Improved Quantification of 18F-FDG PET during 131I-Rituximab Therapy on Mouse Lymphoma Models after 131I Prompt Emission Correction
Abstract
:1. Introduction
2. Materials and Methods
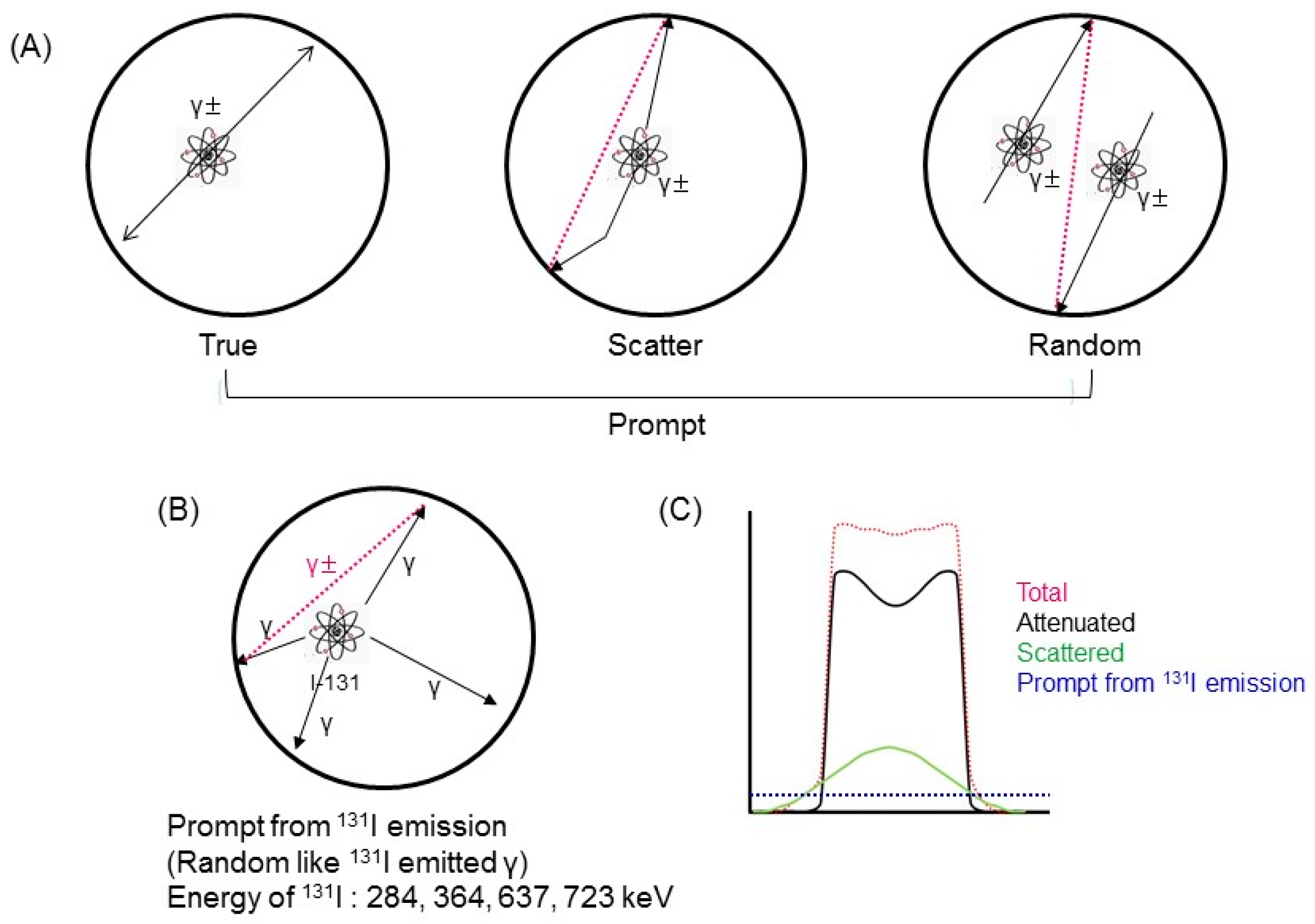
2.1. 131I Prompt Emissions during PET
2.2. GATE Simulation
2.3. Estimation of Prompt Emission Counting Rate due to A Single Gamma Photon from 131I
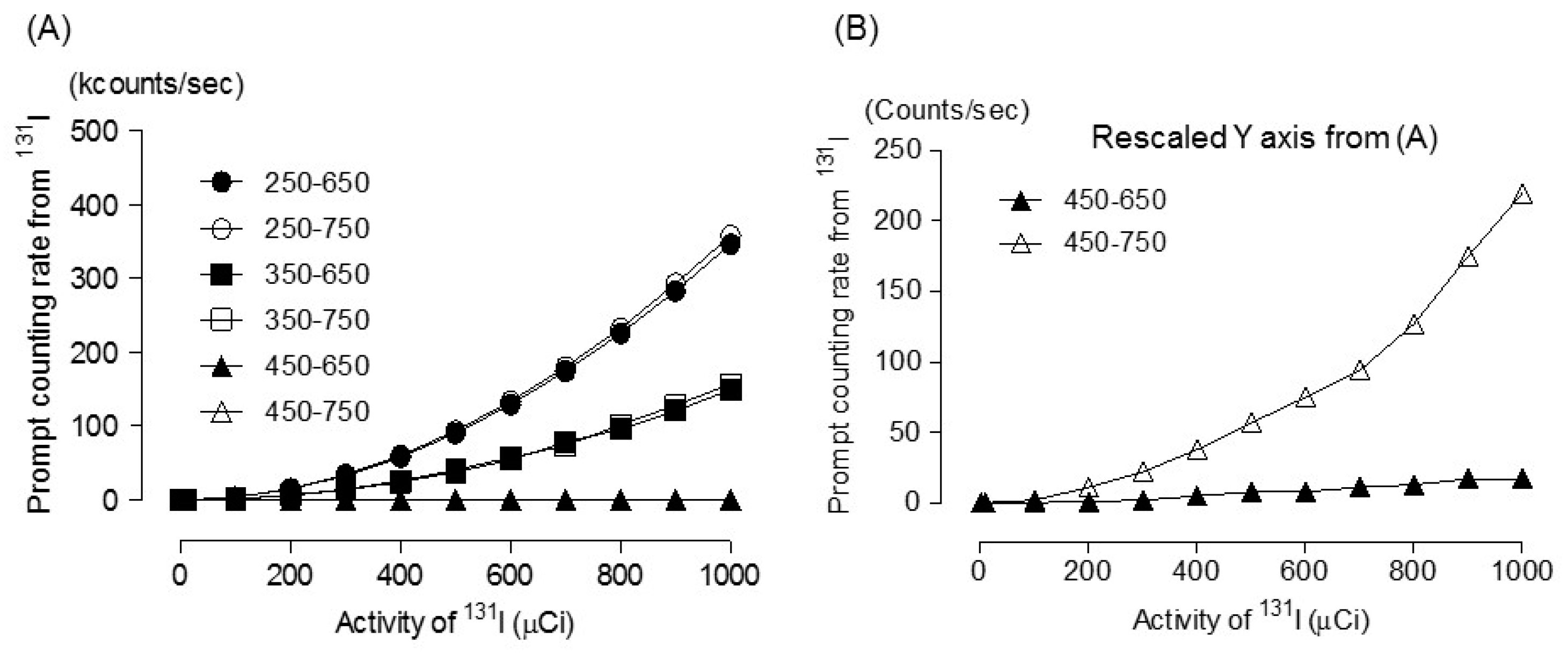
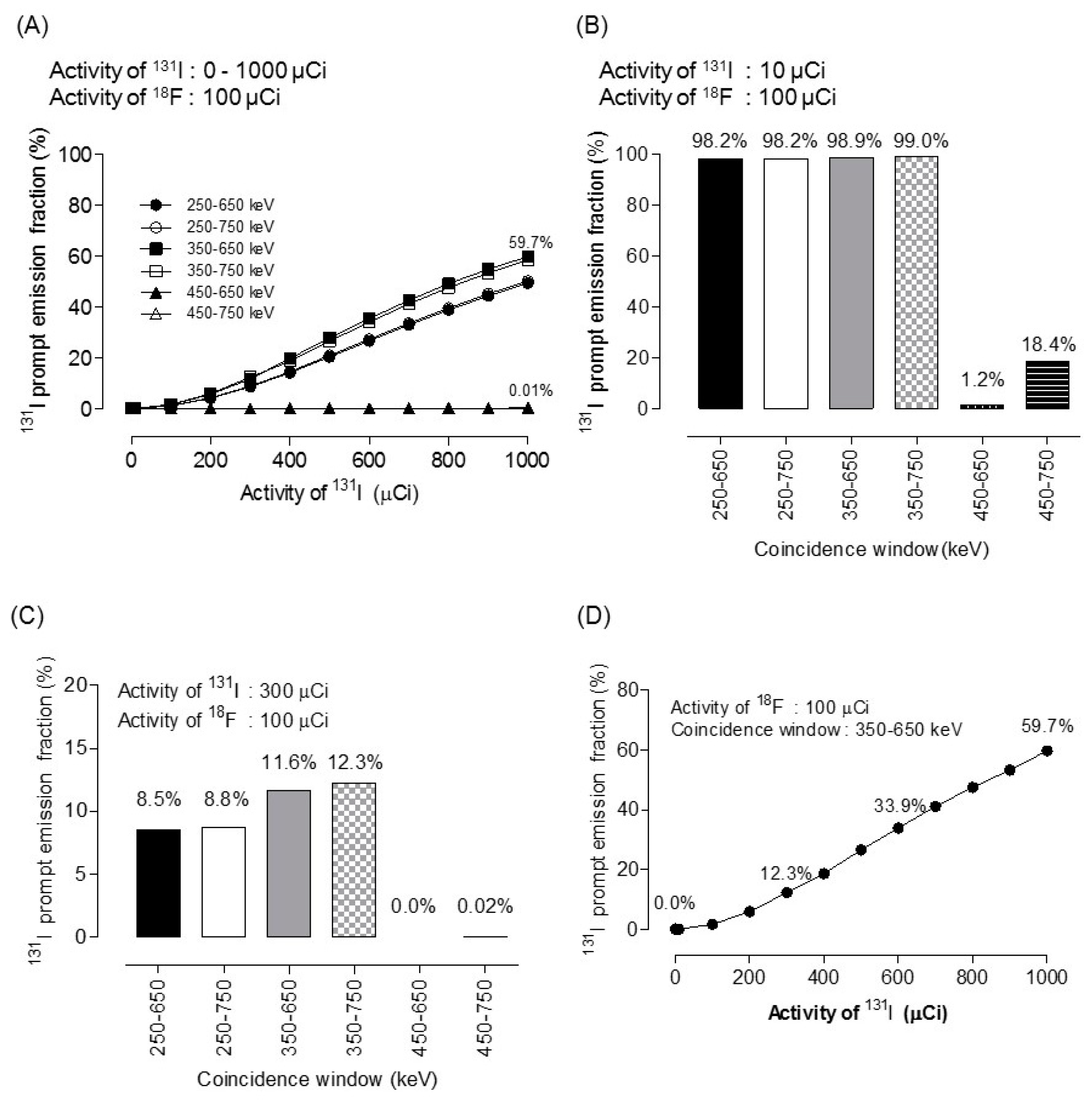
2.4. 131 I Prompt Emission Fraction
2.5. Correction of 131I Prompt Emission on 18F-FDG PET Imaging
2.6. Phantom Study (Spill-Over Ratio)
2.7. In Vivo Study
2.7.1. Lymphoma Animal Model
2.7.2. Preparation of 131I Rituximab
2.7.3. PET Imaging of Lymphoma Mouse Model
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Verel, I.; Visser, G.W.; van Dongen, G.A. The promise of immuno-PET in radioimmunotherapy. J. Nucl. Med. 2005, 46, 164S–171S. [Google Scholar] [PubMed]
- Pouget, J.P.; Navarro-Teulon, I.; Bardies, M.; Chouin, N.; Cartron, G.; Pelegrin, A.; Azria, D. Clinical radioimmunotherapy—The role of radiobiology. Nat. Rev. Clin. Oncol. 2011, 8, 720–734. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.S. Combination Radioimmunotherapy Approaches and Quantification of Immuno-PET. Nucl. Med. Mol. Imaging 2016, 50, 104–111. [Google Scholar] [CrossRef] [PubMed]
- Boerman, O.C.; Oyen, W.J. Immuno-PET of cancer: A revival of antibody imaging. J. Nucl. Med. 2011, 52, 1171–1172. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.J.; Lee, S.S.; Byun, B.H.; Kim, K.M.; Lim, I.; Choi, C.W.; Suh, C.; Kim, W.S.; Nam, S.H.; Lee, S.I.; et al. Repeated radioimmunotherapy with I-131-rituximab for patients with low-grade and aggressive relapsed or refractory B cell non-Hodgkin lymphoma. Cancer Chemother. Pharmacol. 2013, 71, 945–953. [Google Scholar] [CrossRef] [PubMed]
- Quackenbush, R.C.; Horner, T.J.; Williams, V.C.; Giampietro, P.; Lin, T.S. Patients with relapsed follicular lymphoma treated with rituximab versus tositumomab and iodine 131I tositumomab. Leuk. Lymphoma 2015, 56, 779–781. [Google Scholar] [CrossRef]
- Kobayashi, M.; Wakabayashi, H.; Kayano, D.; Konishi, T.; Kojima, H.; Yoneyama, H.; Okuda, K.; Tsushima, H.; Onoguchi, M.; Kawai, K.; et al. Application of a medium-energy collimator for 131I imaging after ablation treatment of differentiated thyroid cancer. Ann. Nucl. Med. 2014, 28, 551–558. [Google Scholar] [CrossRef]
- Hanscheid, H.; Lassmann, M.; Buck, A.K.; Reiners, C.; Verburg, F.A. The limit of detection in scintigraphic imaging with 131I in patients with differentiated thyroid carcinoma. Phys. Med. Biol. 2014, 59, 2353–2368. [Google Scholar] [CrossRef]
- Erdi, Y.E.; Macapinlac, H.; Larson, S.M.; Erdi, A.K.; Yeung, H.; Furhang, E.E.; Humm, J.L. Radiation Dose Assessment for 131I Therapy of Thyroid Cancer Using 124I PET Imaging. Clin. Positron Imaging 1999, 2, 41–46. [Google Scholar] [CrossRef]
- Jentzen, W.; Hobbs, R.F.; Stahl, A.; Knust, J.; Sgouros, G.; Bockisch, A. Pre-therapeutic 124I PET(/CT) dosimetry confirms low average absorbed doses per administered 131I activity to the salivary glands in radioiodine therapy of differentiated thyroid cancer. Eur. J. Nucl. Med. Mol. Imaging 2010, 37, 884–895. [Google Scholar] [CrossRef]
- Jentzen, W.; Hoppenbrouwers, J.; van Leeuwen, P.; van der Velden, D.; van de Kolk, R.; Poeppel, T.D.; Nagarajah, J.; Brandau, W.; Bockisch, A.; Rosenbaum-Krumme, S. Assessment of lesion response in the initial radioiodine treatment of differentiated thyroid cancer using 124I PET imaging. J. Nucl. Med. 2014, 55, 1759–1765. [Google Scholar] [CrossRef] [PubMed]
- Maruoka, Y.; Abe, K.; Baba, S.; Isoda, T.; Kitamura, Y.; Mizoguchi, N.; Akamatsu, G.; Sasaki, M.; Honda, H. Usefulness of partial volume effect-corrected 18F FDG PET/CT for predicting 131I accumulation in the metastatic lymph nodes of patients with thyroid carcinoma. Ann. Nucl. Med. 2013, 27, 873–879. [Google Scholar] [CrossRef] [PubMed]
- Nowosinska, E.; Chan, P.S.; Buscombe, J.R. Use of 18F FDG PET and the short temporal response of Hodgkin’s disease to RIT. World J. Nucl. Med. 2018, 17, 171–177. [Google Scholar] [CrossRef] [PubMed]
- Byun, B.H.; Na, I.I.; Cheon, G.J.; Kang, H.J.; Kim, K.M.; Lee, S.S.; Ryoo, B.Y.; Choi, C.W.; Lim, S.M.; Yang, S.H. Clinical significance of 18F FDG uptake by primary sites in patients with diffuse large B cell lymphoma in the head and neck: A pilot study. Ann. Nucl. Med. 2008, 22, 645–651. [Google Scholar] [CrossRef]
- An, Y.S.; Yoon, J.K.; Lee, S.J.; Jeong, S.H.; Lee, H.W. Clinical significance of post-treatment 18F fluorodeoxyglucose uptake in cervical lymph nodes in patients with diffuse large B-cell lymphoma. Eur. Radiol. 2016, 26, 4632–4639. [Google Scholar] [CrossRef]
- Kesavan, M.; Boucek, J.; MacDonald, W.; McQuillan, A.; Turner, J.H. Imaging of Early Response to Predict Prognosis in the First-Line Management of Follicular Non-Hodgkin Lymphoma with 131I -Rituximab Radioimmunotherapy. Diagnostics 2017, 7, 26. [Google Scholar] [CrossRef]
- Schrepfer, T.; Haerle, S.K.; Strobel, K.; Schaefer, N.; Halg, R.A.; Huber, G.F. The value of 18F fluorodeoxyglucose positron emission tomography/computed tomography for staging of primary extranodal head and neck lymphomas. Laryngoscope 2010, 120, 937–944. [Google Scholar] [CrossRef]
- Kim, J.S.; Yu, A.R. Determination of single gamma photon fraction of 124I PET. J. Instrum. 2015, 10. [Google Scholar] [CrossRef]
- Yu, A.R.; Kim, H.J.; Lim, S.M.; Kim, J.S. Comparison of Imaging Characteristics of 124I PET for Determination of Optimal Energy Window on the Siemens Inveon PET. Biomed. Res. Int. 2016, 2016. [Google Scholar] [CrossRef]
- Gregory, R.A.; Hooker, C.A.; Partridge, M.; Flux, G.D. Optimization and assessment of quantitative 124I imaging on a Philips Gemini dual GS PET/CT system. Eur. J. Nucl. Med. Mol. Imaging 2009, 36, 1037–1048. [Google Scholar] [CrossRef]
- Moncayo, V.M.; Garcia, E.V. Prompt-gamma compensation in 82Rb myocardial perfusion 3D PET/CT: Effect on clinical practice. J. Nucl. Cardiol. 2018, 25, 606–608. [Google Scholar] [CrossRef] [PubMed]
- Esteves, F.P.; Nye, J.A.; Khan, A.; Folks, R.D.; Halkar, R.K.; Garcia, E.V.; Schuster, D.M.; Lerakis, S.; Raggi, P.; Votaw, J.R. Prompt-gamma compensation in 82Rb myocardial perfusion 3D PET/CT. J. Nucl. Cardiol. 2010, 17, 247–253. [Google Scholar] [CrossRef] [PubMed]
- Werling, A.; Bublitz, O.; Doll, J.; Adam, L.E.; Brix, G. Fast implementation of the single scatter simulation algorithm and its use in iterative image reconstruction of PET data. Phys. Med. Biol. 2002, 47, 2947–2960. [Google Scholar] [CrossRef] [PubMed]
- National Electrical Manufacturers Association. Performance Measurements of Small Animal Positron Emission Tomographs. In NEMA Standards Publication NU 4-2008; NEMA: Rosslyn, VA, USA, 2008. [Google Scholar]
- Kim, J.S.; Lee, J.S.; Park, M.J.; Hong, S.J.; Lee, D.S. Comparative evaluation of three microPET series systems using Monte Carlo simulation: Sensitivity and scatter fraction. In Proceedings of the IEEE Nuclear Science Symposium Conference Record (NSS ’07), Honolulu, HI, USA, 26 October–3 November 2007; pp. 4534–4535. [Google Scholar]
- Lin, H.H.; Chuang, K.S.; Chen, S.Y.; Jan, M.L. Recovering the triple coincidence of non-pure positron emitters in preclinical PET. Phys. Med. Biol. 2016, 61, 1904–1931. [Google Scholar] [CrossRef]
- Wierts, R.; Conti, M.; Claessen, A.G.; Herrmann, K.; Kemerink, G.J.; Binse, I.; Wildberger, J.E.; Mottaghy, F.M.; Backes, W.H.; Jentzen, W. Impact of prompt gamma coincidence correction on absorbed dose estimation in differentiated thyroid cancer using 124I PET/CT imaging. Nucl. Med. Commun. 2018, 39, 1156–1164. [Google Scholar] [CrossRef]
- Laforest, R.; Liu, X. Cascade removal and microPET imaging with 76Br. Phys. Med. Biol. 2009, 54, 1503–1531. [Google Scholar] [CrossRef]
- Beattie, B.J.; Finn, R.D.; Rowland, D.J.; Pentlow, K.S. Quantitative imaging of 76Br and 86Y with PET: A method for the removal of spurious activity introduced by cascade gamma rays. Med. Phys. 2003, 30, 2410–2423. [Google Scholar] [CrossRef]


| Activity of 131I * (µCi) | Energy Window (keV) | |||
|---|---|---|---|---|
| 350–650 | 350–750 | 450–650 | 450–750 | |
| 1 | 0.00 | 0.00 | 0.00 | 0.00 |
| 10 | 0.01 | 0.01 | 0.00 | 0.00 |
| 100 | 1.45 | 1.60 | 0.00 | 0.00 |
| 200 | 5.59 | 5.88 | 0.00 | 0.01 |
| 300 | 11.64 | 12.25 | 0.00 | 0.02 |
| 400 | 19.70 | 18.65 | 0.00 | 0.04 |
| 500 | 27.85 | 26.53 | 0.01 | 0.05 |
| 600 | 35.31 | 33.92 | 0.01 | 0.07 |
| 700 | 42.53 | 41.12 | 0.01 | 0.09 |
| 800 | 49.08 | 47.54 | 0.01 | 0.12 |
| 900 | 54.81 | 53.30 | 0.02 | 0.17 |
| 1000 | 58.50 | 59.74 | 0.02 | 0.21 |
| 10,000 | 98.96 | 99.01 | 1.28 | 18.48 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, Y.S.; Kim, H.-J.; Kim, J.S. Improved Quantification of 18F-FDG PET during 131I-Rituximab Therapy on Mouse Lymphoma Models after 131I Prompt Emission Correction. Diagnostics 2019, 9, 144. https://doi.org/10.3390/diagnostics9040144
Lee YS, Kim H-J, Kim JS. Improved Quantification of 18F-FDG PET during 131I-Rituximab Therapy on Mouse Lymphoma Models after 131I Prompt Emission Correction. Diagnostics. 2019; 9(4):144. https://doi.org/10.3390/diagnostics9040144
Chicago/Turabian StyleLee, Young Sub, Hee-Joung Kim, and Jin Su Kim. 2019. "Improved Quantification of 18F-FDG PET during 131I-Rituximab Therapy on Mouse Lymphoma Models after 131I Prompt Emission Correction" Diagnostics 9, no. 4: 144. https://doi.org/10.3390/diagnostics9040144
APA StyleLee, Y. S., Kim, H.-J., & Kim, J. S. (2019). Improved Quantification of 18F-FDG PET during 131I-Rituximab Therapy on Mouse Lymphoma Models after 131I Prompt Emission Correction. Diagnostics, 9(4), 144. https://doi.org/10.3390/diagnostics9040144





