Abstract
The purpose of the present study was to evaluate changes of signs and symptoms in patients with meibomian gland dysfunction (MGD) treated with intense regulated pulsed light (IRPL), and to further investigate which parameter could predict positive outcomes of the procedure. Twenty-eight patients who bilaterally received three IRPL sessions at day 1, 15, and 45 satisfied the criteria and were included in the study. Non-invasive break-up time (NIBUT), lipid layer thickness (LLT), meibography, tear osmolarity, and ocular discomfort symptoms were measured before and 30 days after the last IRPL session. Qualified or complete success was defined in the presence of an improvement of symptoms associated with an increase of NIBUT (< or ≥ 20%). After IRPL treatment, median NIBUT and LLT increased from 7.5 to 10.2 s and 2.0 to 3.0, respectively (p <0.001); tear osmolarity decreased from 304.0 to 301.0 mOsm/L (p = 0.002). Subjective symptoms improved after IRPL in 26 patients. Qualified success was reached in 34 eyes, while complete success in 16 eyes. Patients with lower baseline break-up time (BUT) values showed better response to treatment (p = 0.04). In conclusion, IRPL improved signs and symptoms in MGD patients, while lower baseline NIBUT values were predictive of better response to IRPL.
1. Introduction
Dry eye symptoms are among the most common complaints at ophthalmic practices, impairing patient quality of life and restricting daily activities and work productivity [1,2]. Although various ocular and systemic conditions can determine the onset of dry eye [3,4,5,6], the vast majority of cases originates from a deficiency of the meibomian glands (meibomian gland dysfunction, MGD), that is characterized by a chronic and diffuse abnormality of glands with obstruction of terminal duct and qualitative/quantitative changes of glandular secretion [7,8].
The pathogenesis of MGD is arranged in a vicious circle: meibomian gland inflammation or blockage for ductal epithelium hyperkeratinization leads to stasis of the meibum inside the glands. The reduced gland outflow promotes the proliferation of bacteria, increasing the viscosity of the meibum and thus resulting in further blockage of the gland orifices [9]. Most of the treatments currently available are mainly palliative and consist of hygiene measures and tear substitutes. Antibiotics, anti-inflammatory drugs, and immunosuppressant agents are also used with the aim of breaking the vicious circle. However, therapy often provides only short-term and partial relief of symptoms and signs, with compliance issues.
Intense pulsed light (IPL) has been used in dermatology for over a decade for the treatment of rosacea, acne, and various skin lesions (e.g., benign cavernous hemangioma and telangiectasia) [10,11]. IPL consists of a non-coherent and polychromatic light source with a wavelength spectrum of 500–1200 nm, which can be easily modulated through a proper filter and, when directed to the skin, it is absorbed by chromophores and converted into heat, inducing the ablation of blood vessels.
A new-generation of device (E>Eye), designed specifically for periocular application with calibrated and sculpted sequenced light pulses delivered under the shape of regulated train pulses (intense regulated pulsed light, IRPL), has recently become commercially available, and is currently the only medically certified device for treating dry eye owing to MGD [12,13,14,15,16]. During treatment, the protection of the patient’s eye is mandatory and is obtained thanks to the use of protective shields.
Despite recent studies showed both safety and efficacy of IRPL, predictive parameters of patients who will most likely benefit from the treatment have not yet been identified.
The purpose of this study was to evaluate the changes of a comprehensive ocular surface workup, based on both automated objective and subjective parameters, after IRPL treatment in patients with MGD; in addition, ocular surface parameters predictive of positive outcomes of the procedure were also investigated.
2. Materials and Methods
2.1. Materials and Patients
This prospective clinical study was conducted between September 2016 and September 2017 at Carones Ophthalmology Center (Milan, Italy). The study was approved by the local Institutional Review Board (approval date 16 May 2016) and was carried out in accordance with the principles of the Declaration of Helsinki. All participants provided written informed consent before any study procedure. Inclusion criteria were age older than 18 years; clinical signs and subjective symptoms of MGD [17]; willingness to continue ongoing therapy (unpreserved tear substitutes, eyelid hygiene); and Fitzpatrick skin scale from 1 to 4. Exclusion criteria were any ocular surface disease other than MGD (e.g., Sjögren’s syndrome, graft-versus-host disease, allergy); previous IPL and/or vectored thermal pulsation therapies within the past 24 months; previous ocular surgery or eyelid trauma; hypotensive eye drops use; punctal plugs; skin pigmented lesions in the treatment area; pregnancy and breastfeeding; any uncontrolled ocular or systemic disease.
2.2. Ocular Surface Workup
Ocular surface workup was performed before and 30 days after the last IRPL treatment and included the following automated quantitative measurements: non-invasive break-up time (NIBUT), lipid layer thickness (LLT), non-contact infrared meibography, and tear osmolarity. The Ocular Surface Disease Index (OSDI) questionnaire was administered before any procedure to score ocular discomfort symptoms. In addition, patient’s satisfaction after treatment was ascertained by asking the patients whether they perceived improvements from their baseline symptoms according to a 5-grade scale: none = 0, trace = 1, mild = 2, moderate = 3, high = 4.
The I.C.P. Tearscope (SBM Sistemi, Turin, Italy), a lighting system that allows the in vivo visualization of the different layers of the tear film at the slit lamp under magnification × 16–25, was used to measure NIBUT and LLT [18]. The median value of three successive measurements was used for statistical analysis. Lipid layer patterns were classified based on their appearance, and thickness was graded from 0 to 5: absence of lipids (grade 0); open meshwork (grade 1); tight meshwork (grade 2); waves (grade 3); amorphous (grade 4); and color mixing (grade 5) [12,19]. Tear osmolarity was evaluated with TearLab Osmolarity System (TearLab Corporation, San Diego, CA, USA) by obtaining a sample from the lower temporal eyelid tear film meniscus [20].
Non-contact infrared meibography was performed by using the I.C.P. MGD meibography system (SBM Sistemi, Turin, Italy) to acquire infrared images of the meibomian glands after everting the lower eyelid [21,22]. Meibomian gland loss (MGL) was defined as the percentage of gland loss in relation to the total tarsal area of the lid. Images were digitally analyzed and MGL was measured using I.C.P. application.
2.3. Treatment Procedure
IRPL treatments were performed using E>Eye device (E-Swin, Paris, France), set on the proprietary “dry eye mode”. Treatment intensity was determined based on patient’s Fitzpatrick skin type, ranging from 9.8 to 13 J/cm2. Patients received three treatment sessions performed at day 1, 15, and 45, as per manufacturer recommendations [12]. During each treatment, protective eye shields were placed over the eyes and ultrasound gel was applied to the treatment area. Five flashes were applied for each eye starting from the inner canthus and ending on the temporal region below the lower eyelid, with slight overlapping applications. According to our protocol, all patients continued their ongoing therapy (unpreserved tear substitute and eyelid hygiene); in addition, all patients instilled 0.3% cortisol phosphate in hyaluronic acid vehicle eye drops (Cortivis; Medivis, Catania, Italy) twice daily for 10 days after the first session of IRPL.
2.4. Main Outcome Measures
The primary outcomes were the changes of each ocular surface parameter analyzed 30 days after the last IRPL session (at day 75), and the rates of qualified and complete success. In detail, qualified success was defined in the presence of an improvement of symptoms (score ≥2) associated with an increase of NIBUT; complete success was defined in the presence of an improvement of symptoms (score ≥3) associated with an increase of NIBUT ≥20%.
The secondary outcome was the detection of ocular surface parameters predictive of the success of the procedure.
2.5. Statistical Analysis
The SPSS statistical software (SPSS Inc Version 22.0, Chicago, IL, USA) was used for data analysis. Values are expressed as median, interquartile range (IQR), and 95% confidence intervals (CIs). The difference between pre- and post-treatment values for each parameter was calculated and reported as delta (Δ). The non-parametric Wilcoxon test was used to compare variables before and after IRPL treatment. The Mann–Whitney U test was used to compare variables between patients who experienced success after the procedure and those who did not. Spearman’s correlation was run to determine the relationships between variables. A p <0.05 was considered statistically significant.
3. Results
Eighty eyes of 40 patients underwent IRPL treatment during the study period. Of these, 56 eyes of 28 patients (6 males, 22 females; median age 46.0 years, IQR: 17.5) received regular treatment sessions and follow-up visits and were finally included in the analysis. The remaining patients were lost to follow-up (four patients) or did not complete the whole treatment cycle (eight patients). Demographic characteristics and ocular surface parameters at baseline of the included patients are reported in Table 1.

Table 1.
Demographic and clinical characteristics of patients at baseline.
Thirty days after the last IRPL session, median NIBUT significantly increased from 7.5 s (IQR: 5.2, 95% CI: 7.0–8.5) to 10.2 s (IQR: 5.4, 95% CI: 9.5–11.4) (p <0.001; Figure 1, part A), and median LLT grade significantly improved from 2.0 (IQR: 1.0, 95% CI: 1.5–1.9) to 3.0 (IQR: 2.0, 95% CI: 2.6–3.3) (p <0.001, Figure 1, part B). No statistically significant changes were recorded after IRPL treatment for both MGL (Figure 1, part C) and OSDI score (always p >0.05). Median tear osmolarity significantly decreased after treatment from 304.0 mOsm/L (IQR: 9.8, 95% CI: 302.9–308.3) to 301.0 mOsm/L (IQR: 14.0, 95% CI: 298.1–303.2) (p = 0.002, Figure 1, part D). Twenty-six patients (92.9% of the total) showed an improvement of symptoms after treatment (median grade 2.0 (IQR: 2.0, 95% CI: 2.0–2.5)). Figure 2 shows the distribution of patients’ perceived improvement in symptoms according to the 5-grade scale used.
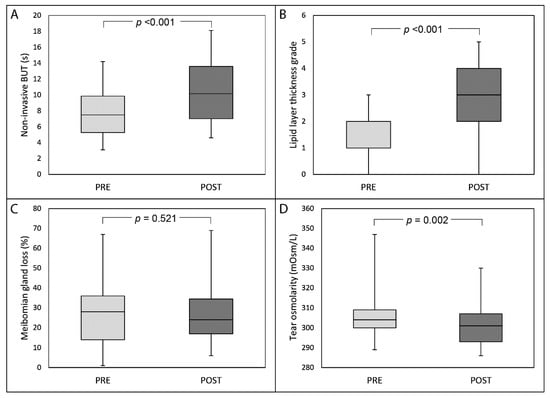
Figure 1.
Box-plot analysis of non-invasive break-up time (NIBUT) (part A), lipid layer thickness grade (part B), meibomian gland loss (part C) and tear osmolarity (part D) before and 30 days after the last session of intense regulated pulsed light treatment.
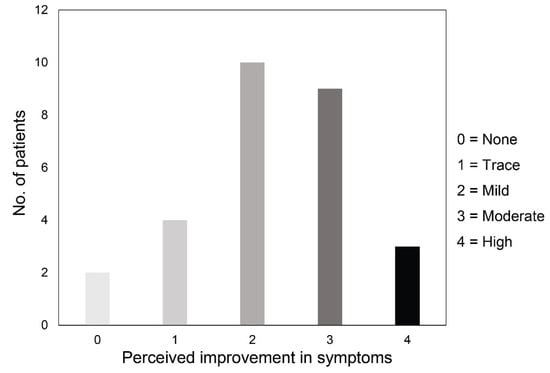
Figure 2.
Distribution of patients according to the 5-grade scale about their perceived improvement in symptoms after intense regulated pulsed light treatment.
Qualified success (improvement of both symptoms and NIBUT) was reached in 34 eyes (60.7% of the total), while complete success (improvement of symptoms (score ≥3) associated with an increase of NIBUT (≥20%)) in 16 eyes (28.6% of the total). Patients who achieved qualified success had significantly lower NIBUT values at baseline compared to the others (respectively 6.7 s (IQR: 3.9, 95% CI:5.4–7.7) vs. 8.7 s (IQR: 5.3, 95% CI:7.5–10.1)) (Figure 3). Conversely, no differences were found at baseline between patients belonging to the success group vs. others for LLT, meibomian gland loss, and tear osmolarity (always p >0.05). A significant negative correlation was found between baseline NIBUT and Δ NIBUT (p <0.001; R = −0.463), baseline osmolarity and Δ osmolarity (p <0.001; R = −0.584), and baseline meibomian gland loss and Δ meibomian gland loss (p <0.001; R = −0.470) (Figure 4).
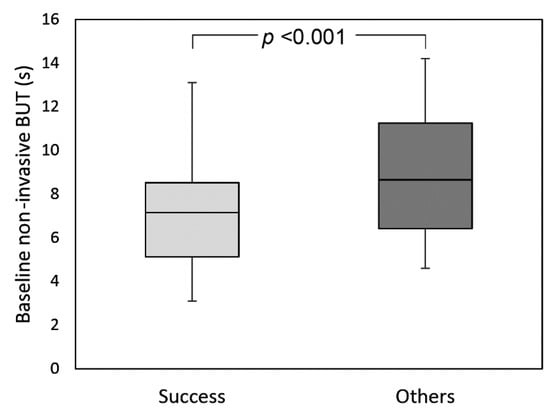
Figure 3.
Box-plot analysis of baseline non-invasive break-up time in patients belonging to the success group and in the others.
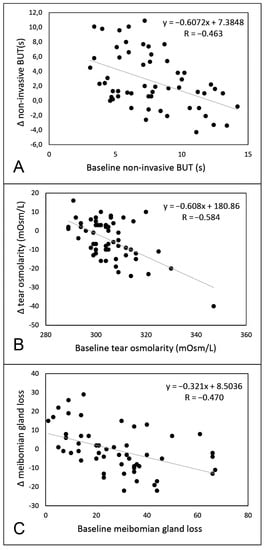
Figure 4.
Linear regression analysis between baseline value and delta (Δ) value for non-invasive break-up time (part A), tear osmolarity (part B), and meibomian gland loss (part C).
No adverse effects related to the treatment were reported at any visit of the study.
4. Discussion
Meibomian gland dysfunction has been identified as the most common cause of dry eye disease [8]. Treatments currently available are mainly palliative solutions, often insufficient to improve clinical signs and overcome patient’s discomfort symptoms. Intense pulsed light was recently introduced in the field of ophthalmology and recent clinical studies showed that it is able to provide an improvement of both signs and symptoms in MGD patients (Table 2) [12,13,14,15,16,23,24,25,26,27,28,29,30,31,32,33,34,35]. IPL can be performed in combination with other therapies, like meibomian gland expression, and thus also represents a promising complementary treatment for MGD. [14,24,26,30,31,35]. This combination of treatments allowed for the successful management of refractory cases of MGD, as demonstrated in a recent multicenter prospective study [35]. It was recently demonstrated that the treatment reduces consistently also tear inflammatory cytokines [33,34]. Although different speculative pathophysiological theories have been proposed to explain the positive effects of intense pulsed light upon dry eye signs and symptoms, the mechanisms of actions are still not fully elucidated. Among these, the coagulation of superficial blood vessels and telangiectasias of eyelids skin induced by light energy, the heating and liquefying of meibomian glands secretions with improved viscosity and outflow, and the decrease of bacterial and parasitic load over eyelids and eyelashes have been proposed [36]. More recently, the enhancement in collagen synthesis and connective tissue remodeling, the reduction in skin epithelial cell turnover, and the modulation of cellular inflammatory markers have also been hypothesized [37].

Table 2.
Clinical studies about the use of intense pulsed light treatment for meibomian gland dysfunction.
To date, most of the available studies reported improvements in terms of lid margin features (e.g., thickening and vascularity, telangiectasia, number of plugged glands) and meibomian gland secretion quality and expressibility [13,14,25,26,28,31]. However, these measures are subjective, and prone to observer bias due to a low degree of standardization. Conversely, a comprehensive ocular surface workup with automated quantitative measurements may overcome these drawbacks, thus improving the objective monitoring of the disease course after treatment [38].
In the present study, NIBUT, LLT, non-contact meibography, and tear osmolarity have been investigated before and after IRPL sessions. Our results confirmed that NIBUT significantly increases after IRPL [12,13,14,15,16,23,25,26,27,29,30,31,32,35]. Since the role of tear film instability as pivotal mechanism of DED onset and persistence has been gaining prominence [39,40], its significant improvement after IRPL therapy represents a major goal of the procedure.
Only few previous studies evaluated LLT changes after IPL, with conflicting results [12,26,29]. Despite the lack of universal consensus on the LLT modifications after IPL therapy, Ahmed and co-authors demonstrated an improvement in lipid content and composition (and in particular of polar lipids that critically impact the health of the ocular surface), contributing to the stability of the tear film [41]. In the present study, we showed a significant increase of LLT by using the same grading scale previously utilized by Craig and collaborators [12].
Tear osmolarity significantly decreased after treatment, in contrast with previous studies which conversely reported no changes of this parameter after treatment [12,14,26]. Since tear osmolarity has been shown by the TFOS Dry Eye Workshop II to be influenced by both tear film stability and lipid layer characteristics [41], the improvement of these two parameters after IRPL could explain this finding. However, it should be pointed out that in our study, as well as in other MGD populations, tear osmolarity values were within the upper range of normality [14,20,42].
In the present study, the area of MGL did not change after IRPL treatment. This finding is in contrast with the only study evaluating before this parameter, which reported a 5% decrease of MGL after IPL in treatment-naïve patients [27]. However, this finding is controversial since a similar improvement was noted in the same study also in control patients treated with eyelid hygiene alone.
Subjective symptoms were investigated in the present study by both OSDI score and a five-grade scale specifically focused on patients’ perceived improvement in symptoms after treatment. Despite the lack of significant decrease of OSDI score after treatment, the vast majority of patients reported an overall improvement of discomfort symptoms, which was classified as moderate to high in almost half of the patients. This amelioration of subjective symptoms is in agreement with previous studies, which employed both validated questionnaires and specific scales of satisfaction [12,13,14,23,24,25,26,32,33,35].
As several options are available in the therapeutic algorithm of MGD, studies aiming at predicting patients who will most likely benefit from IRPL are desirable in order to guide the selection of the best patient population for this type of treatment. Therefore, we tried to identify which factors might predict a positive outcome of IRPL by analyzing the characteristics of patients before treatment. We found that patients with lower values of NIBUT at baseline showed a better response after IRPL treatment. On the other hand, none of the other parameters evaluated at baseline were able to discriminate patients who experienced the most benefit from treatment. In addition, significant negative correlations between each parameter and its variation after IRPL treatment were found, except for LLT. Therefore, those patients presenting with worse baseline value of one parameter experienced a greater improvement of the parameter after treatment.
The major limitation of the study is represented by the lack of a control group, which makes it difficult to rule out whether improvements could be related specifically to the IRPL treatment, the natural fluctuations of the disease course, or the placebo effect. Additionally, the relatively small size of the population might hamper detection of further significance in case of small differences among parameters. For this reason, we included both eyes of each patient in the final analysis, and this may represent a bias. However, since the intraclass correlation coefficients between right and left eyes were high, especially for LLT (0.876) and MGL (0.765) but also for tear osmolarity (0.634) and NIBUT (0.542), further studies with larger sample sizes including only one eye for each patient are desirable in order to confirm these findings.
In conclusion, IRPL for the treatment of patients with dry eye owing to MGD improved NIBUT, LLT, and tear osmolarity, as well as subjective symptoms. Patients with a higher tear film instability at baseline responded better to the procedure and would likely represent the ideal candidates for this treatment.
Author Contributions
Conceptualization, L.V., G.G.; methodology, L.V.; validation, L.V., F.C.; formal analysis, M.P.; investigation, Taroni L., S.S., F.B., A.M., N.D.S., V.S.; visualization, F.B., S.S.; data curation, L.V., M.P., F.C.; writing—original draft preparation, L.T., L.V.; writing—review and editing, G.G.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest. All the authors read and approved the submitted version.
References
- Craig, J.P.; Nichols, K.K.; Akpek, E.K.; Caffery, B.; Dua, H.S.; Joo, C.K.; Liu, Z.; Nelson, J.D.; Nichols, J.J.; Tsubota, K.; et al. TFOS DEWS II Definition and Classification Report. Ocul Surf. 2017, 15, 276–283. [Google Scholar] [CrossRef] [PubMed]
- Uchino, M.; Uchino, Y.; Dogru, M.; Kawashima, M.; Yokoi, N.; Komuro, A.; Sonomura, Y.; Kato, H.; Kinoshita, S.; Schaumberg, D.A.; et al. Dry eye disease and work productivity loss in visual display users: The Osaka study. Am. J. Ophthalmol. 2014, 157, 294–300. [Google Scholar] [CrossRef] [PubMed]
- Sato, E.A.; Matsumoto, Y.; Dogru, M.; Kaido, M.; Wakamatsu, T.; Ibrahim, O.M.; Obata, H.; Tsubota, K. Lacrimal gland in Sjogren’s syndrome. Ophthalmology 2010, 117, 1055. [Google Scholar] [CrossRef] [PubMed]
- Versura, P.; Giannaccare, G.; Pellegrini, M.; Sebastiani, S.; Campos, E.C. Neurotrophic keratitis: Current challenges and future prospects. Eye Brain 2018, 10, 37–45. [Google Scholar] [CrossRef]
- Giannaccare, G.; Bonifazi, F.; Sessa, M.; Fresina, M.; Arpinati, M.; Bandini, G.; Versura, P. Dry eye is already present in haematological patients before hematopoietic stem cell transplantation. Cornea 2016, 35, 638–643. [Google Scholar] [CrossRef]
- Versura, P.; Giannaccare, G.; Vukatana, G.; Mulè, R.; Malavolta, N.; Campos, E.C. Predictive role of tear protein expression in the early diagnosis of Sjogren syndrome. Ann. Clin. Biochem. 2018, 55, 561–570. [Google Scholar] [CrossRef]
- Qiao, J.; Yan, X. Emerging treatment options for meibomian gland dysfunction. Clin. Ophthalmol. 2013, 7, 1797–1803. [Google Scholar] [CrossRef]
- Nichols, K.K.; Foulks, G.N.; Bron, A.J.; Glasgow, B.J.; Dogru, M.; Tsubota, K.; Lemp, M.A.; Sullivan, D.A. The international workshop on meibomian gland dysfunction: Executive summary. Invest. Ophthalmol. Vis. Sci. 2011, 52, 1922–1929. [Google Scholar] [CrossRef]
- Baudouin, C.; Messmer, E.M.; Aragona, P.; Geerling, G.; Akova, Y.A.; Benítez-del-Castillo, J.; Boboridis, K.G.; Merayo-Lloves, J.; Rolando, M.; Labetoulle, M. Revisiting the vicious circle of dry eye disease: A focus on the pathophysiology of meibomian gland dysfunction. Br. J. Ophthalmol. 2016, 100, 300–306. [Google Scholar] [CrossRef]
- Raulin, C.; Greve, B.; Grema, H. IPL technology: A review. Lasers Surg. Med. 2003, 32, 78–87. [Google Scholar] [CrossRef]
- Papageorgiou, P.; Clayton, W.; Norwood, S.; Chopra, S.; Rustin, M. Treatment of rosacea with intense pulsed light: Significant improvement and long-lasting results. Br. J. Derm. 2008, 159, 628–632. [Google Scholar] [CrossRef] [PubMed]
- Craig, J.P.; Chen, Y.H.; Turnbull, P.R. Prospective trial of intense pulsed light for the treatment of meibomian gland dysfunction. Invest. Ophthalmol. Vis. Sci. 2015, 56, 1965–1970. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Lv, H.; Song, H.; Zhang, M.; Liu, Y.; Hu, X.; Li, X.; Wang, W. Evaluation of the Safety and Effectiveness of Intense Pulsed Light in the Treatment of Meibomian Gland Dysfunction. J. Ophthalmol. 2016, 2016, 1910694. [Google Scholar] [CrossRef] [PubMed]
- Albietz, J.M.; Schmid, K.L. Intense pulsed light treatment and meibomian gland expression for moderate to advanced meibomian gland dysfunction. Clin. Exp. Optom. 2018, 101, 23–33. [Google Scholar] [CrossRef] [PubMed]
- Guilloto Caballero, S.; García Madrona, J.L.; Colmenero Reina, E. Effect of pulsed laser light in patients with dry eye syndrome. Arch. Soc. Esp. Oftalmol. 2017, 92, 509–515. [Google Scholar] [CrossRef]
- Vigo, L.; Giannaccare, G.; Sebastiani, S.; Pellegrini, M.; Carones, F. Intense pulsed light treatment of dry eye owing to meibomian gland dysfunction. J. Vis. Exp. 2019. [Google Scholar] [CrossRef]
- Tomlinson, A.; Bron, A.J.; Korb, D.R.; Amano, S.; Paugh, J.R.; Pearce, E.I.; Yee, R.; Yokoi, N.; Arita, R.; Dogru, M. The international workshop on meibomian gland dysfunction: Report of the diagnosis subcommittee. Invest. Ophthalmol. Vis. Sci. 2011, 52, 2006–2049. [Google Scholar] [CrossRef]
- Giannaccare, G.; Vigo, L.; Pellegrini, M.; Sebastiani, S.; Carones, F. Ocular surface workup with automated noninvasive measurements for the diagnosis of meibomian gland dysfunction. Cornea 2018, 37, 740–745. [Google Scholar] [CrossRef]
- Maïssa, C.; Guillon, M. Tear film dynamics and lipid layer characteristics--effect of age and gender. Cont. Lens. Anterior. Eye 2010, 33, 176–182. [Google Scholar] [CrossRef]
- Finis, D.; Hayajneh, J.; König, C.; Borrelli, M.; Schrader, S.; Geerling, G. Evaluation of an automated thermodynamic treatment (LipiFlow®) system for meibomian gland dysfunction: A prospective, randomized, observer-masked trial. Ocul. Surf. 2014, 12, 146–154. [Google Scholar] [CrossRef]
- Ban, Y.; Shimazaki-Den, S.; Tsubota, K.; Shimazaki, J. Morphological evaluation of meibomian glands using noncontact infrared meibography. Ocul. Surf. 2013, 11, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Pult, H.; Riede-Pult, B.H. Non-contact meibography: Keep it simple but effective. Cont. Lens. Anterior. Eye 2012, 35, 77–80. [Google Scholar] [CrossRef] [PubMed]
- Toyos, R.; McGill, W.; Briscoe, D. Intense pulsed light treatment for dry eye disease due to meibomian gland dysfunction; a 3-year retrospective study. Photomed Laser. Surg. 2015, 33, 41–46. [Google Scholar] [CrossRef] [PubMed]
- Vegunta, S.; Patel, D.; Shen, J.F. Combination Therapy of Intense Pulsed Light Therapy and Meibomian Gland Expression (IPL/MGX) Can Improve Dry Eye Symptoms and Meibomian Gland Function in Patients With Refractory Dry Eye: A Retrospective Analysis. Cornea 2016, 35, 318–322. [Google Scholar] [CrossRef] [PubMed]
- Gupta, P.K.; Vora, G.K.; Matossian, C.; Kim, M.; Stinnett, S. Outcomes of intense pulsed light therapy for treatment of evaporative dry eye disease. Can. J. Ophthalmol 2016, 51, 249–253. [Google Scholar] [CrossRef]
- Dell, S.J.; Gaster, R.N.; Barbarino, S.C.; Cunningham, D.N. Prospective evaluation of intense pulsed light and meibomian gland expression efficacy on relieving signs and symptoms of dry eye disease due to meibomian gland dysfunction. Clin. Ophthalmol. 2017, 11, 817–827. [Google Scholar] [CrossRef]
- Yin, Y.; Liu, N.; Gong, L.; Song, N. Changes in the Meibomian Gland After Exposure to Intense Pulsed Light in Meibomian Gland Dysfunction (MGD) Patients. Curr. Eye Res. 2017, 4, 1–6. [Google Scholar] [CrossRef]
- Seo, K.Y.; Kang, S.M.; Ha, D.Y.; Chin, H.S.; Jung, J.W. Long-term effects of intense pulsed light treatment on the ocular surface in patients with rosacea-associated meibomian gland dysfunction. Cont. Lens. Anterior. Eye 2018, 41, 430–435. [Google Scholar] [CrossRef]
- Arita, R.; Fukuoka, S.; Morishige, N. Therapeutic efficacy of intense pulsed light in patients with refractory meibomian gland dysfunction. Ocul. Surf. 2019, 17, 104–110. [Google Scholar] [CrossRef]
- Rong, B.; Tang, Y.; Liu, R.; Tu, P.; Qiao, J.; Song, W.; Yan, X. Long-Term Effects of Intense Pulsed Light Combined with Meibomian Gland Expression in the Treatment of Meibomian Gland Dysfunction. Photomed. Laser Surg. 2018, 36, 562–567. [Google Scholar] [CrossRef]
- Mejia, L.F.; Gil, J.C.; Jaramillo, M. Intense pulsed light therapy: A promising complementary treatment for dry eye disease. Arch. Soc. Esp. Oftalmol. 2019, 94, 331–336. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Lin, S.B.; Cheng, B. Intense Pulsed Light Treatment for Meibomian Gland Dysfunction in Skin Types III/IV. Photobiomodul Photomed Laser. Surg. 2019, 37, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Rong, B.; Tu, P.; Tang, Y.; Song, W.; Toyos, R.; Yan, X. Analysis of Cytokine Levels in Tears and Clinical Correlations After Intense Pulsed Light Treating Meibomian Gland Dysfunction. Am. J. Ophthalmol. 2017, 183, 81–90. [Google Scholar] [CrossRef] [PubMed]
- Choi, M.; Han, S.J.; Ji, Y.W.; Choi, Y.J.; Jun, I.; Alotaibi, M.H.; Ko, B.Y.; Kim, E.K.; Kim, T.; Nam, S.M.; et al. Meibum Expressibility Improvement as a Therapeutic Target of Intense Pulsed Light Treatment in Meibomian Gland Dysfunction and Its Association with Tear Infammatory Cytokines. Sci. Rep. 2019, 9, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Arita, R.; Mizoguchi, T.; Fukuoka, S.; Morishige, N. Multicenter Study of intense pulsed light therapy for patients with refractory meibomian gland dysfunction. Cornea 2018, 37, 1566–1571. [Google Scholar] [CrossRef] [PubMed]
- Vora, G.K.; Gupta, P.K. Intense pulsed light therapy for the treatment of evaporative dry eye disease. Curr. Opin. Ophthalmol. 2015, 26, 314–318. [Google Scholar] [CrossRef]
- Dell, S.J. Intense pulsed light for evaporative dry eye disease. Clin. Ophthalmol. 2017, 11, 1167–1173. [Google Scholar] [CrossRef]
- Roy, N.S.; Wei, Y.; Kuklinski, E.; Asbell, P.A. The Growing Need for Validated Biomarkers and Endpoints for Dry Eye Clinical Research. Invest. Ophthalmol. Vis. Sci. 2017, 58, BIO1–BIO19. [Google Scholar] [CrossRef]
- Tsubota, K.; Yokoi, N.; Shimazaki, J.; Watanabe, H.; Dogru, M.; Yamada, M.; Kinoshita, S.; Kim, H.M.; Tchah, H.W.; Hyon, J.Y.; et al. Asia Dry Eye Society. New Perspectives on Dry Eye Definition and Diagnosis: A Consensus Report by the Asia Dry Eye Society. Ocul. Surf. 2017, 15, 65–76. [Google Scholar] [CrossRef]
- Tsubota, K. Short Tear Film Breakup Time-Type Dry Eye. Invest. Ophthalmol. Vis. Sci. 2018, 59, DES64–DES70. [Google Scholar] [CrossRef]
- Ahmed, S.A.; Taher, I.M.E.; Ghoneim, D.F.; Sawfat, A.E.M. Effect of Intense Pulsed Light Therapy on Tear Proteins and Lipids in Meibomian Gland Dysfunction. J. Ophthalmic Vis. Res. 2019, 14, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Meadows, J.F.; Ramamoorthy, P.; Nichols, J.J.; Nichols, K.K. Development of the 4-3-2-1 meibum expressibility scale. Eye Contact Lens. 2012, 38, 86–92. [Google Scholar] [CrossRef] [PubMed]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).