KRT20, KRT5, ESR1 and ERBB2 Expression Can Predict Pathologic Outcome in Patients Undergoing Neoadjuvant Chemotherapy and Radical Cystectomy for Muscle-Invasive Bladder Cancer
Abstract
1. Introduction
2. Materials and Methods
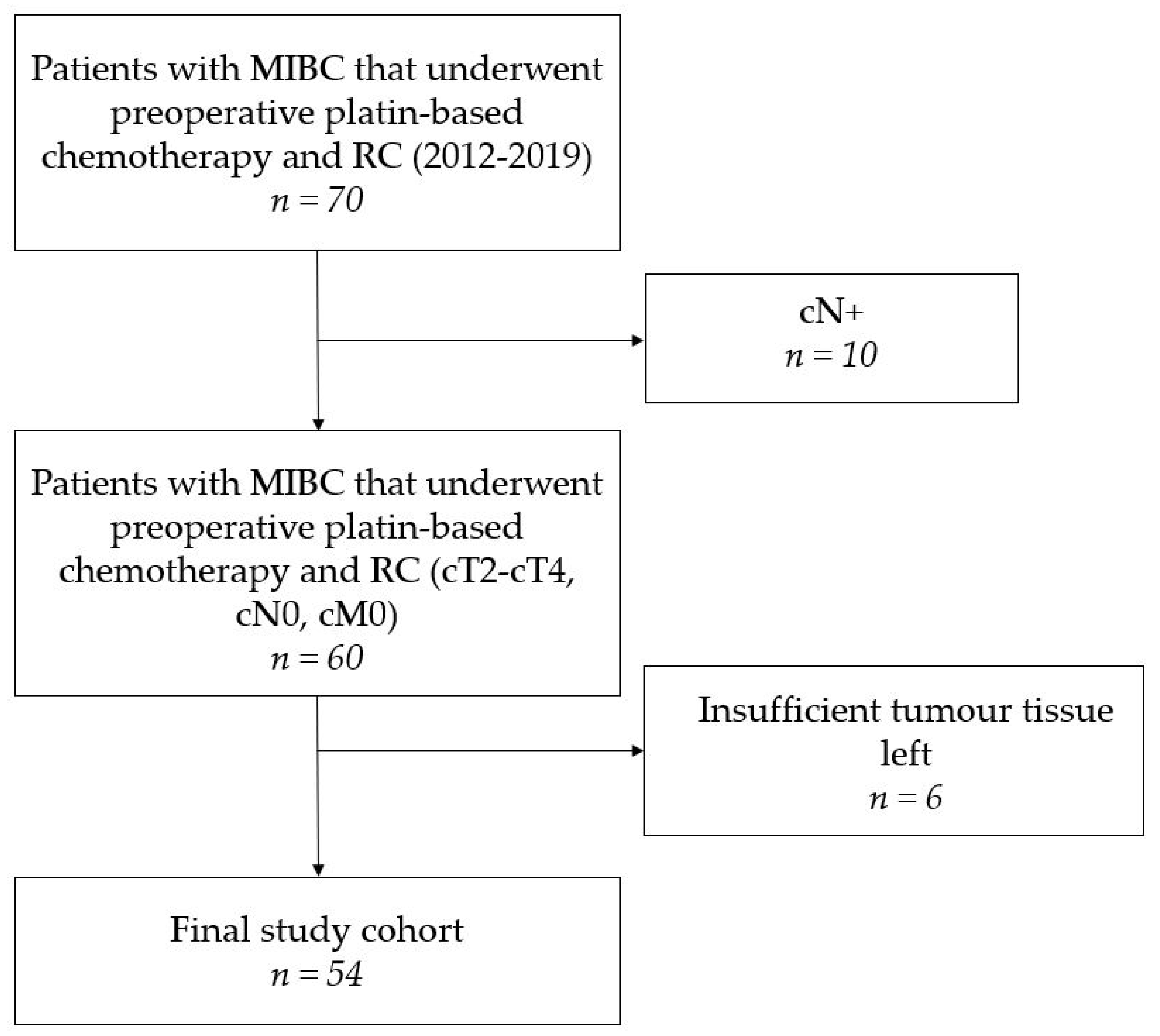
2.1. Study Population
2.2. Pathologic Assessment
2.3. Molecular Pathology
2.4. Statistical Analyses
3. Results
3.1. Patient and Treatment Characteristics
3.2. Elevated Expression of Markers Associated with Luminal Differentiation Favours pCR after NAC
3.3. ERBB2 Expression Correlates with Luminal Marker Expression
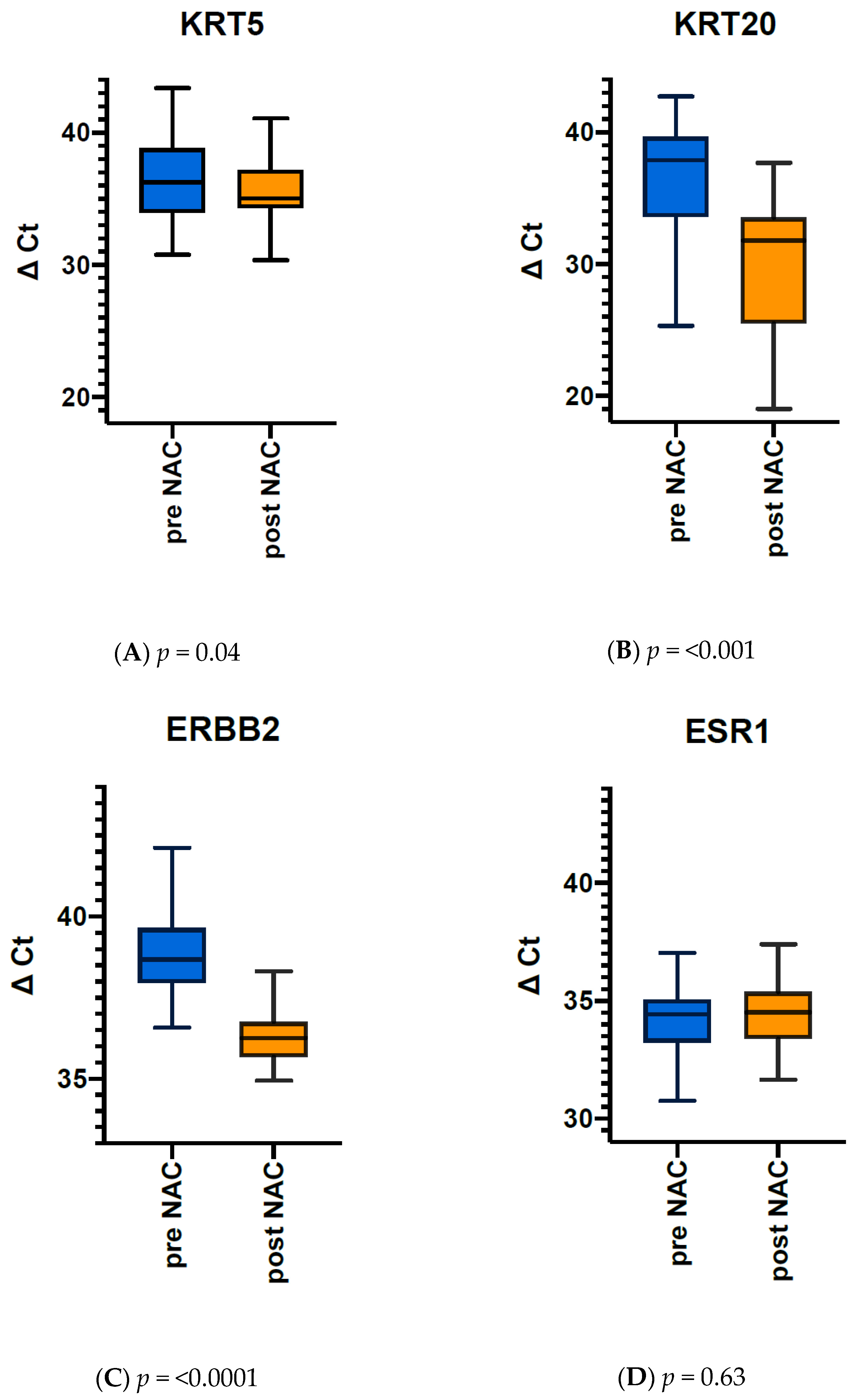
3.4. Comparison of Gene Expression Pre- vs. Post Therapy Reveals Dramatic Downregulation of Luminal Markers KRT20 and ERBB2
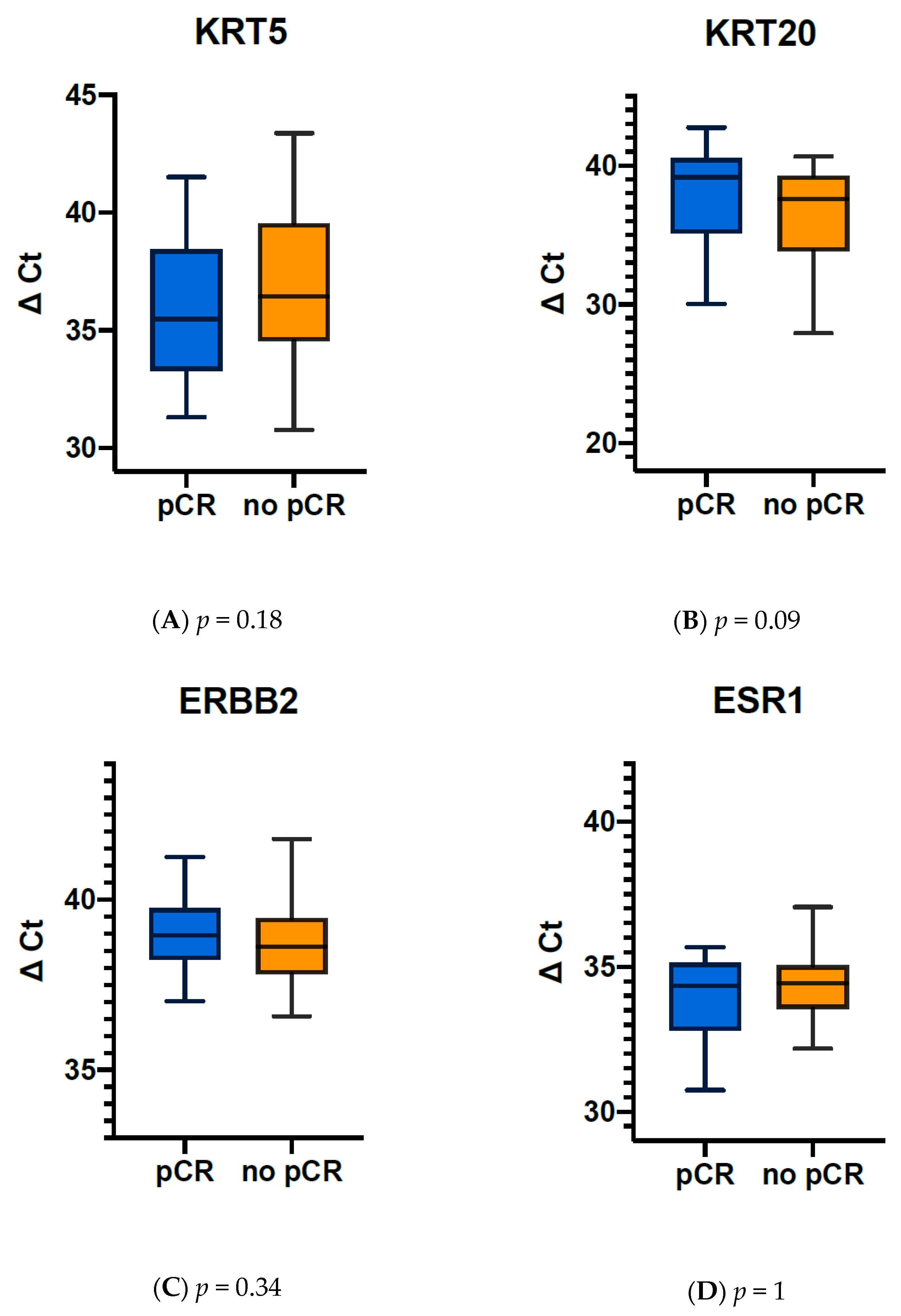
3.5. Elevated KRT20, ERBB2 and ESR1 Expression before NAC Correlate with pCR in MIBC Tumours
3.6. Changes after NAC in ERBB2 and KRT20 Expression Correlate with Each Other
3.7. Subanalyses According to Gender
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Martini, A.; Sfakianos, J.P.; Renström-Koskela, L.; Mortezavi, A.; Falagario, U.G.; Egevad, L.; Hosseini, A.; Mehrazin, R.; Galsky, M.D.; Steineck, G.; et al. The natural history of untreated muscle-invasive bladder cancer. BJU Int. 2020, 125, 270–275. [Google Scholar] [CrossRef] [PubMed]
- Stein, J.P.; Lieskovsky, G.; Cote, R.; Groshen, S.; Feng, A.C.; Boyd, S.; Skinner, E.; Bochner, B.; Thangathurai, D.; Mikhail, M.; et al. Radical cystectomy in the treatment of invasive bladder cancer: Long-term results in 1,054 patients. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2001, 19, 666–675. [Google Scholar] [CrossRef]
- Grossman, H.B.; Natale, R.B.; Tangen, C.M.; Speights, V.O.; Vogelzang, N.J.; Trump, D.L.; White, R.W.D.; Sarosdy, M.F.; Wood, D.P.; Raghavan, D.; et al. Neoadjuvant chemotherapy plus cystectomy compared with cystectomy alone for locally advanced bladder cancer. N. Engl. J. Med. 2003, 349, 859–866. [Google Scholar] [CrossRef]
- Peyton, C.C.; Tang, D.; Reich, R.R.; Azizi, M.; Chipollini, J.; Pow-Sang, J.M.; Manley, B.; Spiess, P.E.; Poch, M.A.; Sexton, W.J.; et al. Downstaging and Survival Outcomes Associated with Neoadjuvant Chemotherapy Regimens among Patients Treated with Cystectomy for Muscle-Invasive Bladder Cancer. JAMA Oncol. 2018, 4, 1535–1542. [Google Scholar] [CrossRef]
- Witjes, J.A.; Lebret, T.; Compérat, E.M.; Cowan, N.C.; De Santis, M.; Bruins, H.M.; Hernández, V.; Espinós, E.L.; Dunn, J.; Rouanne, M.; et al. Updated 2016 EAU Guidelines on Muscle-invasive and Metastatic Bladder Cancer. Eur Urol. 2017, 71, 462–475. [Google Scholar] [CrossRef]
- Milowsky, M.I.; Rumble, R.B.; Booth, C.M.; Gilligan, T.; Eapen, L.J.; Hauke, R.J.; Boumansour, P.; Lee, C.T. Guideline on Muscle-Invasive and Metastatic Bladder Cancer (European Association of Urology Guideline): American Society of Clinical Oncology Clinical Practice Guideline Endorsement. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2016, 34, 1945–1952. [Google Scholar] [CrossRef] [PubMed]
- Galsky, M.D.; Hahn, N.M.; Rosenberg, J.; Sonpavde, G.; Hutson, T.; Oh, W.; Dreicer, R.; Vogelzang, N.; Sternberg, C.; Bajorin, D.F.; et al. A consensus definition of patients with metastatic urothelial carcinoma who are unfit for cisplatin-based chemotherapy. Lancet Oncol. 2011, 12, 211–214. [Google Scholar] [CrossRef]
- Compérat, E.M.; Burger, M.; Gontero, P.; Mostafid, A.H.; Palou, J.; Rouprêt, M.; van Rhijn, B.W.; Shariat, S.F.; Sylvester, R.J.; Zigeuner, R.; et al. Grading of Urothelial Carcinoma and The New “World Health Organisation Classification of Tumours of the Urinary System and Male Genital Organs 2016”. Eur. Urol. Focus 2019, 5, 457–466. [Google Scholar] [CrossRef] [PubMed]
- Svatek, R.S.; Shariat, S.F.; Novara, G.; Skinner, E.C.; Fradet, Y.; Bastian, P.J.; Kamat, A.M.; Kassouf, W.; Karakiewicz, P.I.; Fritsche, H.-M.; et al. Discrepancy between clinical and pathological stage: External validation of the impact on prognosis in an international radical cystectomy cohort. BJU Int. 2011, 107, 898–904. [Google Scholar] [CrossRef]
- Shah, J.B.; McConkey, D.J.; Dinney, C.P.N. New strategies in muscle-invasive bladder cancer: On the road to personalized medicine. Clin. Cancer Res. Am. Assoc. Cancer Res. 2011, 17, 2608–2612. [Google Scholar] [CrossRef]
- Breyer, J.; Wirtz, R.M.; Laible, M.; Schlombs, K.; Erben, P.; Kriegmair, M.C.; Stoehr, R.; Eidt, S.; Denzinger, S.; Burger, M.; et al. ESR1, ERBB2, and Ki67 mRNA expression predicts stage and grade of non-muscle-invasive bladder carcinoma (NMIBC). Virchows Archiv 2016, 469, 547–552. [Google Scholar] [CrossRef] [PubMed]
- Breyer, J.; Markus Wirtz, R.; Otto, W.; Laible, M.; Schlombs, K.; Erben, P.; Kriegmair, M.C.; Stoehr, R.; Eidt, S.; Denzinger, S.; et al. Predictive value of molecular subtyping in NMIBC by RT-qPCR of ERBB2, ESR1, PGR and MKI67 from formalin fixed TUR biopsies. Oncotarget 2017, 8, 67684–67695. [Google Scholar] [CrossRef]
- Choi, W.; Porten, S.; Kim, S.; Willis, D.; Plimack, E.R.; Hoffman-Censits, J.; Roth, B.; Cheng, T.; Tran, M.; Lee, I.-L.; et al. Identification of distinct basal and luminal subtypes of muscle-invasive bladder cancer with different sensitivities to frontline chemotherapy. Cancer Cell 2014, 25, 152–165. [Google Scholar] [CrossRef] [PubMed]
- Eckstein, M.; Wirtz, R.M.; Gross-Weege, M.; Breyer, J.; Otto, W.; Stoehr, R.; Sikic, D.; Keck, B.; Eidt, S.; Burger, M.; et al. Mrna-expression of krt5 and krt20 defines distinct prognostic subgroups of muscle-invasive urothelial bladder cancer correlating with histological variants. Int. J. Mol. Sci. 2018, 19, 3396. [Google Scholar] [CrossRef]
- Denkert, C.; Loibl, S.; Kronenwett, R.; Budczies, J.; Von Törne, C.; Nekljudova, V.; Darb-Esfahani, S.; Solbach, C.; Sinn, B.V.; Petry, C.; et al. RNA-based determination of ESR1 and HER2 expression and response to neoadjuvant chemotherapy. Ann. Oncol. 2012, 24, 632–639. [Google Scholar] [CrossRef] [PubMed]
- Denkert, C.; Huober, J.; Loibl, S.; Prinzler, J.; Kronenwett, R.; Darb-Esfahani, S.; Brase, J.C.; Solbach, C.; Mehta, K.; Fasching, P.A.; et al. HER2 and ESR1 mRNA expression levels and response to neoadjuvant trastuzumab plus chemotherapy in patients with primary breast cancer. Breast Cancer Res. 2013, 15, R11. [Google Scholar] [CrossRef] [PubMed]
- Seiler, R.; Ashab, H.A.D.; Erho, N.; van Rhijn, B.W.G.; Winters, B.; Douglas, J.; Van Kessel, K.E.; van de Putte, E.E.F.; Sommerlad, M.; Wang, N.Q.; et al. Impact of Molecular Subtypes in Muscle-invasive Bladder Cancer on Predicting Response and Survival after Neoadjuvant Chemotherapy [Figure presented]. Eur. Urol. 2017, 72, 544–554. [Google Scholar] [CrossRef]
- Tan, T.Z.; Rouanne, M.; Tan, K.T.; Huang, R.Y.J.; Thiery, J.P. Molecular Subtypes of Urothelial Bladder Cancer: Results from a Meta-cohort Analysis of 2411 Tumors. Eur. Urol. 2019, 75, 423–432. [Google Scholar] [CrossRef]
- Groenendijk, F.H.; de Jong, J.; van de Putte, E.E.F.; Michaut, M.; Schlicker, A.; Peters, D.; Velds, A.; Nieuwland, M.; Heuvel, M.M.V.D.; Kerkhoven, R.M.; et al. ERBB2 Mutations Characterize a Subgroup of Muscle-invasive Bladder Cancers with Excellent Response to Neoadjuvant Chemotherapy. Eur. Urol. 2016, 69, 384–388. [Google Scholar] [CrossRef] [PubMed]
- Damrauer, J.S.; Hoadley, K.A.; Chism, D.D.; Fan, C.; Tiganelli, C.J.; Wobker, S.E.; Yeh, J.J.; Milowsky, M.I.; Iyer, G.; Parker, J.S.; et al. Intrinsic subtypes of high-grade bladder cancer reflect the hallmarks of breast cancer biology. Proc. Natl. Acad. Sci. USA 2014, 111, 3110–3115. [Google Scholar] [CrossRef]
- Hussain, S.A.; Palmer, D.H.; Lloyd, B.; Collins, S.I.; Barton, D.; Ansari, J.; James, N.D. A study of split-dose cisplatin-based neo-adjuvant chemotherapy in muscle-invasive bladder cancer. Oncol. Lett. 2012, 3, 855–859. [Google Scholar] [CrossRef]
- Erben, P.; Sikic, D.; Wirtz, R.M.; Martini, T.; Weis, C.-A.; Breyer, J.; Otto, W.; Keck, B.; Hartmann, A.; Bolenz, C. Analysis of the prognostic relevance of sex-steroid hormonal receptor mRNA expression in muscle-invasive urothelial carcinoma of the urinary bladder. Virchows. Arch. 2018, 474, 209–217. [Google Scholar] [CrossRef] [PubMed]
- Kriegmair, M.; Wirtz, R.; Worst, T.; Breyer, J.; Ritter, M.; Keck, B.; Boehmer, C.; Otto, W.; Eckstein, M.; Weis, C.; et al. Prognostic Value of Molecular Breast Cancer Subtypes based on Her2, ESR1, PGR and Ki67 mRNA-Expression in Muscle Invasive Bladder Cancer. Transl. Oncol. 2018, 11, 467–476. [Google Scholar] [CrossRef] [PubMed]
- Taber, A.; Christensen, E.; Lamy, P.; Nordentoft, I.; Prip, F.; Lindskrog, S.V.; Birkenkamp-Demtröder, K.; Okholm, T.L.H.; Knudsen, M.; Pedersen, J.S.; et al. Molecular correlates of cisplatin-based chemotherapy response in muscle invasive bladder cancer by integrated multi-omics analysis. Nat. Commun. 2020, 11, 4858. [Google Scholar] [CrossRef]
- Kamoun, A.; de Reyniès, A.; Allory, Y.; Sjödahl, G.; Robertson, A.G.; Seiler, R.; Hoadley, K.A.; Al-Ahmadie, H.; Choi, W.; Groeneveld, C.S.; et al. A Consensus Molecular Classification of Muscle-invasive Bladder Cancer. Eur. Urol. 2020, 77, 420–433. [Google Scholar] [CrossRef]
- Noske, A.; Loibl, S.; Darb-Esfahani, S.; Roller, M.; Kronenwett, R.; Müller, B.M.; Steffen, J.; von Toerne, C.; Wirtz, R.; Baumann, I.; et al. Comparison of different approaches for assessment of HER2 expression on protein and mRNA level: Prediction of chemotherapy response in the neoadjuvant GeparTrio trial (NCT00544765). Breast Cancer Res. Treat. 2011, 126, 109–117. [Google Scholar] [CrossRef]
- von Minckwitz, G.; Rezai, M.; Fasching, P.A.; Huober, J.; Tesch, H.; Bauerfeind, I.; Hilfrich, J.; Eidtmann, H.; Gerber, B.; Hanusch, C.; et al. Survival after adding capecitabine and trastuzumab to neoadjuvant anthracycline-taxane-based chemotherapy for primary breast cancer (GBG 40--GeparQuattro). Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2014, 25, 81–89. [Google Scholar] [CrossRef]
- Oudard, S.; Culine, S.; Vano, Y.; Goldwasser, F.; Théodore, C.; Nguyen, T.; Voog, E.; Banu, E.; Vieillefond, A.; Priou, F.; et al. Multicentre randomised phase II trial of gemcitabine+platinum, with or without trastuzumab, in advanced or metastatic urothelial carcinoma overexpressing Her2. Eur. J. Cancer Oxf. Engl. 1990 2015, 51, 45–54. [Google Scholar] [CrossRef] [PubMed]
- Choudhury, N.J.; Campanile, A.; Antic, T.; Yap, K.L.; Fitzpatrick, C.A.; Wade, J.L.; Karrison, T.; Stadler, W.M.; Nakamura, Y.; O’Donnell, P.H. Afatinib Activity in Platinum-Refractory Metastatic Urothelial Carcinoma in Patients With ERBB Alterations. J. Clin. Oncol. 2016, 34, 2165–2171. [Google Scholar] [CrossRef]
- Osborne, C.K.; Bardou, V.; Hopp, T.A.; Chamness, G.C.; Hilsenbeck, S.G.; Fuqua, S.A.W.; Wong, J.; Allred, D.C.; Clark, G.M.; Schiff, R. Role of the estrogen receptor coactivator AIB1 (SRC-3) and HER-2/neu in tamoxifen resistance in breast cancer. J. Natl. Cancer Inst. 2003, 95, 353–361. [Google Scholar] [CrossRef]
- Schiff, R.; Massarweh, S.; Shou, J.; Osborne, C.K. Breast cancer endocrine resistance: How growth factor signaling and estrogen receptor coregulators modulate response. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2003, 9, 447S–454S. [Google Scholar]
| Overall | Male | Female | p | |
|---|---|---|---|---|
| n (%) | 54 (100) | 42 (77.8) | 12 (22.2) | |
| Age (median [Interquartile range IQR]) | 64.00 [59.00, 69.00] | 63.50 [59.00, 69.75] | 66.00 [60.25, 69.00] | 0.835 |
| BMI (median [IQR]) | 26.49 [24.40, 29.72] | 26.93 [24.85, 29.72] | 24.59 [23.45, 26.95] | 0.100 |
| Concomitant CIS, n (%) | 3 (5.7) | 3 (7.3) | 0 (0.0) | 0.799 |
| cT4, n (%) | 4 (7.4) | 3 (5.55) | 1 (1.85) | 1 |
| ASA-score 3, n (%) | 22 (40.7) | 18 (42.9) | 4 (33.3) | 0.796 |
| ESR1 Expression (median [IQR]) | 34.44 [33.24, 35.04] | 34.58 [33.24, 35.04] | 33.91 [33.28, 34.97] | 0.546 |
| ERBB2 Expression (median [IQR]) | 38.69 [38.05, 39.63] | 38.67 [38.21, 39.61] | 38.84 [37.72, 39.61] | 0.851 |
| Overall | Male | Female | p | |
|---|---|---|---|---|
| n (%) | 54 (100) | 42 (77.8) | 12 (22.2) | |
| T category | 0.044 | |||
| ypT0 | 24 (44.4) | 21 (50.0) | 3 (25.0) | |
| ypTis | 5 (9.3) | 3 (7.1) | 2 (16.7) | |
| ypT1 | 4 (7.4) | 3 (7.1) | 1 (8.3) | |
| ypT2 | 11 (20.4) | 10 (23.8) | 1 (8.3) | |
| ypT3 | 8 (14.8) | 3 (7.1) | 5 (41.7) | |
| ypT4 | 2 (3.7) | 2 (4.8) | 0 (0.0) | |
| N category | 0.125 | |||
| pN0 | 45 (84.9) | 37 (90.2) | 8 (66.7) | |
| pN1 | 2 (3.8) | 1 (2.4) | 1 (8.3) | |
| pN2 | 5 (9.4) | 2 (4.9) | 3 (25.0) | |
| pN3 | 1 (1.9) | 1 (2.4) | 0 (0.0) | |
| Concomitant CIS | 8 (14.8) | 6 (14.3) | 2 (16.7) | 1 |
| ypT0 | 24 (44.4) | 21 (50.0) | 3 (25.0) | 0.227 |
| pCR | 22 (40.7) | 21 (50.0) | 1 (8.3) | 0.024 |
| local downstaging (≤pT1) | 33 (61.1) | 27 (64.3) | 6 (50.0) | 0.684 |
| Downstaging (≤pT1pN0) | 30 (55.6) | 26 (61.9) | 4 (33.3) | 0.154 |
| ESR1 expression (median [IQR]) | 34.50 [33.38, 35.29] | 34.23 [33.25, 35.00] | 35.56 [34.28, 36.03] | 0.015 |
| ERBB2 expression (median [IQR]) | 36.26 [35.69, 36.72] | 36.20 [35.58, 36.72] | 36.42 [36.13, 36.69] | 0.129 |
| (A) | |||
| KRT20 (≥40.485 ΔCt) ERBB2 (≥38.84 ΔCt) ESR1 (≥34.485 ΔCt) | n (%) | ||
| no pCR | pCR | ||
| no | 16 (84.2) | 3 (15.8) | 19 (100) |
| yes | 16 (45.7) | 19 (54.3) | 35 (100) |
| total | 32 (59.3) | 22 (40.7) | 54 (100) |
| Fisher’s p = 0.009 | |||
| (B) | |||
| KRT20 ≥ 40.485 ΔCt | n(%) | Total | |
| no pCR | pCR | ||
| no | 31 (66) | 16 (34) | 47 (100 |
| yes | 1 (14.3) | 6 (85.7) | 7 (100) |
| total | 32 (59.3) | 22 (40.7) | 54 (100) |
| Fisher’s p = 0.014 | |||
| (C) | |||
| ESR1 ≥ 34.485 ΔCt | n(%) | Total | |
| no pCR | pCR | ||
| no | 24 (66.7) | 12 (33.3) | 36 (100) |
| yes | 8 (44.4) | 10 (55.6) | 18 (100) |
| total | 32 (59.3) | 22 (40.7) | 54 (100) |
| Fisher’s p = 0.148 | |||
| mRNA | mRNA | Spearman ρ | p |
|---|---|---|---|
| ERBB2 | KRT20 | 0.5597 | <0.0001 * |
| ERBB2 | KRT5 | −0.4098 | 0.0021 * |
| KRT20 | KRT5 | −0.3156 | 0.0201 * |
| ERBB2 | ESR1 | 0.2476 | 0.0711 |
| ESR1 | KRT20 | −0.0305 | 0.8265 |
| ESR1 | KRT5 | −0.0180 | 0.8975 |
| Δ-ΔCt | Δ-ΔCt | Spearman ρ | p |
|---|---|---|---|
| ERBB2 | KRT20 | 0.33501 | 0.0133 * |
| ERBB2 | KRT5 | −0.15109 | 0.2755 |
| KRT20 | KRT5 | 0.13547 | 0.3287 |
| ERBB2 | ESR1 | 0.1235 | 0.3736 |
| KRT20 | ESR1 | 0.2143 | 0.1197 |
| ESR1 | KRT5 | −0.0002 | 0.9989 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jütte, H.; Reike, M.; Wirtz, R.M.; Kriegmair, M.; Erben, P.; Tully, K.; Weyerer, V.; Eckstein, M.; Hartmann, A.; Eidt, S.; et al. KRT20, KRT5, ESR1 and ERBB2 Expression Can Predict Pathologic Outcome in Patients Undergoing Neoadjuvant Chemotherapy and Radical Cystectomy for Muscle-Invasive Bladder Cancer. J. Pers. Med. 2021, 11, 473. https://doi.org/10.3390/jpm11060473
Jütte H, Reike M, Wirtz RM, Kriegmair M, Erben P, Tully K, Weyerer V, Eckstein M, Hartmann A, Eidt S, et al. KRT20, KRT5, ESR1 and ERBB2 Expression Can Predict Pathologic Outcome in Patients Undergoing Neoadjuvant Chemotherapy and Radical Cystectomy for Muscle-Invasive Bladder Cancer. Journal of Personalized Medicine. 2021; 11(6):473. https://doi.org/10.3390/jpm11060473
Chicago/Turabian StyleJütte, Hendrik, Moritz Reike, Ralph M. Wirtz, Maximilian Kriegmair, Philipp Erben, Karl Tully, Veronika Weyerer, Markus Eckstein, Arndt Hartmann, Sebastian Eidt, and et al. 2021. "KRT20, KRT5, ESR1 and ERBB2 Expression Can Predict Pathologic Outcome in Patients Undergoing Neoadjuvant Chemotherapy and Radical Cystectomy for Muscle-Invasive Bladder Cancer" Journal of Personalized Medicine 11, no. 6: 473. https://doi.org/10.3390/jpm11060473
APA StyleJütte, H., Reike, M., Wirtz, R. M., Kriegmair, M., Erben, P., Tully, K., Weyerer, V., Eckstein, M., Hartmann, A., Eidt, S., Wezel, F., Bolenz, C., Tannapfel, A., Noldus, J., & Roghmann, F. (2021). KRT20, KRT5, ESR1 and ERBB2 Expression Can Predict Pathologic Outcome in Patients Undergoing Neoadjuvant Chemotherapy and Radical Cystectomy for Muscle-Invasive Bladder Cancer. Journal of Personalized Medicine, 11(6), 473. https://doi.org/10.3390/jpm11060473









