19. [Google Scholar] [CrossRef]
- Wissel, J.; Verrier, M.; Simpson, D.M.; Charles, D.; Guinto, P.; Papapetropoulos, S.; Sunnerhagen, K.S. Post-stroke spasticity: Predictors of early development and considerations for therapeutic intervention. PM&R 2015, 7, 60–67. [Google Scholar]
- Li, S.; Francisco, G.E. New insights into the pathophysiology of post-stroke spasticity. Front. Hum. Neurosci. 2015, 9, 192. [Google Scholar] [CrossRef] [PubMed]
- Burke, D.; Knowles, L.; Andrews, C.; Ashby, P. Spasticity, decerebrate rigidity and the clasp-knife phenomenon: An experimental study in the cat. Brain 1972, 95, 31–48. [Google Scholar] [CrossRef]
- Schreiner, L.; Lindsley, D.; Magoun, H. Role of brain stem facilitatory systems in maintenance of spasticity. J. Neurophysiol. 1949, 12, 207–216. [Google Scholar] [CrossRef]
- Jang, S.H.; Seo, J.P. The distribution of the cortical origin of the corticoreticular pathway in the human brain: A diffusion tensor imaging study. Somatosens. Mot. Res. 2014, 31, 204–208. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Chen, Y.-T.; Francisco, G.E.; Zhou, P.; Rymer, W.Z. A unifying pathophysiological account for post-stroke spasticity and disordered motor control. Front. Neurol. 2019, 10, 468. [Google Scholar] [CrossRef] [PubMed]
- Moura, L.M.; Luccas, R.; de Paiva, J.P.Q.; Amaro, E.; Leemans, A.; de Leite, C.C.; Otaduy, M.C.G.; Conforto, A.B. Diffusion Tensor Imaging Biomarkers to Predict Motor Outcomes in Stroke: A Narrative Review. Front. Neurol. 2019, 10. [Google Scholar] [CrossRef] [PubMed]
- Jang, S.H.; Lee, S.J. Corticoreticular tract in the human brain: A mini-review. Front. Neurol. 2019, 10, 1188. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.; Winstein, C. Can Neurological Biomarkers of Brain Impairment Be Used to Predict Poststroke Motor Recovery? A Systematic Review. Neurorehabilit. Neural Repair 2017, 31, 3–24. [Google Scholar] [CrossRef] [PubMed]
- Stinear, C.M. Prediction of motor recovery after stroke: Advances in biomarkers. Lancet Neurol. 2017, 16, 826–836. [Google Scholar] [CrossRef]
- Yeo, S.S.; Chang, M.C.; Kwon, Y.H.; Jung, Y.J.; Jang, S.H. Corticoreticular pathway in the human brain: Diffusion tensor tractography study. Neurosci. Lett. 2012, 508, 9–12. [Google Scholar] [CrossRef] [PubMed]
- Jang, S.H.; Seo, J.P. The anatomical location of the corticoreticular pathway at the subcortical white matter in the human brain: A diffusion tensor imaging study. Somatosens. Mot. Res. 2015, 32, 106–109. [Google Scholar] [CrossRef] [PubMed]
- Jang, S.H.; Chang, C.H.; Lee, J.; Kim, C.S.; Seo, J.P.; Yeo, S.S. Functional role of the corticoreticular pathway in chronic stroke patients. Stroke 2013, 44, 1099–1104. [Google Scholar] [CrossRef]
- Jang, S.H.; Do Lee, H. Gait deterioration due to neural degeneration of the corticoreticular pathway: A case report. Neural Regen. Res. 2016, 11, 687. [Google Scholar] [PubMed]
- Lee, H.D.; Jang, S.H. Injury of the corticoreticular pathway in patients with mild traumatic brain injury: A diffusion tensor tractography study. Brain Inj. 2015, 29, 1219–1222. [Google Scholar] [PubMed]
- Jun, S.; Hong, B.; Kim, Y.; Lim, S. Does Motor Tract Integrity at 1 Month Predict Gait and Balance Outcomes at 6 Months in Stroke Patients? Brain Sci. 2021, 11, 867. [Google Scholar] [CrossRef]
- Rw, B.; Smith, M. Interrater reliability of a modified Ashworth scale of muscle spasticity. Phys. Ther. 1987, 67, 206–207. [Google Scholar]
- Gregson, J.M.; Leathley, M.J.; Moore, A.P.; Smith, T.L.; Sharma, A.K.; Watkins, C.L. Reliability of measurements of muscle tone and muscle power in stroke patients. Age Ageing 2000, 29, 223–228. [Google Scholar] [CrossRef]
- Woolsey, T.A.; Hanaway, J.; Gado, M.H. The Brain Atlas: A Visual Guide to the Human Central Nervous System; John Wiley & Sons: Hoboken, NJ, USA, 2017. [Google Scholar]
- BucY, P.C.; Keplinger, J.E.; Siqueira, E.B. Destruction of the “pyramidal tract” in man. J. Neurosurg. 1964, 21, 385–398. [Google Scholar] [CrossRef]
- Van Gijn, J. The Babinski sign and the pyramidal syndrome. J. Neurol. Neurosurg. Psychiatry 1978, 41, 865–873. [Google Scholar] [CrossRef][Green Version]
- Phillips, C.G.; Porter, R. Corticospinal neurones. Their role in movement. Monogr. Physiol. Soc. 1977, 462. Available online: https://pascal-francis.inist.fr/vibad/index.php?action=getRecordDetail&idt=PASCAL7850377830 (accessed on 9 September 2020).
- Sherman, S.J.; Koshland, G.; Laguna, J. Hyper-reflexia without spasticity after unilateral infarct of the medullary pyramid. J. Neurol. Sci. 2000, 175, 145–155. [Google Scholar] [CrossRef]
- Barlow, S.J. Identifying the brain regions associated with acute spasticity in patients diagnosed with an ischemic stroke. Somatosens. Mot. Res. 2016, 33, 104–111. [Google Scholar] [CrossRef]
- Lee, K.B.; Hong, B.Y.; Kim, J.S.; Sul, B.; Yoon, S.C.; Ji, E.-K.; Son, D.B.; Hwang, B.Y.; Lim, S.H. Which brain lesions produce spasticity? An observational study on 45 stroke patients. PLoS ONE 2019, 14, e0210038. [Google Scholar] [CrossRef]
- Picelli, A.; Tamburin, S.; Gajofatto, F.; Zanette, G.; Praitano, M.; Saltuari, L.; Corradini, C.; Smania, N. Association between Severe Upper Limb Spasticity and Brain Lesion Location in Stroke Patients. BioMed Res. Int. 2014, 2014, 162754. [Google Scholar] [CrossRef] [PubMed]
- Cheung, D.K.; Climans, S.A.; Black, S.E.; Gao, F.; Szilagyi, G.M.; Mochizuki, G. Lesion characteristics of individuals with upper limb spasticity after stroke. Neurorehabilit. Neural Repair 2016, 30, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Kwon, H.G.; Jang, S.H. Delayed gait disturbance due to injury of the corticoreticular pathway in a patient with mild traumatic brain injury. Brain Inj. 2014, 28, 511–514. [Google Scholar] [CrossRef]
- Jang, S.H.; Kwon, H.G. Delayed gait recovery with recovery of an injured corticoreticulospinal tract in a chronic hemiparetic patient: A case report. Medicine 2016, 95, e5277. [Google Scholar] [CrossRef] [PubMed]
- Jang, S.H.; Do Lee, H. Recovery of the corticoreticulospinal tract injured by a subfalcine herniation in a patient with traumatic brain injury. Am. J. Phys. Med. Rehabil. 2016, 95, e60–e61. [Google Scholar] [CrossRef] [PubMed]
| Total (n = 70) | Control (n = 41) | Spasticity (n = 29) | p | |
|---|---|---|---|---|
| Age, mean ± SD (years) | 61.6 ± 11.5 | 61.7 ± 12.3 | 61.5 ± 10.3 | 0.934 |
| Sex | 0.932 | |||
| Male, n (%) | 43 (61.4%) | 25 (61.0%) | 18 (62.1%) | |
| Female, n (%) | 27 (38.6%) | 16 (39.0%) | 11 (37.9%) | |
| Infarction side | 0.939 | |||
| Lt., n (%) | 31 (44.3%) | 18 (43.9%) | 13 (44.8%) | |
| Rt., n (%) | 39 (55.7%) | 23 (56.1%) | 16 (55.2%) | |
| Lesion, n | 0.530 | |||
| MCA | 52 (74.3%) | 29 (70.7%) | 23 (79.3%) | |
| LSA | 10 (14.3%) | 7 (17.1%) | 3 (10.3%) | |
| Multiple infarction | 8 (11.4%) | 5 (12.2%) | 3 (10.3%) | |
| NIHSS score, mean | 6.8 ± 5.6 | 5.9 ± 4.9 | 8.6 ± 6.1 | 0.032 * |
| MR DTI after onset, mean ± SD (days) | 27.1 ± 7.0 | 26.8 ± 7.9 | 27.6 ± 5.7 | 0.679 |
| MAS, n (%) | <0.001 * | |||
| 0 | 41 (58.6%) | 41 (100%) | - | |
| 1 | 10 (14.3%) | - | 10 (34.5%) | |
| 1+ | 12 (17.1%) | - | 12 (41.4%) | |
| 2 | 5 (7.1%) | - | 5 (17.2%) | |
| 3 | 2 (2.9%) | - | 2 (6.9%) | |
| 4 | 0 (0.0%) | - | 0 (0.0%) |
| ROIs of FA | Control | Spasticity | p | |
|---|---|---|---|---|
| CST | iFA | 0.371 ± 0.547 | 0.308 ± 0.529 | <0.01 * |
| cFA | 0.396 ± 0.514 | 0.390 ± 0.689 | 0.91 | |
| p | <0.01 * | <0.01 * | ||
| rFA | 0.952 ± 0.106 | 0.802 ± 0.147 | <0.01 * | |
| CRP | iFA | 0.403 ± 0.026 | 0.394 ± 0.036 | 0.27 |
| cFA | 0.412 ± 0.035 | 0.395 ± 0.032 | 0.04 * | |
| p | 0.02 * | 0.92 | ||
| rFA | 0.981 ± 0.056 | 1.000 ± 0.053 | 0.18 | |
| ROIs of FA | Control | Spasticity | p | |
|---|---|---|---|---|
| Pontine RF | iFA | 0.512 ± 0.046 | 0.501 ± 0.057 | 0.39 |
| cFA | 0.526 ± 0.057 | 0.515 ± 0.052 | 0.42 | |
| p | 0.01 * | 0.03 * | ||
| rFA | 0.978 ± 0.062 | 0.975 ± 0.063 | 0.84 | |
| Pontomedullar junction RF | iFA | 0.364 ± 0.031 | 0.354 ± 0.037 | 0.24 |
| cFA | 0.372 ± 0.036 | 0.354 ± 0.036 | 0.05 | |
| p | 0.17 | 0.98 | ||
| rFA | 0.985 ± 0.089 | 1.003 ± 0.084 | 0.39 | |
| Medullar RF | iFA | 0.346 ± 0.029 | 0.343 ± 0.045 | 0.69 |
| cFA | 0.350 ± 0.035 | 0.330 ± 0.039 | 0.03 * | |
| p | 0.33 | 0.02 * | ||
| rFA | 0.992 ± 0.073 | 1.040 ± 0.085 | 0.02 * | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ko, S.-H.; Kim, T.; Min, J.H.; Kim, M.; Ko, H.-Y.; Shin, Y.-I. Corticoreticular Pathway in Post-Stroke Spasticity: A Diffusion Tensor Imaging Study. J. Pers. Med. 2021, 11, 1151. https://doi.org/10.3390/jpm11111151
Ko S-H, Kim T, Min JH, Kim M, Ko H-Y, Shin Y-I. Corticoreticular Pathway in Post-Stroke Spasticity: A Diffusion Tensor Imaging Study. Journal of Personalized Medicine. 2021; 11(11):1151. https://doi.org/10.3390/jpm11111151
Chicago/Turabian StyleKo, Sung-Hwa, Taehyung Kim, Ji Hong Min, Musu Kim, Hyun-Yoon Ko, and Yong-Il Shin. 2021. "Corticoreticular Pathway in Post-Stroke Spasticity: A Diffusion Tensor Imaging Study" Journal of Personalized Medicine 11, no. 11: 1151. https://doi.org/10.3390/jpm11111151
APA StyleKo, S.-H., Kim, T., Min, J. H., Kim, M., Ko, H.-Y., & Shin, Y.-I. (2021). Corticoreticular Pathway in Post-Stroke Spasticity: A Diffusion Tensor Imaging Study. Journal of Personalized Medicine, 11(11), 1151. https://doi.org/10.3390/jpm11111151






