Survival Impact of Current-Smoking-Related COPD or COPD with Acute Exacerbation on Bladder Preservation through Concurrent Chemoradiotherapy for Muscle-Invasive Bladder Urothelial Carcinoma
Abstract
1. Introduction
2. Patients and Methods
2.1. Study Population
2.2. Inclusion and Exclusion Criteria
2.3. PSM and Covariates
2.4. Statistics
3. Results
3.1. PSM and Study Cohort
3.2. All-Cause Mortality, COPD Death, and Bladder Cancer Death
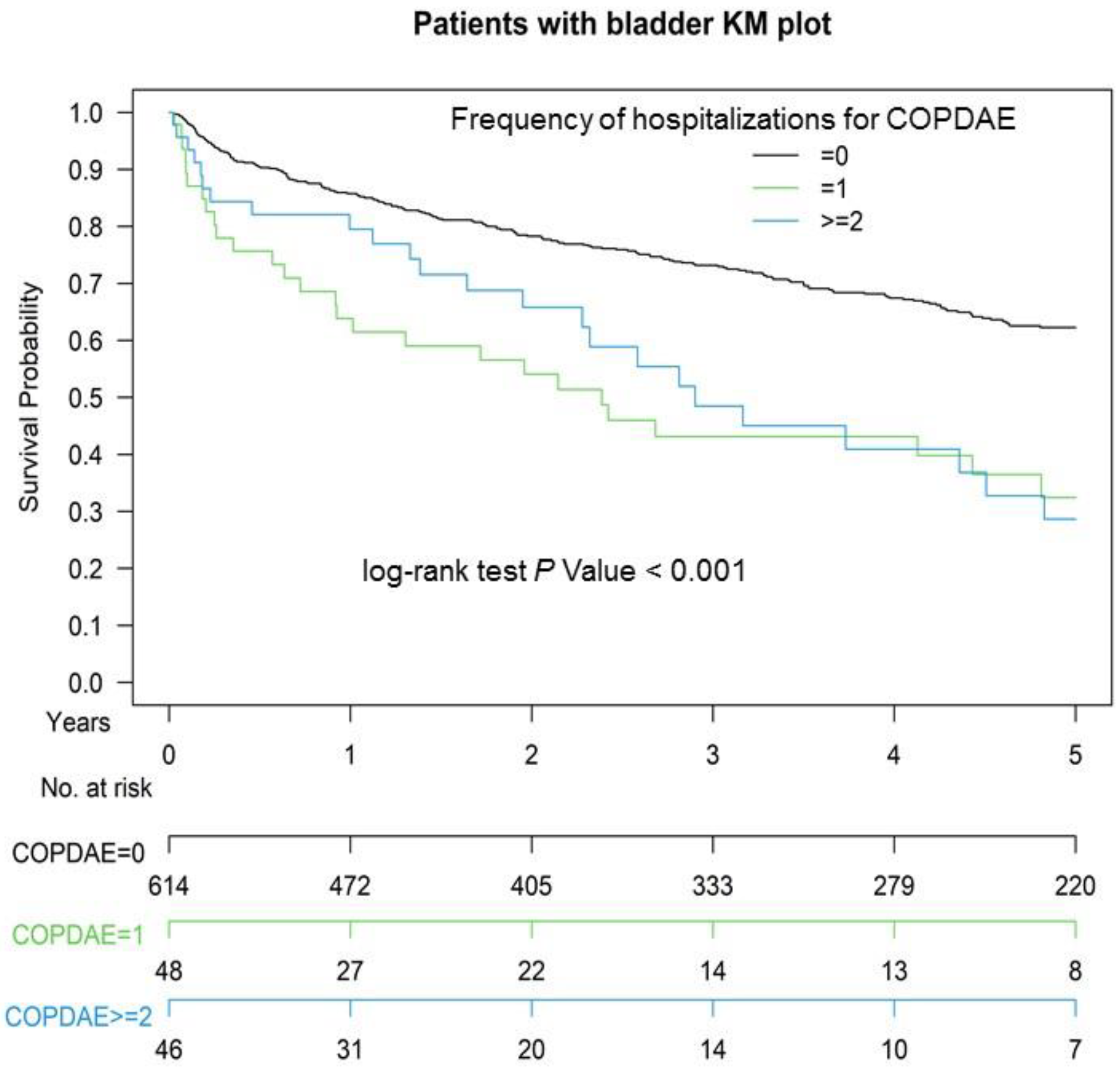
3.3. Kaplan–Meier OS among Non-COPD, COPD, and Hospitalization for COPDAE
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer Statistics, 2021. Ca Cancer J. Clin. 2021, 71, 7–33. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization; The Global Cancer Observatory. International Agency for Research on Cancer Globocan. Available online: https://gco.iarc.fr/today/data/factsheets/populations/900-world-fact-sheets.pdf (accessed on 3 December 2020).
- Taiwan Cancer Registry Annual Report. 2018. Available online: http://tcr.cph.ntu.edu.tw/main.php?Page=N2 (accessed on 29 December 2020).
- Donat, S.M.; Shabsigh, A.; Savage, C.; Cronin, A.M.; Bochner, B.; Dalbagni, G.; Herr, H.W.; Milowsky, M.I. Potential Impact of Postoperative Early Complications on the Timing of Adjuvant Chemotherapy in Patients Undergoing Radical Cystectomy: A High-Volume Tertiary Cancer Center Experience. Eur. Urol. 2009, 55, 177–186. [Google Scholar] [CrossRef] [PubMed]
- Chang, S.S.; Bochner, B.H.; Chou, R.; Dreicer, R.; Kamat, A.M.; Lerner, S.P.; Lotan, Y.; Meeks, J.J.; Michalski, J.M.; Morgan, T.M.; et al. Treatment of Non-Metastatic Muscle-Invasive Bladder Cancer: AUA/ASCO/ASTRO/SUO Guideline. J. Urol. 2017, 198, 552–559. [Google Scholar] [CrossRef] [PubMed]
- NCCN Clinical Practice Guidelines in Oncology: Prostate Cancer. Available online: https://www.nccn.org/professionals/physician_gls/pdf/prostate.pdf (accessed on 21 April 2021).
- Giacalone, N.J.; Shipley, W.U.; Clayman, R.H.; Niemierko, A.; Drumm, M.; Heney, N.M.; Michaelson, M.D.; Lee, R.J.; Saylor, P.J.; Wszolek, M.F.; et al. Long-term Outcomes After Bladder-preserving Tri-modality Therapy for Patients with Muscle-invasive Bladder Cancer: An Updated Analysis of the Massachusetts General Hospital Experience. Eur. Urol. 2017, 71, 952–960. [Google Scholar] [CrossRef] [PubMed]
- Efstathiou, J.A.; Spiegel, D.Y.; Shipley, W.U.; Heney, N.M.; Kaufman, D.S.; Niemierko, A.; Coen, J.J.; Skowronski, R.Y.; Paly, J.J.; McGovern, F.J.; et al. Long-Term Outcomes of Selective Bladder Preservation by Combined-Modality Therapy for Invasive Bladder Cancer: The MGH Experience. Eur. Urol. 2012, 61, 705–711. [Google Scholar] [CrossRef]
- Cacciamani, G.E.; Ghodoussipour, S.; Mari, A.; Gill, K.S.; Desai, M.; Artibani, W.; Gill, P.S.; Shariat, S.F.; Gill, I.S.; Djaladat, H. Association between Smoking Exposure, Neoadjuvant Chemotherapy Response and Survival Outcomes following Radical Cystectomy: Systematic Review and Meta-Analysis. J. Urol. 2020, 204, 649–660. [Google Scholar] [CrossRef] [PubMed]
- Boeri, L.; Soligo, M.; Frank, I.; Boorjian, S.A.; Thompson, R.H.; Tollefson, M.; Quevedo, F.J.; Cheville, J.C.; Karnes, R.J. Cigarette smoking is associated with adverse pathological response and increased disease recurrence amongst patients with muscle-invasive bladder cancer treated with cisplatin-based neoadjuvant chemotherapy and radical cystectomy: A single-centre experien. BJU Int. 2019, 123, 1011–1019. [Google Scholar] [CrossRef]
- Burney, P.; Patel, J.; Minelli, C.; Gnatiuc, L.; Amaral, A.F.S.; Kocabaş, A.; Cherkaski, H.H.; Gulsvik, A.; Nielsen, R.; Bateman, E.; et al. Prevalence and Population-Attributable Risk for Chronic Airflow Obstruction in a Large Multinational Study. Am. J. Respir. Crit. Care Med. 2021, 203, 1353–1365. [Google Scholar] [CrossRef]
- Tager, I.B.; Speizer, F.E. Risk estimates for chronic bronchitis in smokers: A study of male-female differences. Am. Rev. Respir. Dis. 1976, 113, 619–625. [Google Scholar] [CrossRef]
- Xu, X.; Weiss, S.T.; Rijcken, B.; Schouten, J.P. Smoking, changes in smoking habits, and rate of decline in FEV1: New insight into gender differences. Eur. Respir. J. 1994, 7, 1056–1061. [Google Scholar]
- Doll, R.; Peto, R. Mortality in relation to smoking: 20 years’ observations on male British doctors. Br. Med. J. 1976, 2, 1525–1536. [Google Scholar] [CrossRef]
- Wang, C.; Xu, J.; Yang, L.; Xu, Y.; Zhang, X.; Bai, C.; Kang, J.; Ran, P.; Shen, H.; Wen, F.; et al. Prevalence and risk factors of chronic obstructive pulmonary disease in China (the China Pulmonary Health [CPH] study): A national cross-sectional study. Lancet 2018, 391, 1706–1717. [Google Scholar] [CrossRef]
- Collaborators, T.U.B.O.D.; Mokdad, A.H.; Ballestros, K.; Echko, M.; Glenn, S.; Olsen, H.E.; Mullany, E.; Lee, A.; Khan, A.R.; Ahmadi, A.; et al. The State of US Health, 1990–2016: Burden of Diseases, Injuries, and Risk Factors among US States. JAMA 2018, 319, 1444–1472. [Google Scholar] [CrossRef] [PubMed]
- Riesco-Miranda, J.A.; Alcazar-Navarrete, B.; Carrero, J.A.T.; Campuzano, A.; Gutierrez, M.J.P.; Ferrer, J.L.L. Active smoking and COPD phenotype: Distribution and impact on prognostic factors. Int. J. Chronic Obstr. Pulm. Dis. 2017, 12, 1989–1999. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Lu, C.-Y.; Chen, H.-M.; Wu, S.-Y. Neoadjuvant Chemotherapy or Endocrine Therapy for Invasive Ductal Carcinoma of the Breast with High Hormone Receptor Positivity and Human Epidermal Growth Factor Receptor 2 Negativity. JAMA Netw. Open 2021, 4, e211785. [Google Scholar] [CrossRef] [PubMed]
- Chang, S.-C.; Hsu, C.-H.; Lin, Y.-C.; Wu, S.-Y. Effects of 1-Year Hospital Volume on Surgical Margin and Biochemical-Failure-Free Survival in Patients Undergoing Robotic versus Nonrobotic Radical Prostatectomy: A Nationwide Cohort Study from the National Taiwan Cancer Database. Cancers 2021, 13, 488. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Lu, C.-Y.; Qin, L.; Chen, H.-M.; Wu, S.-Y. Breast-conserving surgery with or without irradiation in women with invasive ductal carcinoma of the breast receiving preoperative systemic therapy: A cohort study. Breast 2020, 54, 139–147. [Google Scholar] [CrossRef]
- Liu, W.-C.; Liu, H.-E.; Kao, Y.-W.; Qin, L.; Lin, K.-C.; Fang, C.-Y.; Tsai, L.-L.; Shia, B.-C.; Wu, S.-Y. Definitive radiotherapy or surgery for early oral squamous cell carcinoma in old and very old patients: A propensity-score-matched, nationwide, population-based cohort study. Radiother. Oncol. 2020, 151, 214–221. [Google Scholar] [CrossRef]
- Lin, K.-C.; Chen, T.-M.; Yuan, K.S.-P.; Wu, A.T.H.; Wu, S.-Y. Assessment of Predictive Scoring System for 90-Day Mortality Among Patients with Locally Advanced Head and Neck Squamous Cell Carcinoma Who Have Completed Concurrent Chemoradiotherapy. JAMA Netw. Open 2020, 3, e1920671. [Google Scholar] [CrossRef]
- Charlson, M.; Szatrowski, T.P.; Peterson, J.; Gold, J. Validation of a combined comorbidity index. J. Clin. Epidemiol. 1994, 47, 1245–1251. [Google Scholar] [CrossRef]
- Chen, J.-H.; Yen, Y.-C.; Yang, H.-C.; Liu, S.-H.; Yuan, S.-P.; Wu, L.-L.; Lee, F.-P.; Lin, K.-C.; Lai, M.-T.; Wu, C.-C.; et al. Curative-Intent Aggressive Treatment Improves Survival in Elderly Patients with Locally Advanced Head and Neck Squamous Cell Carcinoma and High Comorbidity Index. Medicine 2016, 95, e3268. [Google Scholar] [CrossRef] [PubMed]
- Austin, P.C. The performance of different propensity score methods for estimating marginal hazard ratios. Stat. Med. 2012, 32, 2837–2849. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.-L.; Collins, G.S.; Spence, J.; Daurès, J.-P.; Devereaux, P.J.; Landais, P.; Le Manach, Y. Double-adjustment in propensity score matching analysis: Choosing a threshold for considering residual imbalance. BMC Med Res. Methodol. 2017, 17, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Kim, H.J.; Lonjon, G.; Zhu, Y.; written on behalf of AME Big-Data Clinical Trial Collaborative Group. Balance diagnostics after propensity score matching. Ann. Transl. Med. 2019, 7, 16. [Google Scholar] [CrossRef] [PubMed]
- Austin, P.C. The use of propensity score methods with survival or time-to-event outcomes: Reporting measures of effect similar to those used in randomized experiments. Stat. Med. 2013, 33, 1242–1258. [Google Scholar] [CrossRef] [PubMed]
- Freedman, N.D.; Silverman, D.T.; Hollenbeck, A.R.; Schatzkin, A.; Abnet, C.C. Association Between Smoking and Risk of Bladder Cancer Among Men and Women. JAMA 2011, 306, 737–745. [Google Scholar] [CrossRef]
- Cumberbatch, M.G.; Rota, M.; Catto, J.W.; La Vecchia, C. The Role of Tobacco Smoke in Bladder and Kidney Carcinogenesis: A Comparison of Exposures and Meta-analysis of Incidence and Mortality Risks. Eur. Urol. 2016, 70, 458–466. [Google Scholar] [CrossRef]
- Global Initiative for Chronic Obstructive Lung Disease. Global Strategy for the Diagnosis, Management and Prevention of Chronic Obstructive Pulmonary Disease (2018 Report); Global Initiative for Chronic Obstructive Lung Disease: Madison, WI, USA, 2018. [Google Scholar]
- Mak, R.H.; Hunt, D.; Shipley, W.U.; Efstathiou, J.A.; Tester, W.J.; Hagan, M.P.; Kaufman, D.S.; Heney, N.M.; Zietman, A.L. Long-Term Outcomes in Patients with Muscle-Invasive Bladder Cancer After Selective Bladder-Preserving Combined-Modality Therapy: A Pooled Analysis of Radiation Therapy Oncology Group Protocols 8802, 8903, 9506, 9706, 9906, and 0233. J. Clin. Oncol. 2014, 32, 3801–3809. [Google Scholar] [CrossRef]
- James, N.D.; Hussain, S.; Hall, E.; Jenkins, P.; Tremlett, J.; Rawlings, C.; Crundwell, M.; Sizer, B.; Sreenivasan, T.; Hendron, C.; et al. Radiotherapy with or without Chemotherapy in Muscle-Invasive Bladder Cancer. N. Engl. J. Med. 2012, 366, 1477–1488. [Google Scholar] [CrossRef]
- Rödel, C.; Grabenbauer, G.G.; Kühn, R.; Papadopoulos, T.; Dunst, J.; Meyer, M.; Schrott, K.M.; Sauer, R. Combined-Modality Treatment and Selective Organ Preservation in Invasive Bladder Cancer: Long-Term Results. J. Clin. Oncol. 2002, 20, 3061–3071. [Google Scholar] [CrossRef]
- Ploussard, G.; Daneshmand, S.; Efstathiou, J.A.; Herr, H.W.; James, N.D.; Rödel, C.M.; Shariat, S.F.; Shipley, W.U.; Sternberg, C.N.; Thalmann, G.N.; et al. Critical Analysis of Bladder Sparing with Trimodal Therapy in Muscle-invasive Bladder Cancer: A Systematic Review. Eur. Urol. 2014, 66, 120–137. [Google Scholar] [CrossRef]
- Nishioka, T.; Luo, L.-Y.; Shen, L.; He, H.; Mariyannis, A.; Dai, W.; Chen, C. Nicotine increases the resistance of lung cancer cells to cisplatin through enhancing Bcl-2 stability. Br. J. Cancer 2014, 110, 1785–1792. [Google Scholar] [CrossRef]
- Chang, X.; Ravi, R.; Pham, V.; Bedi, A.; Chatterjee, A.; Sidransky, D. Adenylate Kinase 3 Sensitizes Cells to Cigarette Smoke Condensate Vapor Induced Cisplatin Resistance. PLoS ONE 2011, 6, e20806. [Google Scholar] [CrossRef] [PubMed]
- Simon, F.; Schwenk-Zieger, S.; Becker, S.; Unger, K.; Gires, O.; Baumeister, P. Cigarette Smoke Reduces the Efficacy of Cisplatin in Head and Neck Cancer Cells–Role of ABCG2. Anticancer. Res. 2020, 40, 1277–1284. [Google Scholar] [CrossRef]
- Bachir, B.G.; Kassouf, W. Cause–effect? Understanding the risk factors associated with bladder cancer. Expert Rev. Anticancer Ther. 2012, 12, 1499–1502. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kowalkowski, M.A.; Goltz, H.H.; Petersen, N.J.; Amiel, G.E.; Lerner, S.P.; Latini, D.M. Educational opportunities in bladder cancer: Increasing cystoscopic adherence and the availability of smoking-cessation programs. J. Cancer Educ. 2014, 29, 739–745. [Google Scholar] [CrossRef][Green Version]
- Kartalou, M.; Essigmann, J.M. Mechanisms of resistance to cisplatin. Mutat. Res. Mol. Mech. Mutagen. 2001, 478, 23–43. [Google Scholar] [CrossRef]
- Kim, P.H.; Kent, M.; Zhao, P.; Sfakianos, J.P.; Bajorin, D.F.; Bochner, B.H.; Dalbagni, G. The impact of smoking on pathologic response to neoadjuvant cisplatin-based chemotherapy in patients with muscle-invasive bladder cancer. World J. Urol. 2013, 32, 453–459. [Google Scholar] [CrossRef]
- Gao, Y.; Guan, W.; Liu, Q.; Wang, H.; Zhu, Y.; Chen, R.; Zhang, G. Impact of COPD and emphysema on survival of patients with lung cancer: A meta-analysis of observational studies. Respirology 2015, 21, 269–279. [Google Scholar] [CrossRef]
- Saji, H.; Miyazawa, T.; Sakai, H.; Kimura, Y.; Tsuda, M.; Wakiyama, Y.; Marushima, H.; Kojima, K.; Nakamura, H. Survival significance of coexisting chronic obstructive pulmonary disease in patients with early lung cancer after curative surgery. Thorac. Cancer 2017, 9, 19–24. [Google Scholar] [CrossRef]
- Chiu, K.-C.; Lin, W.-C.; Chang, C.-L.; Wu, S.-Y. Impact of Chronic Obstruction Pulmonary Disease on Survival in Patients with Advanced Stage Lung Squamous Cell Carcinoma Undergoing Concurrent Chemoradiotherapy. Cancers 2021, 13, 3231. [Google Scholar] [CrossRef] [PubMed]
- Van Gestel, Y.R.B.M.; E Hoeks, S.; Sin, D.D.; Huzeir, V.; Stam, H.; Mertens, F.W.; Van Domburg, R.T.; Bax, J.J.; Poldermans, D. COPD and cancer mortality: The influence of statins. Thorax 2009, 64, 963–967. [Google Scholar] [CrossRef][Green Version]
- Man, S.F.P.; Connett, J.E.; Anthonisen, N.R.; Wise, R.A.; Tashkin, D.F.; Sin, D.D. C-reactive protein and mortality in mild to moderate chronic obstructive pulmonary disease. Thorax 2006, 61, 849–853. [Google Scholar] [CrossRef] [PubMed]
- Danesh, J.; Lewington, S.; Thompson, S.G.; Lowe, G.D.; Kosatis, J.B.; Wilson, A.C.; Folsom, A.R.; Wu, K.; Collins, R. Plasma Fibrinogen Level and the Risk of Major Cardiovascular Diseases and Nonvascular Mortality. JAMA Fibrinogen Studies Collaboration. 2005, 294, 1799–1809. [Google Scholar] [CrossRef] [PubMed]
- Postma, D.S.; Bush, A.; Berge, M.V.D. Risk factors and early origins of chronic obstructive pulmonary disease. Lancet 2014, 385, 899–909. [Google Scholar] [CrossRef]
- Tan, W.C.; Sin, D.D.; Bourbeau, J.; Hernandez, P.; Chapman, K.R.; Cowie, R.; FitzGerald, J.M.; Marciniuk, D.D.; Maltais, F.; Buist, A.S.; et al. Characteristics of COPD in never-smokers and ever-smokers in the general population: Results from the CanCOLD study. Thorax 2015, 70, 822–829. [Google Scholar] [CrossRef] [PubMed]
- Mannino, D.M.; Buist, A.S.; Petty, T.L.; Enright, P.L.; Redd, S.C. Lung function and mortality in the United States: Data from the First National Health and Nutrition Examination Survey follow up study. Thorax 2003, 58, 388–393. [Google Scholar] [CrossRef] [PubMed]
- Divo, M.; Cote, C.; de Torres, J.P.; Casanova, C.; Marin, J.M.; Pinto-Plata, V.; Zulueta, J.J.; Cabrera, C.; Zagaceta, J.; Hunninghake, G.; et al. Comorbidities and Risk of Mortality in Patients with Chronic Obstructive Pulmonary Disease. Am. J. Respir. Crit. Care Med. 2012, 186, 155–161. [Google Scholar] [CrossRef] [PubMed]
- Nussbaumer-Ochsner, Y.; Rabe, K.F. Systemic Manifestations of COPD. Chest 2011, 139, 165–173. [Google Scholar] [CrossRef]
- Huber, M.B.; Wacker, M.E.; Vogelmeier, C.F.; Leidl, R. Comorbid Influences on Generic Health-Related Quality of Life in COPD: A Systematic Review. PLoS ONE 2015, 10, e0132670. [Google Scholar] [CrossRef]
- Thieblemont, C.; Fendler, J.P.; Trillet-Lenoir, V.; Petris, C.; Chauvin, F.; Brunat-Mentigny, M.; Devaux, Y.; Devonec, M.; Gérard, J.P.; Perrin, P. Prognostic factors of survival in infiltrating urothelial bladder carcinoma. A retrospective study of 158 patients treated by radical cystectomy. Bull Cancer 1996, 83, 139–146. [Google Scholar] [PubMed]
- Pollack, A.; Zagars, G.K.; Swanson, D.A. Muscle-invasive bladder cancer treated with external beam radiotherapy: Prognostic factors. Int. J. Radiat. Oncol. 1994, 30, 267–277. [Google Scholar] [CrossRef]
- Fung, C.Y.; Shipley, W.U.; Young, R.H.; Griffin, P.P.; Convery, K.M.; Kaufman, D.S.; Althausen, A.F.; Heney, N.M.; Prout, G.R. Prognostic factors in invasive bladder carcinoma in a prospective trial of preoperative adjuvant chemotherapy and radiotherapy. J. Clin. Oncol. 1991, 9, 1533–1542. [Google Scholar] [CrossRef]
- Chiang, Y.; Cheng, J.C.-H.; Huang, C.-Y.; Tsai, Y.-C.; Lin, C.-C.; Hsu, C.-H.; Cheng, A.-L.; Pu, Y.-S. A role of multimodality bladder-preserving therapy in patients with muscle-invasive bladder cancer plus hydronephrosis with or without pelvic nodal involvement. J. Formos. Med Assoc. 2016, 116, 689–696. [Google Scholar] [CrossRef]
- Shipley, W.U.; A Winter, K.; Kaufman, D.S.; Lee, W.R.; Heney, N.M.; Tester, W.R.; Donnelly, B.J.; Venner, P.M.; A Perez, C.; Murray, K.J.; et al. Phase III trial of neoadjuvant chemotherapy in patients with invasive bladder cancer treated with selective bladder preservation by combined radiation therapy and chemotherapy: Initial results of Radiation Therapy Oncology Group 89-03. J. Clin. Oncol. 1998, 16, 3576–3583. [Google Scholar] [CrossRef]
- Byun, S.; Kim, J.H.; Oh, Y.K.; Kim, B.H. Concurrent chemoradiotherapy improves survival outcome in muscle-invasive bladder cancer. Radiat. Oncol. J. 2015, 33, 294–300. [Google Scholar] [CrossRef][Green Version]
- Browman, G.P.; Wong, G.; Hodson, I.; Sathya, J.; Russell, R.; McAlpine, L.; Skingley, P.; Levine, M.N. Influence of Cigarette Smoking on the Efficacy of Radiation Therapy in Head and Neck Cancer. N. Engl. J. Med. 1993, 328, 159–163. [Google Scholar] [CrossRef] [PubMed]
- Tammemagi, C.M.; Neslund-Dudas, C.; Simoff, M.; Kvale, P. Smoking and Lung Cancer Survival: The role of comorbidity and treatment. Chest 2004, 125, 27–37. [Google Scholar] [CrossRef] [PubMed]

| Never Smokers without COPD | Current Smokers with COPD | p | |||
|---|---|---|---|---|---|
| N = 472 | (100%) | N = 236 | (100%) | ||
| Age (mean ± SD) | (76.14 ± 8.22) | (76.22 ± 9.63) | 0.844 | ||
| Age (years) | 1.000 | ||||
| ≤65 | 58 | 12.29% | 29 | 12.29% | |
| 66–74 | 146 | 30.93% | 73 | 30.93% | |
| 75–85 | 178 | 37.71% | 89 | 37.71% | |
| >85 | 90 | 19.07% | 45 | 19.07% | |
| Sex | 1.000 | ||||
| Female | 116 | 24.58% | 58 | 24.58% | |
| Male | 356 | 75.42% | 178 | 75.42% | |
| Diabetes | 0.515 | ||||
| No | 321 | 68.01% | 154 | 65.25% | |
| Yes | 151 | 31.99% | 82 | 34.75% | |
| Hyperlipidemia | 0.796 | ||||
| No | 324 | 68.64% | 165 | 69.92% | |
| Yes | 148 | 31.36% | 71 | 30.08% | |
| Hypertension | 0.795 | ||||
| No | 330 | 69.92% | 162 | 68.64% | |
| Yes | 142 | 30.08% | 74 | 31.36% | |
| AMI | 1.000 | ||||
| No | 446 | 94.49% | 223 | 94.49% | |
| Yes | 26 | 5.51% | 13 | 5.51% | |
| Cardiovascular diseases | 0.453 | ||||
| No | 398 | 84.32% | 193 | 81.78% | |
| Yes | 74 | 15.68% | 43 | 18.22% | |
| Ischemic stroke | 0.363 | ||||
| No | 415 | 87.92% | 201 | 85.17% | |
| Yes | 57 | 12.08% | 35 | 14.83% | |
| Kidney or bladder stones | 0.202 | ||||
| No | 349 | 73.94% | 163 | 69.07% | |
| Yes | 123 | 26.06% | 73 | 30.93% | |
| CCI score | 0.952 | ||||
| 0 | 228 | 48.31% | 116 | 49.15% | |
| ≥1 | 244 | 51.69% | 120 | 50.85% | |
| AJCC clinical tumor stage | 1.000 | ||||
| cT2a | 114 | 24.15% | 57 | 24.15% | |
| cT2b | 118 | 25.00% | 59 | 25.00% | |
| cT3 | 166 | 35.17% | 83 | 35.17% | |
| cT4 | 74 | 15.68% | 37 | 15.68% | |
| AJCC clinical nodal stage | 1.000 | ||||
| cN0 | 292 | 61.86% | 146 | 61.86% | |
| cN1 | 136 | 28.81% | 68 | 28.81% | |
| cN2 | 44 | 9.32% | 22 | 9.32% | |
| Surgical consolidation after CCRT | 1.000 | ||||
| No | 354 | 75.00% | 177 | 75.00% | |
| Yes | 118 | 25.00% | 59 | 25.00% | |
| Bladder preservation rate | 1.000 | ||||
| No | 164 | 34.75% | 82 | 34.75% | |
| Yes | 308 | 65.25% | 154 | 65.25% | |
| Cisplatin-based regimen (cumulative total dose of cisplatin, mg/m2) | 0.631 | ||||
| Median (Q1, Q3) | 211.23 | (206.43–276.21) | 213.54 | (210.12–281.52) | |
| Radiotherapy (total dose, Gy) | 1.000 | ||||
| Median (Q1, Q3) | 63.00 | (61.20–64.80) | 63.00 | (61.20–64.80) | |
| Hospitalization frequency for COPDAE (within 1 year before CCRT) | <0.001 | ||||
| 0 | 472 | 100.00% | 142 | 60.17% | |
| 1 | 0 | 0.00% | 48 | 20.34% | |
| ≥2 | 0 | 0.00% | 46 | 19.49% | |
| Follow-up time Years (mean ± SD) | (5.71 ± 2.27) | (4.36 ± 2.19) | <0.001 | ||
| COPD death | <0.001 | ||||
| Yes | 0 | 0% | 7 | 2.97% | |
| Bladder cancer death | <0.001 | ||||
| Yes | 207 | 43.86% | 133 | 56.36% | |
| All-cause death | <0.001 | ||||
| Yes | 269 | 56.99% | 177 | 75.00% | |
| Crude HR (95% CI) | Adjusted HR * (95% CI) | p | |||
|---|---|---|---|---|---|
| COPD status (ref. non-COPD) | |||||
| COPD | 2.18 | (1.30–3.64) | 1.89 | (1.12–3.18) | 0.017 |
| Hospitalization frequency for COPDAE before CCRT (ref. = 0) | |||||
| 1 | 3.64 | (2.20–6.03) | 3.26 | (1.95–5.46) | <0.001 |
| ≥2 | 7.93 | (4.55–13.79) | 6.33 | (3.55–11.28) | <0.001 |
| Sex (ref. Female) | |||||
| Male | 0.85 | (0.67–1.09) | 1.02 | (0.78–1.34) | 0.865 |
| Age (years; ref. ≤65 years) | |||||
| 66–74 | 1.24 | (0.97–1.57) | 1.02 | (0.77–1.33) | 0.910 |
| 75–85 | 2.45 | (0.76–3.59) | 1.26 | (0.80–1.98) | 0.313 |
| >85 | 2.70 | (0.70–4.28) | 1.28 | (0.76–2.15) | 0.355 |
| CCI score (ref. = 0) | |||||
| ≥1 | 1.58 | (0.86–3.23) | 1.26 | (0.87–2.62) | 0.821 |
| Diabetes (ref.: No) | |||||
| Yes | 1.25 | (0.90–1.73) | 1.19 | (0.87–1.61) | 0.317 |
| Hyperlipidemia (ref.: No) | |||||
| Yes | 1.19 | (0.93–1.54) | 1.07 | (0.81–1.42) | 0.630 |
| Hypertension (ref.: No) | |||||
| Yes | 1.06 | (0.83–1.36) | 1.09 | (0.84–1.43) | 0.520 |
| AMI (ref.: No) | |||||
| Yes | 1.28 | (0.96–1.73) | 1.18 | (0.92–1.44) | 0.740 |
| Cardiovascular diseases (ref.: No) | |||||
| Yes | 1.06 | (0.79–1.42) | 0.96 | (0.85–1.28) | 0.441 |
| Ischemic stroke (ref.: No) | |||||
| Yes | 1.26 | (0.81–1.96) | 1.21 | (0.88–1.33) | 0.463 |
| Kidney or bladder stones (ref.: No) | |||||
| Yes | 1.11 | (0.86–1.43) | 0.94 | (0.72–1.22) | 0.643 |
| AJCC clinical tumor stages (ref. cT2a) | |||||
| cT2b | 1.92 | (0.72–5.16) | 1.31 | (0.47–3.63) | 0.602 |
| cT3 | 1.96 | (0.92–4.17) | 1.39 | (0.64–3.05) | 0.405 |
| cT4 | 2.12 | (0.98–4.59) | 1.13 | (0.50–2.53) | 0.767 |
| AJCC clinical nodal stages (ref. cN0) | |||||
| cN1 | 1.11 | (0.86–1.43) | 0.93 | (0.72–1.21) | 0.597 |
| cN2 | 1.06 | (0.83–1.36) | 1.08 | (0.83–1.41) | 0.568 |
| Surgical consolidation after CCRT (ref.: No) | |||||
| Yes | 1.06 | (0.64–1.14) | 1.07 | (0.60–1.11) | 0.191 |
| Bladder preservation (ref.: No) | |||||
| Yes | 0.86 | (0.68–1.09) | 0.92 | (0.79–1.03) | 0.255 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, J.; Chang, S.-C.; Chiang, M.-F.; Chiu, K.-C.; Wu, S.-Y. Survival Impact of Current-Smoking-Related COPD or COPD with Acute Exacerbation on Bladder Preservation through Concurrent Chemoradiotherapy for Muscle-Invasive Bladder Urothelial Carcinoma. J. Pers. Med. 2021, 11, 958. https://doi.org/10.3390/jpm11100958
Zhang J, Chang S-C, Chiang M-F, Chiu K-C, Wu S-Y. Survival Impact of Current-Smoking-Related COPD or COPD with Acute Exacerbation on Bladder Preservation through Concurrent Chemoradiotherapy for Muscle-Invasive Bladder Urothelial Carcinoma. Journal of Personalized Medicine. 2021; 11(10):958. https://doi.org/10.3390/jpm11100958
Chicago/Turabian StyleZhang, Jiaqiang, Shyh-Chyi Chang, Ming-Feng Chiang, Kuo-Chin Chiu, and Szu-Yuan Wu. 2021. "Survival Impact of Current-Smoking-Related COPD or COPD with Acute Exacerbation on Bladder Preservation through Concurrent Chemoradiotherapy for Muscle-Invasive Bladder Urothelial Carcinoma" Journal of Personalized Medicine 11, no. 10: 958. https://doi.org/10.3390/jpm11100958
APA StyleZhang, J., Chang, S.-C., Chiang, M.-F., Chiu, K.-C., & Wu, S.-Y. (2021). Survival Impact of Current-Smoking-Related COPD or COPD with Acute Exacerbation on Bladder Preservation through Concurrent Chemoradiotherapy for Muscle-Invasive Bladder Urothelial Carcinoma. Journal of Personalized Medicine, 11(10), 958. https://doi.org/10.3390/jpm11100958






