BRCA1/2 Molecular Assay for Ovarian Cancer Patients: A Survey through Italian Departments of Oncology and Molecular and Genomic Diagnostic Laboratories
Abstract
1. Introduction
2. Materials and Methods
- Core organization of the BRCA1/2 testing process for ovarian cancer patients;
- Pipelines that were most frequently followed to request the test and which professionals are mainly involved;
- The testing approaches followed (somatic vs. germline testing) depending on the type of setting;
- The process of the referral to genetic counseling of both patients and healthy family members takes place;
- The volume of activity of the diagnostic laboratories and their procedures for the execution of the BRCA1/2 assay.
3. Results
3.1. Survey on Departments of Oncology
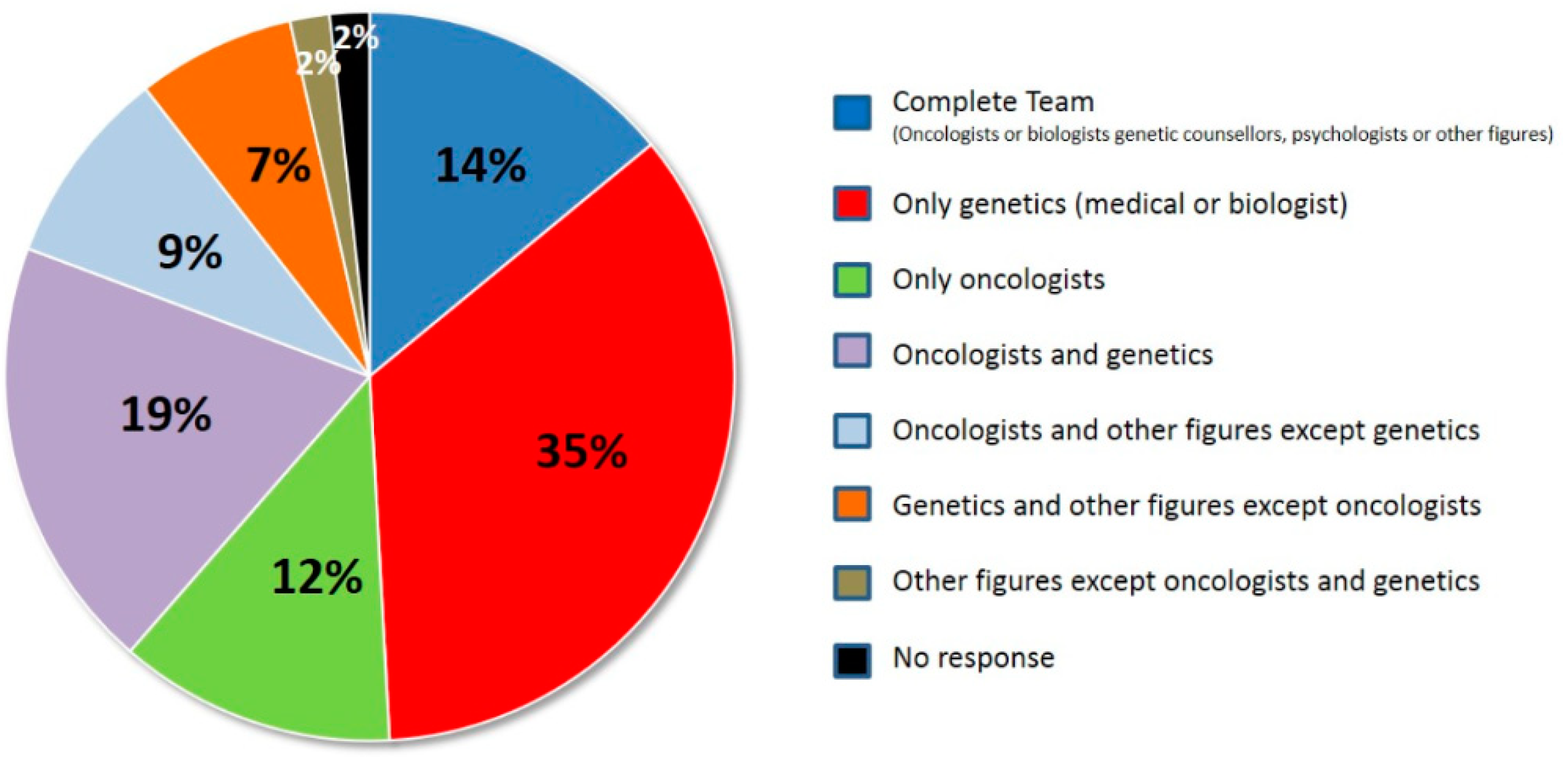
3.2. Genetic Units: Presence Inside the Hospitals and Their Organization
3.3. Germline and Somatic BRCA1/2 Testing
3.4. Genetic Counselling and Genetic Testing for Relatives of PV Carries
3.5. Survey on Diagnostic Laboratories
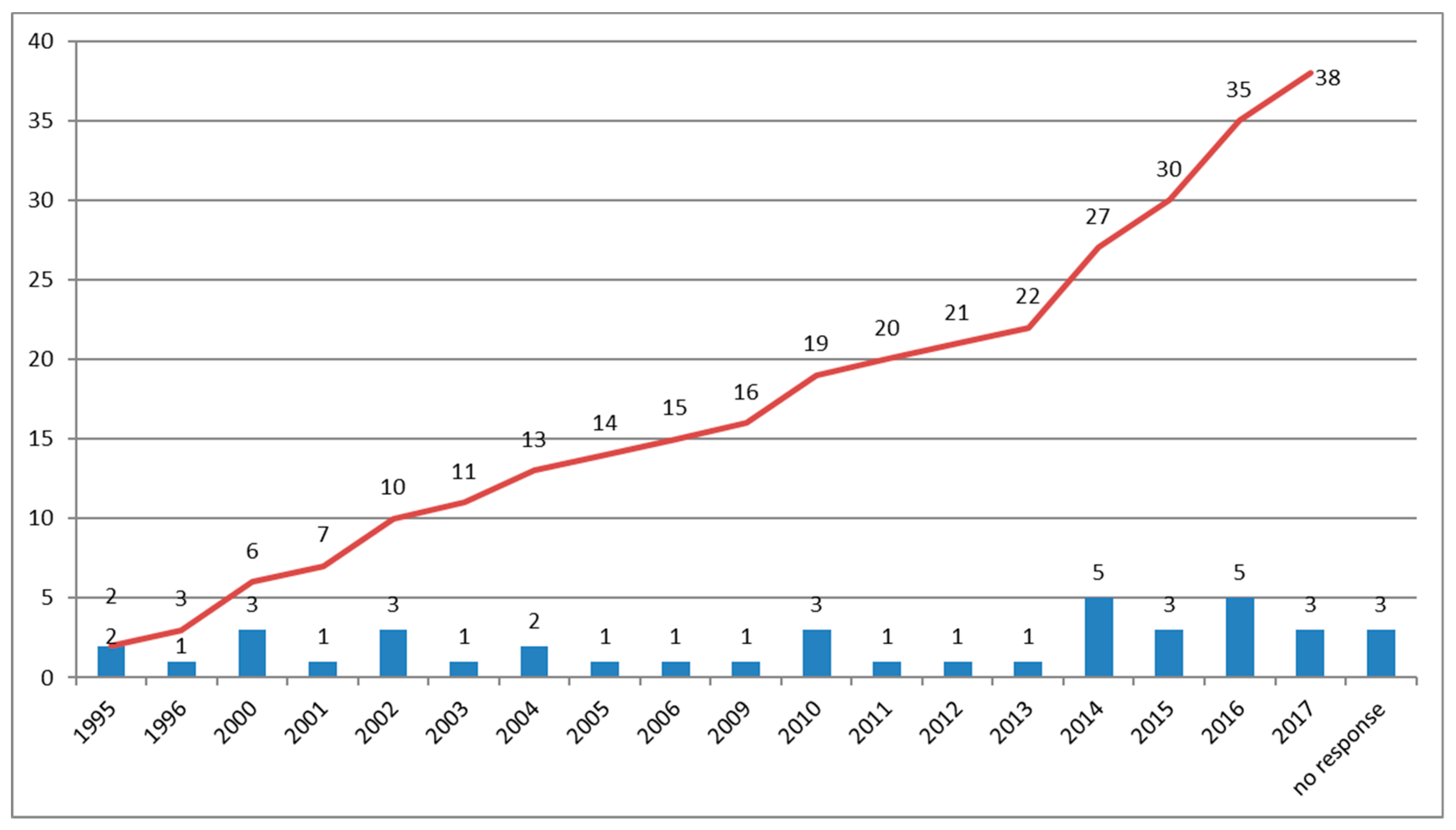
3.6. Duration of BRCA1/2 Testing Activities and Types of Germline Assays
3.7. Technologies and Kit Employed for Germline BRCA1/2 Test
3.8. Yearly Volume of Germline BRCA1/2 Testing
3.9. Somatic BRCA1/2 Testing on Tumor Specimens
3.10. Variant Classification and Interpretation
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Norquist, B.M.; Harrell, M.I.; Brady, M.F.; Walsh, T.; Lee, M.K.; Gulsuner, S.; Bernards, S.S.; Casadei, S.; Yi, Q.; Burger, R.A.; et al. Inherited Mutations in Women with Ovarian Carcinoma. JAMA Oncol. 2016, 2, 482–490. [Google Scholar] [CrossRef] [PubMed]
- I numeri del cancro. Available online: https://www.aiom.it/wp-content/uploads/2018/10/2018_NumeriCancro-operatori.pdf (accessed on 1 March 2019).
- Capoluongo, E.; Scambia, G.; Nabholtz, J.M. Main implications related to the switch to BRCA1/2 tumor testing in ovarian cancer patients: A proposal of a consensus. Oncotarget 2018, 9, 19463–19468. [Google Scholar] [CrossRef] [PubMed]
- Scaglione, G.L.; Concolino, P.; De Bonis, M.; De Paolis, E.; Minucci, A.; Ferrandina, G.; Scambia, G.; Capoluongo, E. Whole Germline BRCA2 Gene Deletion: How to Learn from CNV In Silico Analysis. Int. J. Mol. Sci. 2018, 19, 961. [Google Scholar] [CrossRef] [PubMed]
- Hoskins, P.J. Inadequate Rates of BRCA Testing with its Negative Consequences for Women with Epithelial Ovarian Cancer and their Families: An Overview of the Literature. Clin. Oncol. (R. Coll. Radiol.) 2018, 30, 472–483. [Google Scholar] [CrossRef] [PubMed]
- Childers, C.P.; Childers, K.K.; Maggard-Gibbons, M.; Macinko, J. National Estimates of Genetic Testing in Women with a History of Breast or Ovarian Cancer. J. Clin. Oncol. 2017, 35, 3800–3806. [Google Scholar] [CrossRef] [PubMed]
- Pinto, C.; Bella, M.; Capoluongo, E.; Carrera, P.; Clemente, C.; Colombo, N.; Cortesi, L.; De Rosa, G.; Fenizia, F.; Genuardi, M.; et al. Recommendations for the implementation of BRCA testing in the care and treatment pathways of ovarian cancer patients. Future Oncol. 2016, 12, 2071–2075. [Google Scholar] [CrossRef] [PubMed]
- Libro bianco AIOM. Available online: https://librobianco.aiom.it/ (accessed on 1 September 2017).
- Plon, S.E.; Eccles, D.M.; Easton, D.; Foulkes, W.D.; Genuardi, M.; Greenblatt, M.S.; Hogervorst, F.B.; Hoogerbrugge, N.; Spurdle, A.B.; Tavtigian, S.V. IARC Unclassified Genetic Variants Working Group. Sequence variant classification and reporting: Recommendations for improving the interpretation of cancer susceptibility genetic test results. Hum. Mutat. 2008, 11, 1282–1291. [Google Scholar] [CrossRef] [PubMed]
- Richards, S.; Aziz, N.; Bale, S.; Bick, D.; Das, S.; Gastier-Foster, J.; Grody, W.W.; Hegde, M.; Lyon, E.; Spector, E.; et al. Standards and guidelines for the interpretation of sequence variants: A joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet. Med. 2015, 17, 405–424. [Google Scholar] [CrossRef]
- de Belvis, A.G.; Ferrè, F.; Specchia, M.L.; Valerio, L.; Fattore, G.; Ricciardi, W. The financial crisis in Italy: Implications for the healthcare sector. Health Policy 2012, 106, 10–16. [Google Scholar] [CrossRef]
- Gori, S.; Barberis, M.; Bella, M.A.; Buttitta, F.; Capoluongo, E.; Carrera, P.; Colombo, N.; Cortesi, L.; Genuardi, M.; Gion, M.; et al. Recommendations for the implementation of BRCA testing in ovarian cancer patients and their relatives. Crit. Rev. Oncol. Hematol. 2019, 40, 67–72. [Google Scholar] [CrossRef] [PubMed]
- Capoluongo, E.; Ellison, G.; López-Guerrero, J.A.; Penault-Llorca, F.; Ligtenberg, M.J.L.; Banerjee, S.; Singer, C.; Friedman, E.; Markiefka, B.; Schirmacher, P.; et al. Guidance Statement on BRCA1/2 Tumor Testing in Ovarian Cancer Patients. Semin. Oncol. 2017, 44, 187–197. [Google Scholar] [CrossRef] [PubMed]
- Pan, V.; Yashar, B.M.; Pothast, R.; Wicklund, C. Expanding the genetic counseling workforce: Program directors’ views on increasing the size of genetic counseling graduate programs. Genet. Med. 2016, 18, 842–849. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Axilbund, J.E.; O’Leary, E.; Michalski, S.T.; Evans, R.; Lincoln, S.E.; Esplin, E.D.; Nussbaum, R.L. Underdiagnosis of Hereditary Breast and Ovarian Cancer in Medicare Patients: Genetic Testing Criteria Miss the Mark. Ann. Surg. Oncol. 2018, 25, 2925–2931. [Google Scholar] [CrossRef] [PubMed]
- Pilarski, R. The Role of BRCA Testing in Hereditary Pancreatic and Prostate Cancer Families. Am. Soc. Clin. Oncol. Educ. Book. 2019, 39, 79–86. [Google Scholar] [CrossRef] [PubMed]
- Boussios, S.; Karihtala, P.; Moschetta, M.; Karathanasi, A.; Sadauskaite, A.; Rassy, E.; Pavlidis, N. Combined Strategies with Poly (ADP-Ribose) Polymerase (PARP) Inhibitors for the Treatment of Ovarian Cancer: A Literature Review. Diagnostics 2019, 1, 9. [Google Scholar] [CrossRef] [PubMed]
- Boussios, S.; Karathanasi, A.; Cooke, D.; Neille, C.; Sadauskaite, A.; Moschetta, M.; Zakynthinakis-Kyriakou, N.; Pavlidis, N. PARP Inhibitors in Ovarian Cancer: The Route to “Ithaca”. Diagnostics 2019, 18, 9. [Google Scholar] [CrossRef] [PubMed]
| N Lab | % Lab | Pipelines |
|---|---|---|
| 3 | 7.3% | NGS |
| 1 | 2.3% | NGS, MAQ |
| 4 | 9.8% | NGS, MLPA |
| 1 | 2.3% | NGS, ION TORRENT analysis of CNV |
| 3 | 7.3% | NGS, Sanger |
| 1 | 2.3% | NGS, Sanger, MAQ |
| 21 | 51.2% | NGS, Sanger, MLPA |
| 1 | 2.3% | NGS, Sanger, MLPA, NGS + MLPA * + DHPLC ** |
| 3 | 7.3% | Sanger, MLPA |
| 1 | 2.3% | Sanger, QMPSF (for re-arrangements) |
| 2 | 5.8% | no response |
| Results | Test Performed in 2016 | Test Performed in Breast Cancer Patients | Test Performed in Ovarian Cancer Patients | Test Performed in Other Cancer (No Breast, No Ovarian) ° | Test Performed in Healthy Patients |
|---|---|---|---|---|---|
| Number of test | 12,559 | 6313 | 2648 | 172 | 1712 |
| Mean (range) | 339 | 175 | 76 | 7 | 54 |
| Median | 254 (17–1660) | 136 (8–643) | 35 (5–1000) | 2 (1–28) | 22 (4–500) |
| Centers responding to the questionnaires (out of 41) | 37 | 36 | 35 | 26 | 32 |
| Results | Number of Tests Performed in 2016 | Test in Ovarian Cancer Patients | Test in Breast Cancer Patients | Test in Other Cancers ° |
|---|---|---|---|---|
| Total | 699 | 573 | 76 | 50 |
| Mean | 30 | 32 | 6 | 5 |
| Median (range) | 19 (0–200) | 20 (5–180) | 0 (0–40) | 0 (0–25) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Capoluongo, E.; La Verde, N.; Barberis, M.; Bella, M.A.; Buttitta, F.; Carrera, P.; Colombo, N.; Cortesi, L.; Genuardi, M.; Gion, M.; et al. BRCA1/2 Molecular Assay for Ovarian Cancer Patients: A Survey through Italian Departments of Oncology and Molecular and Genomic Diagnostic Laboratories. Diagnostics 2019, 9, 146. https://doi.org/10.3390/diagnostics9040146
Capoluongo E, La Verde N, Barberis M, Bella MA, Buttitta F, Carrera P, Colombo N, Cortesi L, Genuardi M, Gion M, et al. BRCA1/2 Molecular Assay for Ovarian Cancer Patients: A Survey through Italian Departments of Oncology and Molecular and Genomic Diagnostic Laboratories. Diagnostics. 2019; 9(4):146. https://doi.org/10.3390/diagnostics9040146
Chicago/Turabian StyleCapoluongo, Ettore, Nicla La Verde, Massimo Barberis, Maria Angela Bella, Fiamma Buttitta, Paola Carrera, Nicoletta Colombo, Laura Cortesi, Maurizio Genuardi, Massimo Gion, and et al. 2019. "BRCA1/2 Molecular Assay for Ovarian Cancer Patients: A Survey through Italian Departments of Oncology and Molecular and Genomic Diagnostic Laboratories" Diagnostics 9, no. 4: 146. https://doi.org/10.3390/diagnostics9040146
APA StyleCapoluongo, E., La Verde, N., Barberis, M., Bella, M. A., Buttitta, F., Carrera, P., Colombo, N., Cortesi, L., Genuardi, M., Gion, M., Guarneri, V., Lorusso, D., Marchetti, A., Marchetti, P., Normanno, N., Pasini, B., Pensabene, M., Pignata, S., Radice, P., ... Gori, S. (2019). BRCA1/2 Molecular Assay for Ovarian Cancer Patients: A Survey through Italian Departments of Oncology and Molecular and Genomic Diagnostic Laboratories. Diagnostics, 9(4), 146. https://doi.org/10.3390/diagnostics9040146











