1. Introduction
COPD has a significant impact on individuals and society. Moreover, COPD represents an economic burden on the healthcare system. Statistics Canada [
1], ranked COPD as the fifth leading cause of death in the country. Studies show that people with COPD are vulnerable to many natural events, environmental factors, and sudden worsening of any of the symptoms associated with this disease. Recent years have witnessed a widespread increase in the number of telemedicine projects. This kind of intervention can open a window onto the COPD patient’s life to assist with self-management and prevent declines. Telehealth refers to the remote monitoring and care of patients outside of the hospital setting. Typically, these systems are used with certain chronic diseases that are associated with frequent relapses. The early detection of worsening symptoms will help patients avoid severe problems and lengthy hospital stays [
2]. The role of telemedicine in COPD is still being discussed. In 2018, Dr. Jean Bourbeau, a senior scientist at the research institute of McGill University Health Center, said that “telemedicine, both its application and results, is still controversial in COPD and the monitoring of physiological parameters does not solve the problem of predicting exacerbations that could lead to early therapy and prevention of hospital admissions” [
3]. Recent studies cast serious doubts on the research findings in this domain [
4,
5,
6].
However, pulmonologists still believe that telemonitoring can play an extremely important role if used properly. Brian Carlin, a pulmonary specialist and former chairman of the COPD Alliance, confirms that recognizing the main triggers in each patient is the best health protection plan to prevent flare-ups and thereby slow the progression of the disease; this is through maintaining an active lifestyle in a controlled environment without being exposed to such triggers. Specialists in this field suggested that “it may be more valuable to build the telemonitoring and telecommunication technology in the management of COPD on what we already know” [
3]. This requires working on three different levels: first, find the safe range of environmental factors; second, adjust the normal limits of relevant biomarkers; and third, determine the external influences (e.g., food, excessive physical effort, climatic factors) on the patient’s body. The main contribution of this approach resides in the intelligent monitoring and control of persistent changes in the physiological parameters and the ambient environment. This work was part of a concentrated effort to create adaptive safe ranges for personalized biomarkers, where the normal values of these vital signs are often affected by the medical profile, the type of current exercise, the place, and the weather. Environmental factors are also one of the COPD irritants, where cumulative exposure to a multitude of climate hazards such as improper humidity levels or extreme weather temperatures, both indoor and outdoor air pollution, in addition to the abnormal concentrations of oxygen in the atmosphere, may threaten a patient’s lung health. Developing dynamic alarm thresholds is an important contribution because that would promote the services provided and increase the value of telemonitoring in self-management. Moreover, a customized threshold will help decrease the proportion of false alarms and differentiate between true exacerbation and normal variation. To achieve these goals, there is a need to develop a comprehensive representation of knowledge to capture the real context of the patient in order to avoid misdiagnosis and allow the dynamic reconfiguration of the health disorders threshold.
Rule-based ontology to support context-aware systems offers potential solutions to the multiscale nature of COPD. Many context-aware architectures have been proposed, but there is no published and validated research on the effectiveness of these computing paradigms within the context of COPD. In a previous work [
7], we designed an ontological reasoning framework that provides a rules-driven, context-aware system for COPD patients. In this article, we will present the validation of that proposition, and demonstrating its efficiency through simulated examples of real-life scenarios and empirical data about the environment, activities, symptoms, and physiological parameters. For this purpose, we explain in detail the methods for extracting the medical rules of different contextual events. The paper examines the normal ranges of vital parameters during different activities of daily living, and sets a threshold limit for the environmental conditions, whether indoors or outdoors, which is adapted to suit each patient’s medical profile.
The rest of the paper is organized as follows. In
Section 2, we review the existing telemonitoring platforms and ontology-based models, especially those designed for chronic pulmonary disease, and show the weaknesses that make them non-viable. In
Section 3, we review the steps involved in this research and highlight our vision to deal with COPD. This hypothesis is based on comprehensive ontology and medical rules. In
Section 4, we explain how we extract the SWRL rules using data analysis. In
Section 5, we discuss the data collection process, then describe our implementation in detail and provide a performance analysis and results in
Section 6,
Section 7, and
Section 8. Finally, the work is concluded in
Section 9.
2. Related Works
For almost two decades now, the use of medical ontologies has no longer been limited to defining medical terminologies such as the systematized nomenclature of medicine—clinical terms (SNOMED CT) or the unified medical language system (UMLS), but has also become one of the most powerful solutions for tackling serious health problems and supporting the management of large amounts of complex data. Ontologies have also been used in hundreds of research projects concerned with medical issues such as diagnosis, self-management, and treatment [
8,
9,
10,
11,
12].The ontological approach proved its effectiveness in the remote healthcare arena; for instance, Lasierra [
13] and Rubio et al. [
14] have presented robust examples of ontology usage in the telemonitoring domain for generic and specific chronic diseases. Lasierra proposed an autonomic computing ontology for integrated management at home using medical sensors. Rubio provides a formal representation of knowledge to describe the effect of technological context variations on clinical data quality and its impact on a patient’s treatment. Another example can be found in [
15]: Benyahia et al. developed a generic ontology for monitoring patients diagnosed with chronic diseases. The proposed architecture aims to detect any anomalies or dangerous situations by collecting physiological and lifestyle data. Hristoskova et al. [
16] presented an ontology-based ambient intelligence framework that supports real-time physiological monitoring of patients suffering from congestive heart failure. Ryu et al. [
17] proposed a ubiquitous healthcare context model using an ontology; the model extracts the contextual information for implementing the healthcare service, taking into consideration the medical references and environments. Jong et al. [
18] has designed an interactive healthcare system with wearable sensors that provides personalized services with formal ontology-driven specifications. In the same setting, an ontology-based context-aware framework for customized care has been presented by Ko et al. [
19] as a form of wearable biomedical technology. An interesting projection of ontology in this domain can be found in [
20], in which the authors built a context-aware mobile service aiming at supporting mobile caregivers and sharing information to improve the quality of life of people living with chronic diseases.
In addition to this obvious interest in ontology, most healthcare projects related to computer-assisted medical decision-making are often modeled using rule-based approaches. Semantic web rule language (SWRL) has emerged over existing W3C web ontology language (OWL) axioms to promote the expressiveness of the semantic web. The combination of OWL and SWRL specifications provides further inference capabilities beyond the inductive classification of description logics, with 78 built-in functions categorized across the comparisons, mathematics, Boolean values, strings, date, time and duration, URIs, and lists [
21]. In the medical environment, there are several uses of rules; for example, if‒then rules can be used for chaining or mapping ontologies properties to achieve knowledge integration. By applying rules, the pattern of behaviors of all entities can be expressed, which would produce new facts and tailored services. Some examples of the incorporation of rules in healthcare ontologies as an essential component of decision support applications can be found in [
22,
23,
24,
25]. These rules are written in specific terms to infer useful information and then provide personalized care services to chronic patients according to their situations. For example, [
22] established a set of predefined rules to trigger alarms when critical threshold levels are exceeded, while [
23,
24,
25] used ontology-based rules for ubiquitous computing that allow for monitoring health anytime and anywhere. Furthermore, a few research projects have studied the use of SWRL to aid in diagnosis. These include [
26] and [
27], which provided rule-based ontologies to diagnose heart diseases and diabetes, respectively.
The use of ontology in COPD is only restricted to certain aspects of patients’ lives [
8,
11]. For example, the authors of [
12] developed an ontology inspired by the autonomic computing paradigm that provides configurable services to support home-based care. The authors of [
14] proposed a predictive model to extract relevant attributes and enable the early detection of deteriorations, but the proposed ontology aims at describing the basic structure of the application. Although a significant amount of research has been done to assess the importance of telehealth in COPD, the concept of integrated care services is still in its infancy. The use of semantic mapping between the physiological parameters, environmental factors, symptoms, physical activity, and patient-specific data to construct a telemonitoring system for COPD using ontologies was not found in the literature. This work will be the first building block for creating a comprehensive primary e-healthcare delivery system, capable of organizing various daily life scenarios for COPD patients in a healthy and safe environment.
3. Context-Aware System
Pervasive computing is considered one of the most impactful scientific achievements of the last decade. This conception created a revolution in end-user interactions through the concept of context awareness. Pervasive computing offers a new opportunity to redesign the pattern of conventional solutions as it can easily tailor its processes based on existing contextual situations. Many theoretical architectures have been proposed to enable context-aware computing in pervasive settings, especially in the healthcare domain. The overall architecture of context-aware systems and development process can be found in [
7,
28]. In this work, we distinguish four main components: context acquisition, representation, reasoning, and application. Context acquisition functions allow for querying physical devices to obtain contextual data. Given the various characteristics of contextual information such as heterogeneity, dynamicity, and imperfection, it is essential to define a model to describe these data. There are three main types of approaches to represent the context: the key-value approach, the object-oriented approach, and the ontology-based approach. On top of the context information, reasoning schemes are implemented to develop applications and services for particular needs.
In this work, an ontology-driven rules base for an expert medical system is described and applied. The proposed system provides an intelligent monitoring infrastructure to keep track of the physical status of patients, suggest recommendations, and deliver interventions in a timely manner.
This process involves observing and controlling the behavior of physiological parameters and the surrounding environment. Consequently, the system adapts the safe ranges for the vital signs in proportion to demographic factors, medical profile, physical activity, and external ambiance. The presented project demonstrated its benefits not only in terms of real-time responses, but also in predicting the body’s changes in advance based on the effects of potential triggers. Due to the complexity of this domain of knowledge, we established a scenario-based approach to coordinate the evolution of such a decision-making system. The construction of scenarios is governed by executing a sequential modular strategy and evidence-based rules. This architecture is designed and implemented in four distinct layers: the acquisition layer is dedicated to collecting and properly transmitting different sorts of data, such as the medical profile of the COPD patient, biomarkers, and environmental information, whether gathered from wearable or fixed monitoring sensors. The semantic layer or the ontological schema has been used to interpret complex information and translate the real context of the patient into machine-understandable and accessible language. The generic representation of the semantic layer consists of a set of interdependent ontologies and many concepts related to the pulmonary disease, environment, devices, personal and medical information of the patient [
3]. At the macro level, the telemonitoring system aims to detect all the possible hazardous events that could influence COPD patient. Since OWL has expressivity limitations on representing many types of contextual information, especially if‒then statements, our ontologies have been extended with forward-chaining rules. These rules were expressed in the semantic web rule language (SWRL) to describe all implications and consequences. The proposed rules are extracted from data analysis, existing medical guidelines, and the opinions of pneumologists. Practically, these rules are used by an inference engine to derive new facts, detect events, and predict potential risks. The novelty of these rules lies in the dynamic structure, which has the capacity to configure and reconfigure the secure boundaries according to the current circumstances and contexts.
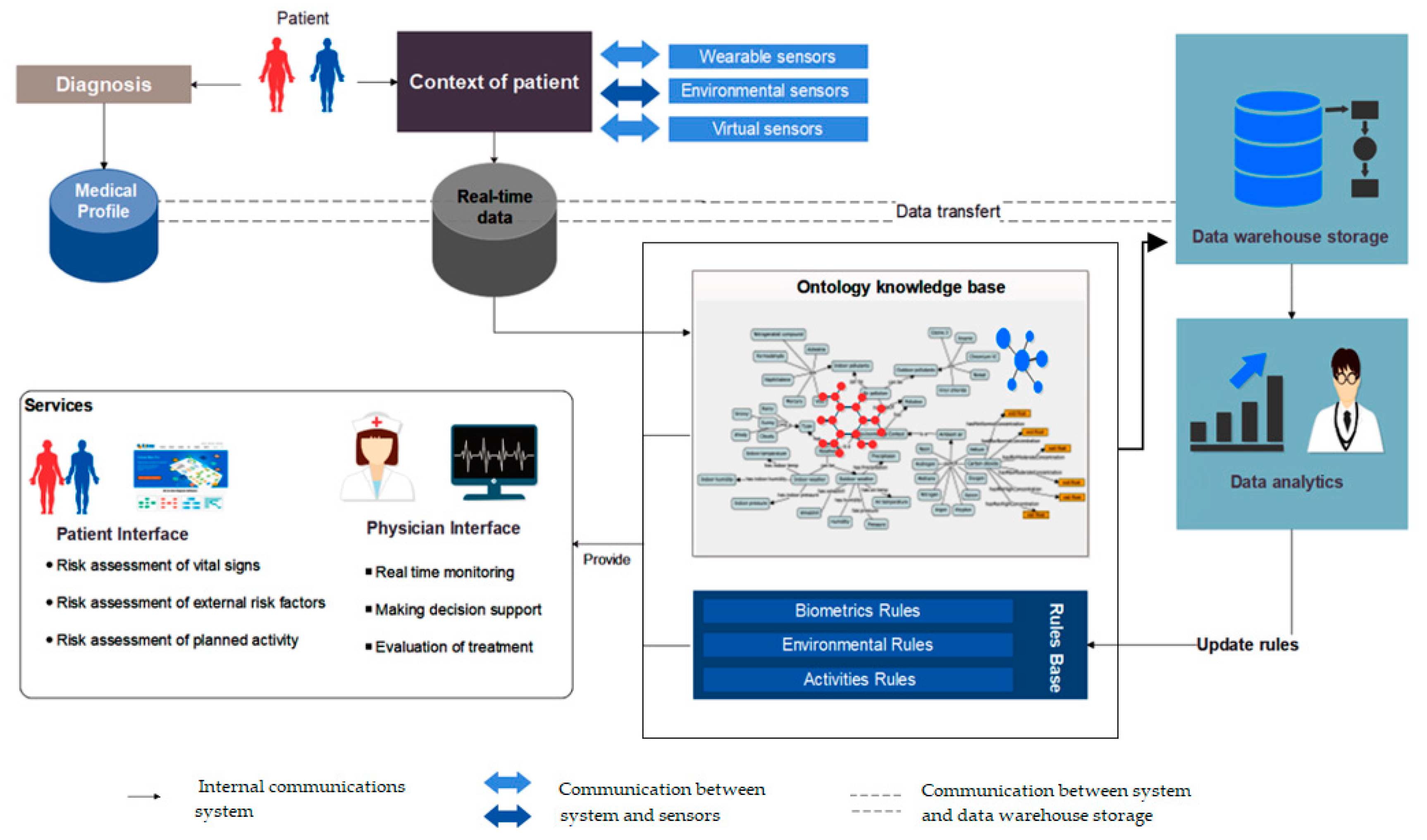
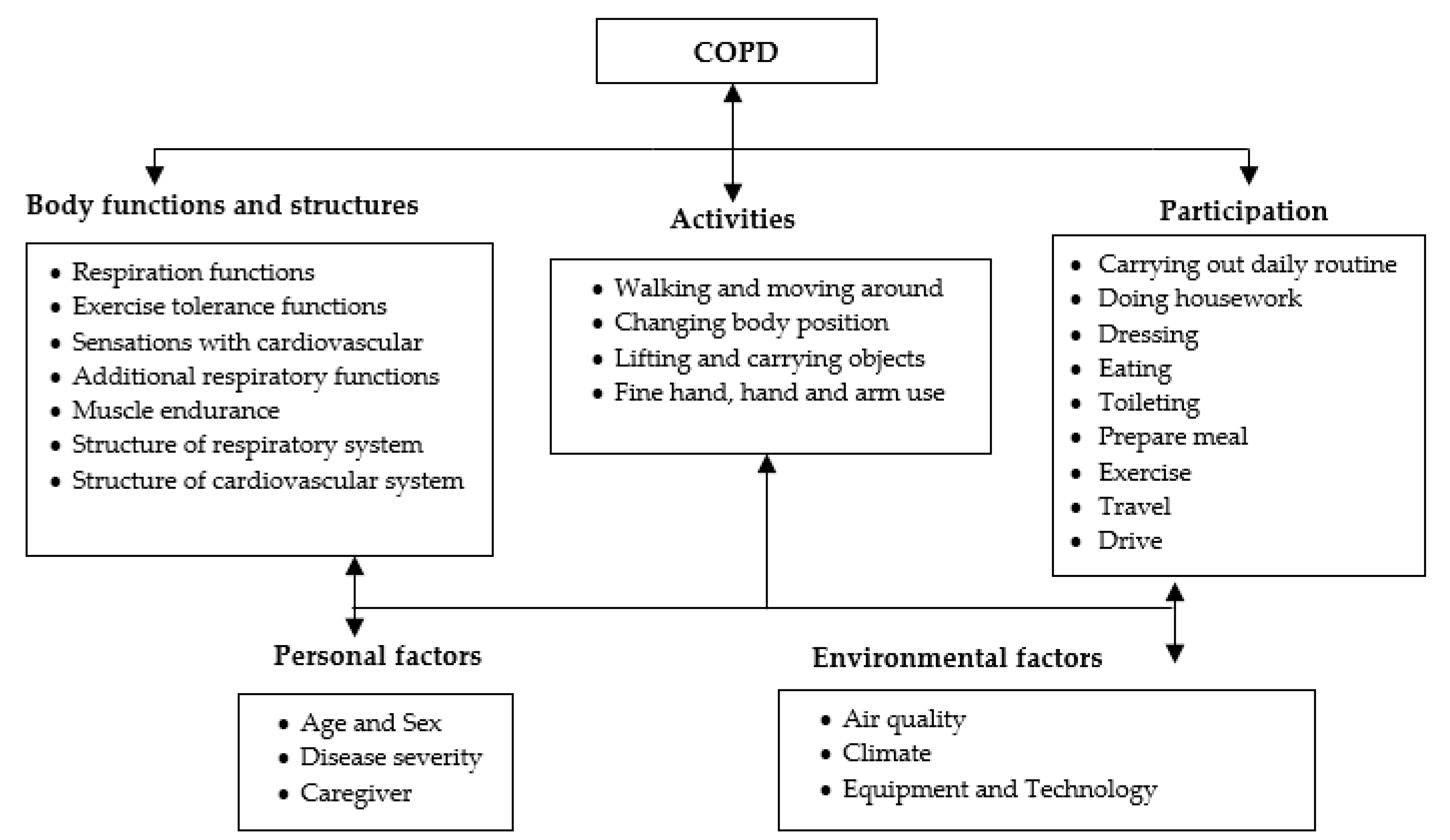
Figure 1 provides an explanation of the relationships between the constituent entities of the system in a simplified manner. The operational environment of this system is divided into four main parts. The first part is the collection of data. These data are distributed between the patient’s medical profile, obtained at the diagnostic stage, and the contextual information that we can obtain from virtual and real sensors. All this information must be kept in customized databases and kept consistent with the purpose, whether in the form of electronic medical records or conventional databases to manage the real-time context. The second part is the leading core of this system, where all the interpretation processes are carried out. This part is an ontology knowledge base, backed by complex medical rules that aim to draw a semi-complete representation of the patient’s life scenarios in a semantic programming language. In practice, the first and second parts are highly intertwined, so the collected data serve as input to the ontology, which in turn extracts the existing scenario and applies the rules of protection to ensure that patients are notified early on of any potential risk-related events.
The third part is related to system functions; the services offered by the system are divided into two main categories: (1) patient services, which include assessment of the vital signs, evaluation of the external risk factors, and estimation of the overall effects of activities. (2) caregivers’ services, which are supposed to provide multi-task interfaces for real-time monitoring, decision-making, and treatment evaluation. The last part controls all background processes. Dynamicity is one of the main features of this system. It develops its facts continuously since it sends raw and derived information periodically to the data warehouses for further analysis. This cycle allows the system to update its rules and promote personalized health services. In the next section, we will learn about the methods of extracting the medical rules of COPD.
6. Implementation
As seen in the previous section, the experimental available datasets consist of values collected at different observational units. So, we synchronized the time scale between these data without affecting the accuracy, and mapped the simulated representation of the real-life activity with corresponding vital signs, according to the medical file. Therefore, the new dataset consists of three fully connected observations, the activities that have been generated using the MATLAB, the environmental conditions that are captured by real sensors, and the biomarkers that are obtained from medical sources. The simulated scenarios revolve around creating sequential records over a 12-month period for COPD patients with different levels of disease severity and autonomy. Each record is a description of the activities performed, the environmental factors, and the vital signs at a certain time. The objective of this process was to create a dataset of the COPD parameters associated with variation in the bodily activity and surrounding factors. The obtained databases consist of a total of 104 parameters, giving distinct advantages over previous studies even though the accuracy is unknown. For example, this dataset utilizes the best available environmental characteristics dataset for weather conditions and outdoor pollutants in Canada; it also uses real-indoor observations taken from a credible research project. The sequences of daily living activities were generated through Markov models and algorithms for probabilistic logic, while the medical profile has been issued by reliable medical sources. To prove the advantages of this work, we conducted many experimental simulations for the whole scenario of biological reactions to physical activities and external influences over 12 months in 100 virtual patients. First, we considered the scenarios of COPD patients having the same level of dependency but in different stages of the disease. Secondly, we simulated COPD in elderly patients who have lost some of their physical ability by excluding some kinds of autonomous activities of daily living tasks. Thirdly, for accuracy reasons, we created our simulations to compare the efficiency of this system during the winter, spring, summer, and autumn. We evaluated the performance of the proposed system for the identification of abnormal situations or patterns that may pose serious health risks for COPD patients. This evaluation consists of quantifying the level of computing or compliance with the expected time and the capacities of rules repository. COPD patients’ needs change according to their medical profiles and the type of hazards. The adaptation services with these changes are an essential point to test in such a remote healthcare system; therefore, it is of paramount importance to deliver a quick and accurate response to a sudden decline in vital parameters and general health status. Using a specific amount of data, the system categorizes the monitored conditions as either normal or abnormal. In the next section, we briefly describe the implementation steps of this project using Protégé that was developed by the Stanford Center for biomedical informatics research at the Stanford University School of Medicine.
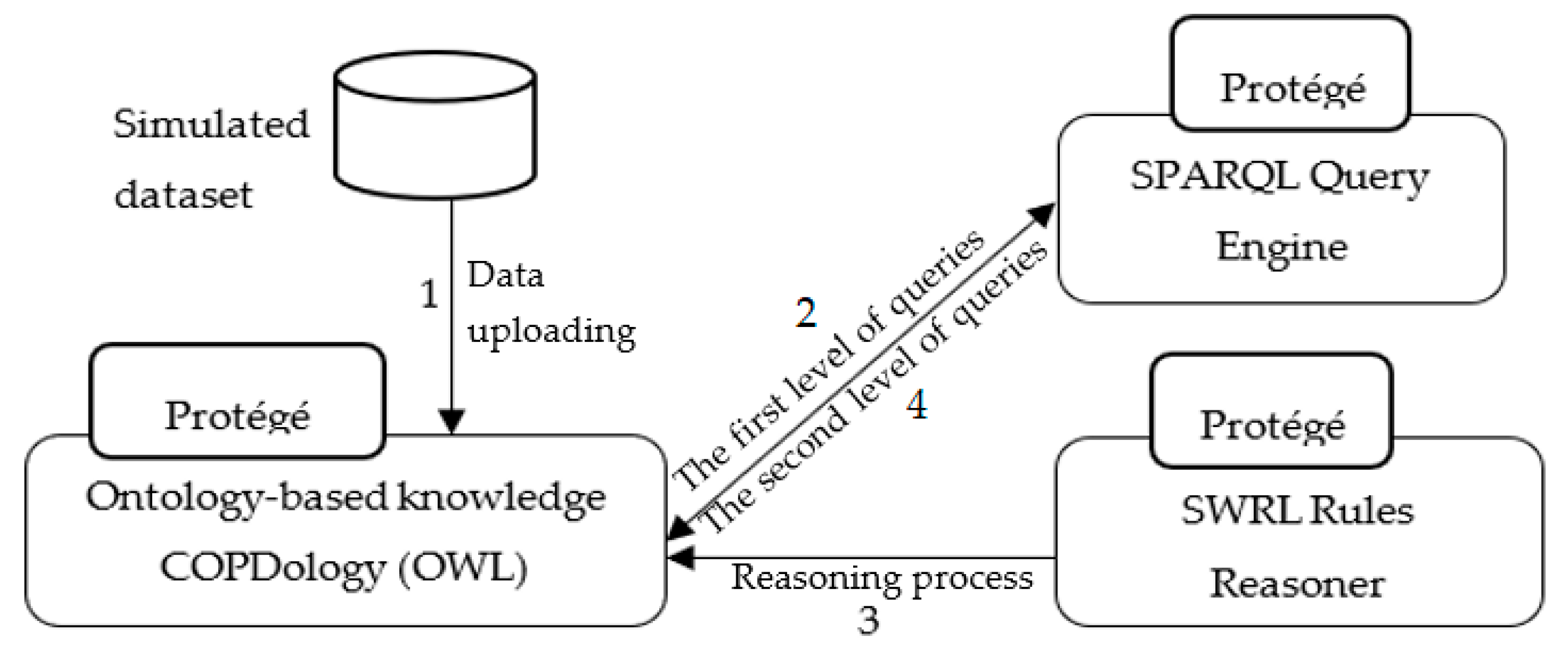
The initial implementation presented in the
Figure 12 is carried out in the following manner: (1) the simulated data, stored in Excel spreadsheet files, will be uploaded directly to Ontology-based knowledge using Cellfie plugin. (2) The SPARQL query engine accesses the Knowledge Base to retrieve information from a patient’s profile regarding current location and activity, etc. (3) The SWRL rule reasoner adds additional information such as normal ranges, and appropriate environment, to the Knowledge Base. Moreover, the SWRL rule reasoner performs reasoning on the updated Knowledge Base of COPD domain and newly inferred facts are added to the Knowledge Base. (4) The SPARQL query engine accesses the Knowledge Base to retrieve notifications and recommendations according to the patient’s context.
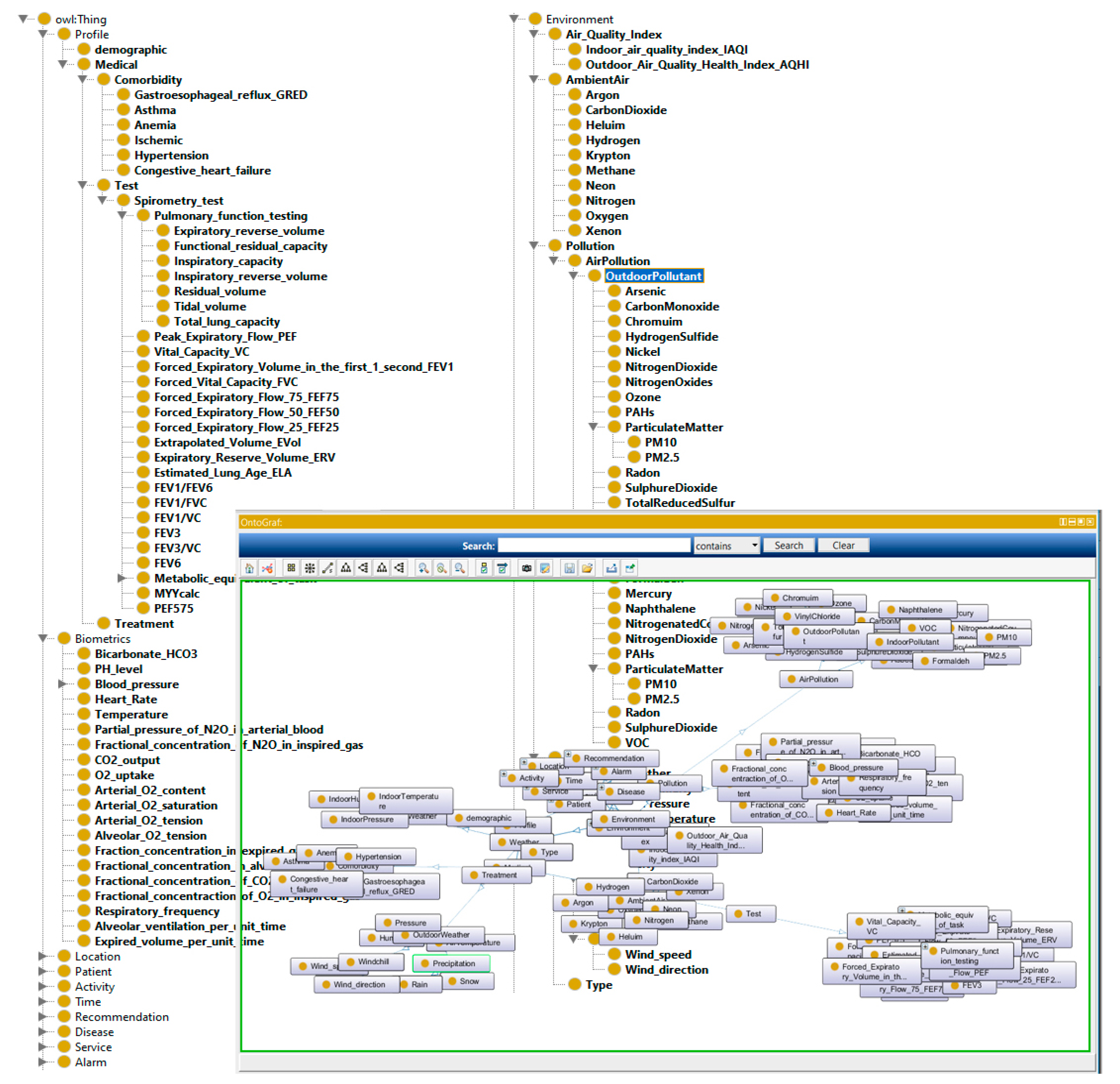
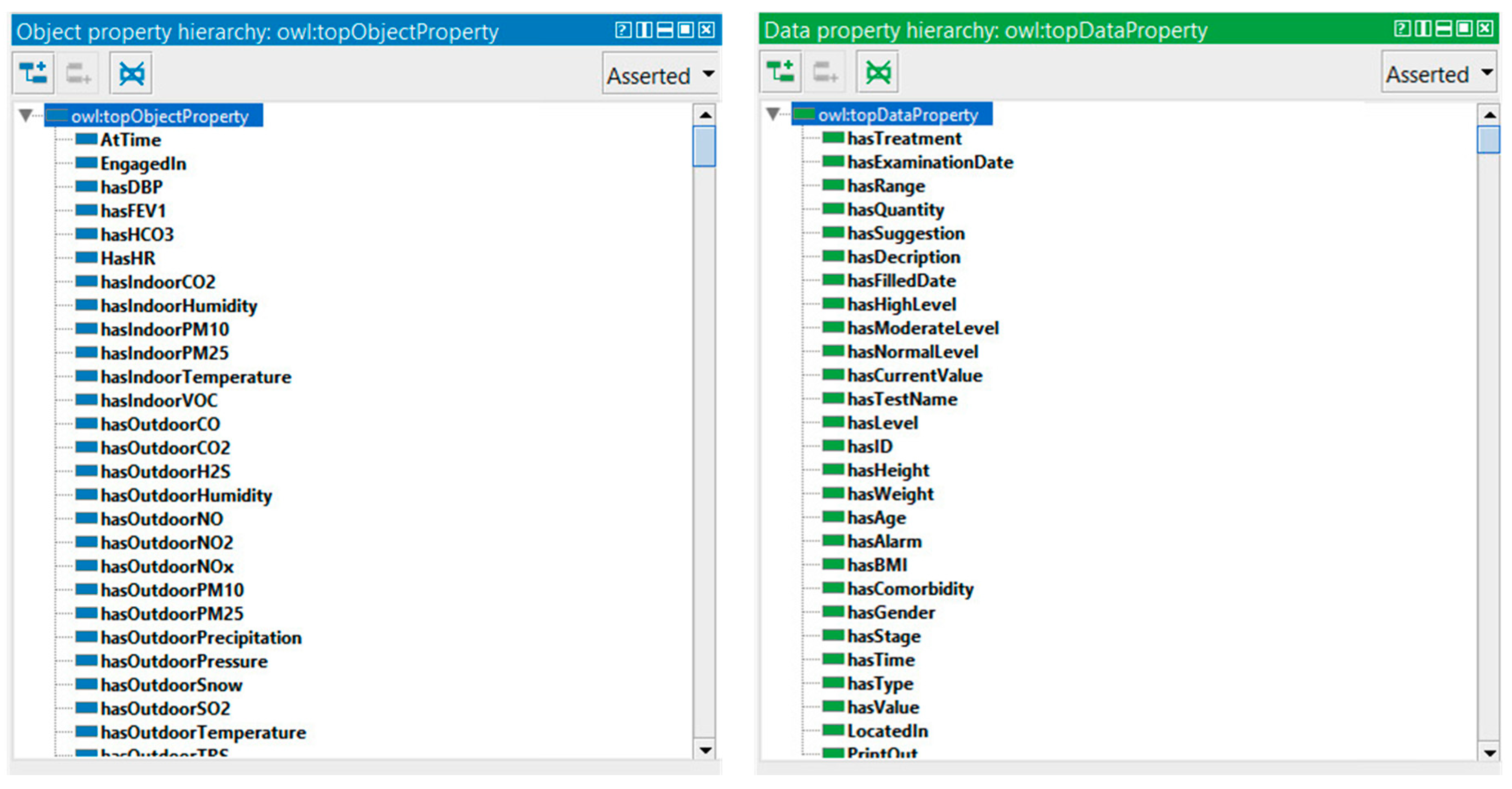
We constructed eight ontologies for monitoring COPD patient and representing machine-understandable Knowledge Base. As shown in
Figure 13, the developed ontologies consist of concepts related to the personal and medical profile, physical examinations, laboratory test, location, activity, environment, time, recommendations, and diseases.
In OWL, properties are used to describe relationships between individuals (instances) or to attribute an XML data type (such as string, integer, etc.) to individuals. There are two main types of properties, object properties that relate individuals from two classes, and datatype properties that are used to save real data values for the individuals of classes in a specific data format. Data type and object properties play a fundamental role in the ontology. Our COPDology consists of hundreds of object and data properties.
These properties are used to define the profile of patient (e.g., hasAge, hasBMI, hasGender), to recognize location and activity (e.g., LocatedAt, EngagedIn), to characterize environment (e.g., hasIndoorTemperature, hasOutdoorPressure), or to trigger alarms and provide suggestions (e.g., hasAlarm, hasSuggestion).
Figure 14 shows an example of the properties defined in our ontology.
The instances were instantiated automatically in Protégé from the gathered dataset. As mentioned before, the data simulates patient conditions and environment characteristics. These individuals can provide a list of medical records, profiles, physiological signs, safe thresholds, potential risks, recommendations, and explanations to the user when running the system.
Figure 15 shows an example of instances created in the ontology. This example, from left to right, illustrates symptoms, answered questions, alarms, suggestions, patient identifier, and treatment.
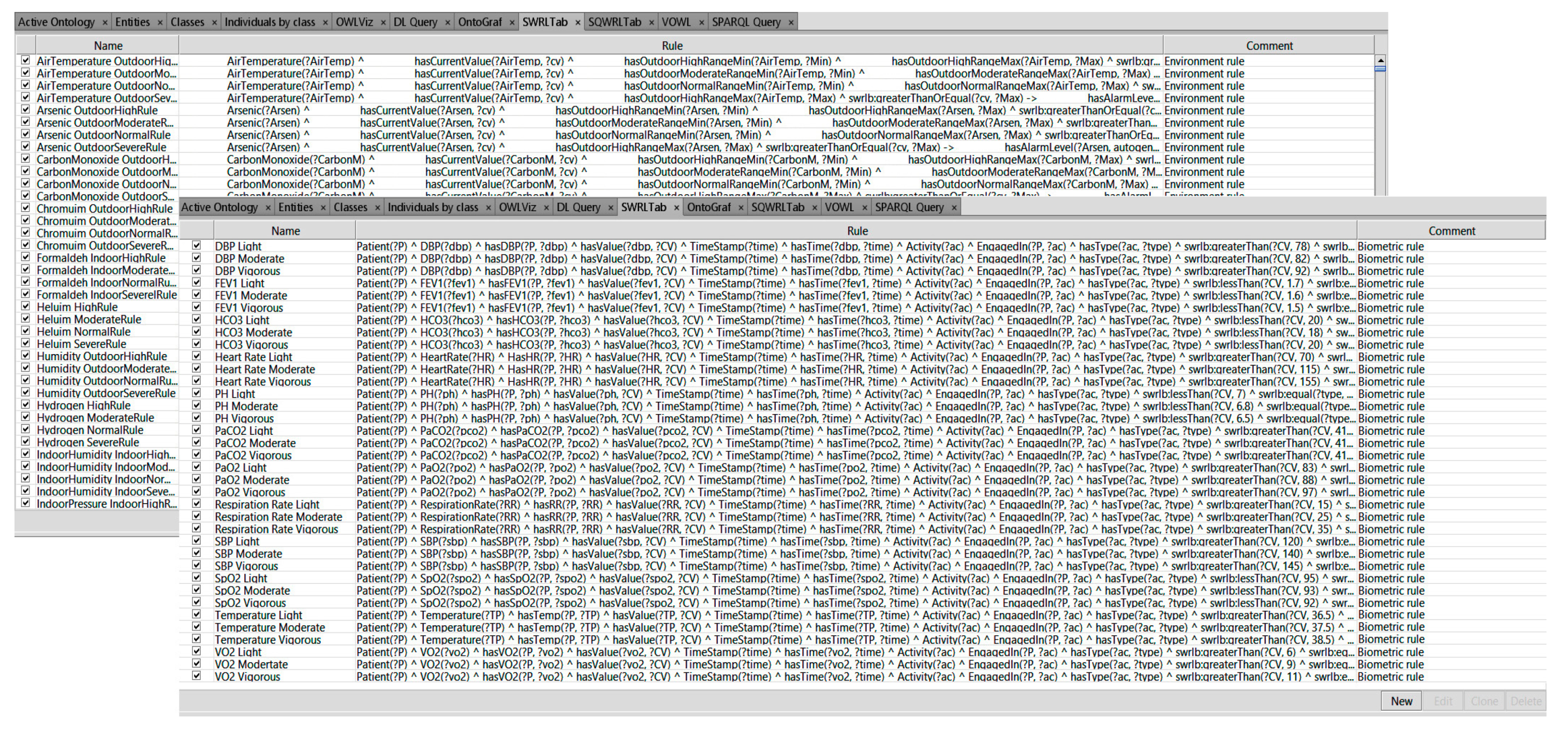
Thousands of SWRL rules were used to manage the status of almost 600 COPD medical profiles in various circumstances. Both chronic and multichronic patient profiles were configured by using the proposed rules. These configured profiles could be used to monitor the safe conditions of patients with chronic or multi chronic illnesses. Based on the medical information found in the guidelines and other information provided by physicians, we created 20,328 rules using forward chaining of inference. These rules use concepts/axioms defined in our COPDology. The rules are set to achieve different goals such as (i) verifying the profile of patients; (ii) detecting the location; (iii) evaluating the patient’s status and surrounding conditions; and (iv) providing the corresponding service for patients. For more details, please refer to the reasoning section of our previous work [
7].
Figure 16 shows the list of SWRL rules that we created in the SWRL tab to monitor the patient.
The use of SWRL rules in combination with COPDology instances has been studied in this article to provide personalized care services. Having reasoning techniques of ontologies and rules that contain the asserted and inferred statements, we used SPARQL to retrieve and derive contextual information from the knowledge base. Although SPARQL is classified as a query language, it can be considered more than this since it provides different query forms that allow its functionalities to be extended. For example, SPARQL can provide functions to verify whether certain constraints currently hold in an RDF triple store (ASK), or to specify inference rules using (CONSTRUCT), not only, but SPARQL can also be used to perform DELETE or INSERT operations. In contrast, the simplest form of a basic graph pattern (WHERE) can establish complex patterns by using UNION, OPTIONAL, and FILTER clauses. Therefore, the same SPARQL rule engine can be a used to execute functions and clinical monitoring rules, providing great flexibility in the definition of both types of data. Furthermore, SPARQL has also been studied to complement OWL expressiveness for arithmetic functions that will be included in the COPDology. This functionality indicates how information can be mathematically processed. A total of 11 arithmetic functions (ABS, AVG, division, multiplication, cell, floor, count, min, max, round, and sum) have been used to compensate for the OWL limitations. The aim of these queries is to retrieve any information relating to an identified instance, such as a sign, symptom, treatment, alarm, recommendation, decision, etc. In this section, we will present some queries that could be used to display important information and illustrate the implementation of the main functions.
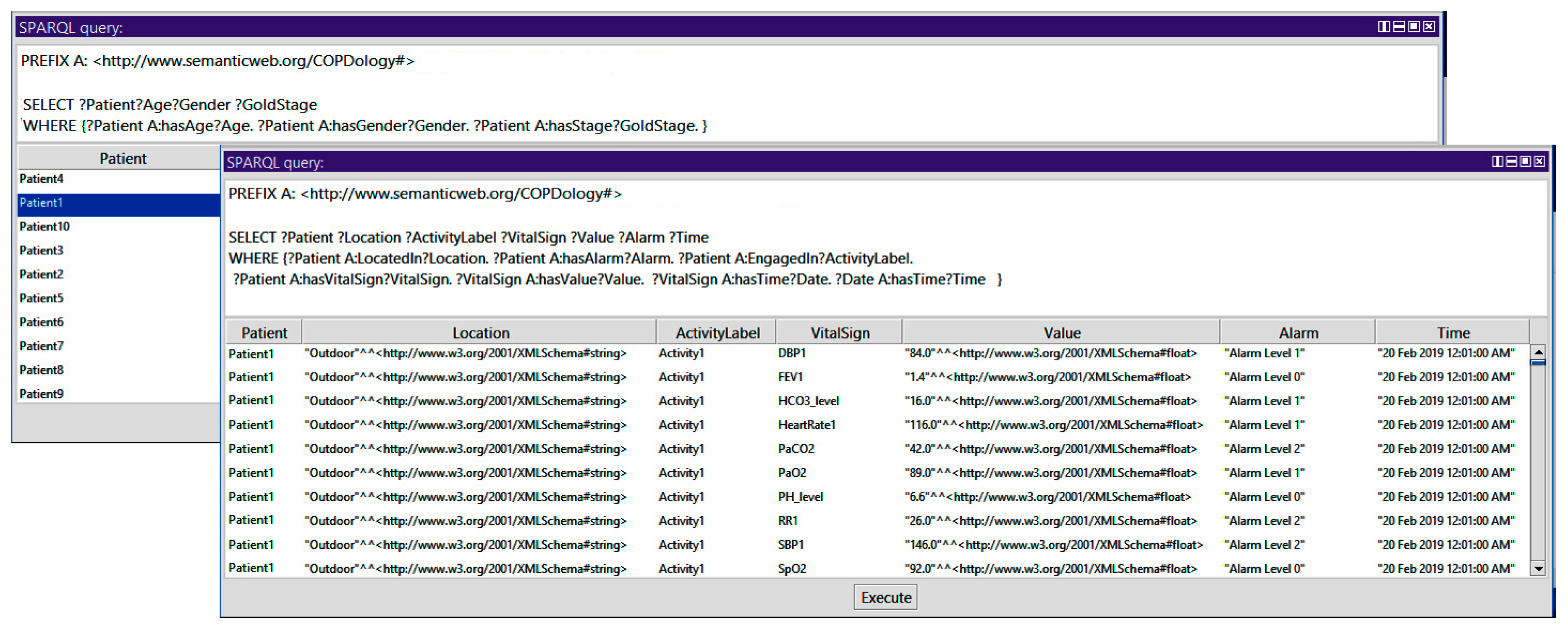
Query 1: How to Show Patient, Age, Gender, Stage, Location, Activity, Vital Signs, Alarm, and Time
In the query presented in the
Figure 17, we display the identifier of all patients and their corresponding information. We use the triple pattern of Patient hasAge Age, Patient hasGender Gender, and Patient hasStage GoldStage. We use the SELECT query to get the information of the patient identifier, age, gender, and stage. In a similar way, we used the triple pattern of Patient LocatedIn Location, Patient hasAlarm Alarm, Patient EngagedIn ActivityLabel, Patient hasVitalSign VitalSign, HasValue Value, VitalSign hasTime Date, and Date hasTime Time. We use the SELECT query to get the information of location, activity, vital, signs, value, alarm, and time.
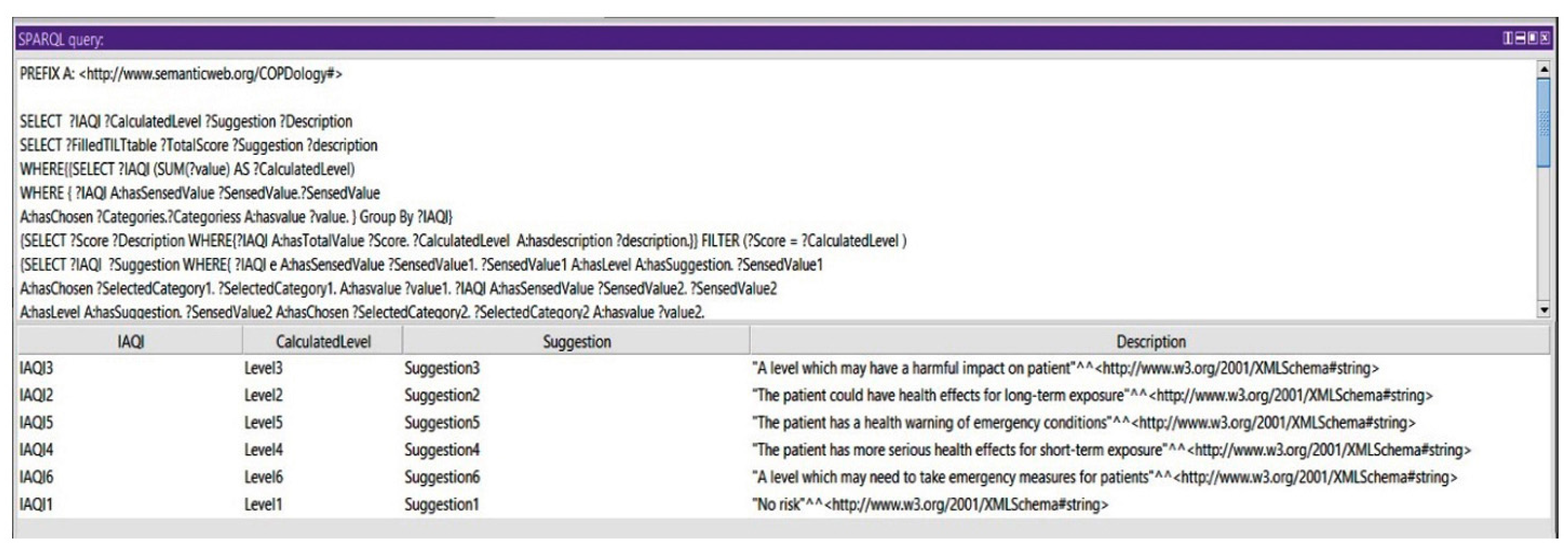
Query 2: Show the Suggestions of All Calculated Levels of IAQI
Based on the indoor air quality index, we could query for the suggestions of all the IAQI levels. This query could show an aspect of the system in monitoring the environmental conditions.
Figure 18 shows the calculated levels of IAQI and their corresponding suggestions.
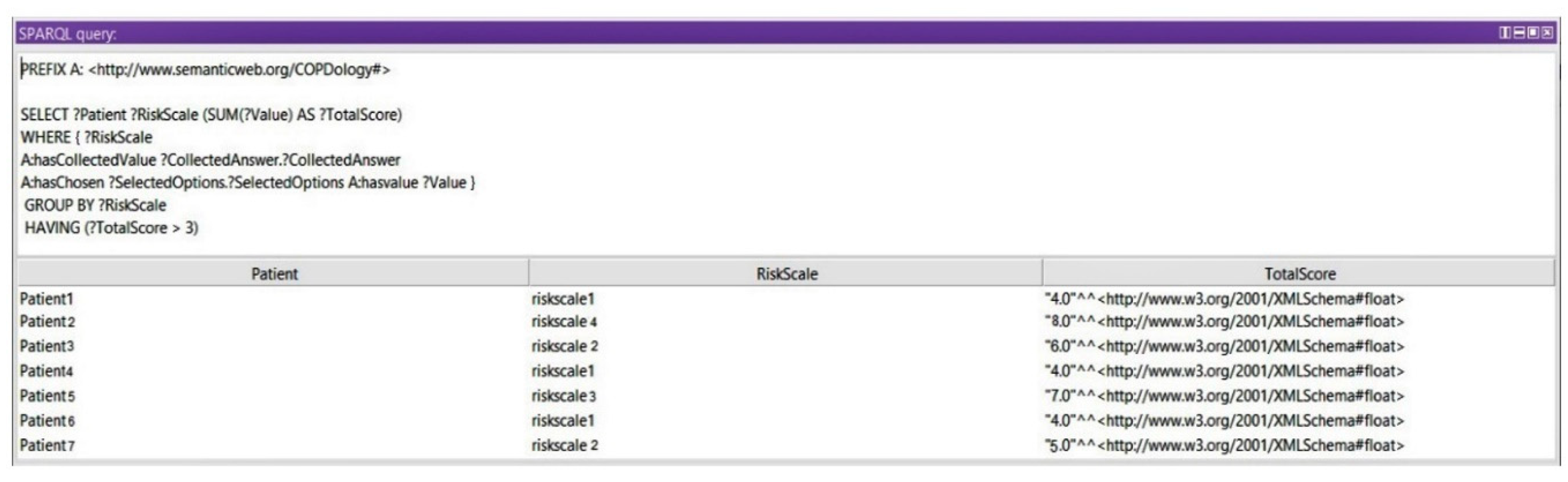
Query 3: Show Risk Scales
In this query, we display the medical assessment of the patient at a certain moment based on the Ottawa COPD Risk Score. Any value between 3 and 9 could reflect a dangerous situation for a patient. In this query we get the risk of exacerbation after calculating the total scores of signs and symptoms. Based on the grouped levels and the total calculated score, we use a subquery to query the risk scale of the queried total scores (see
Figure 19).
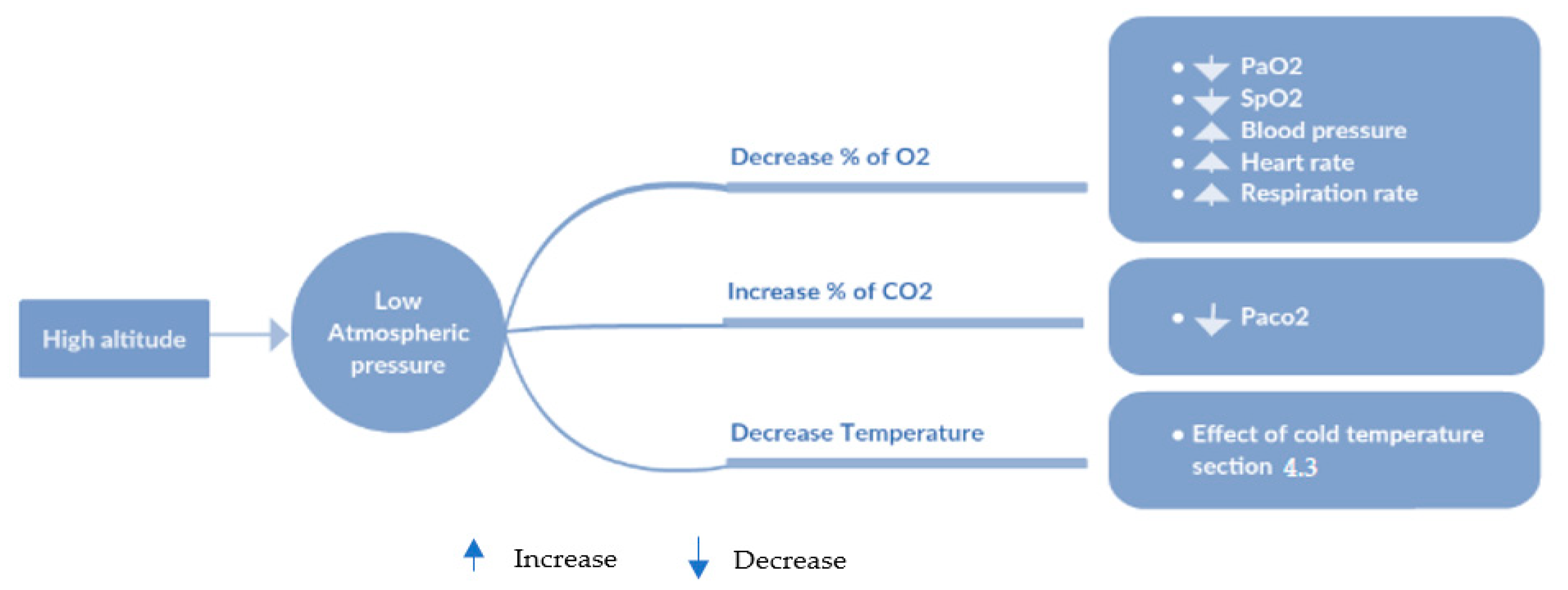
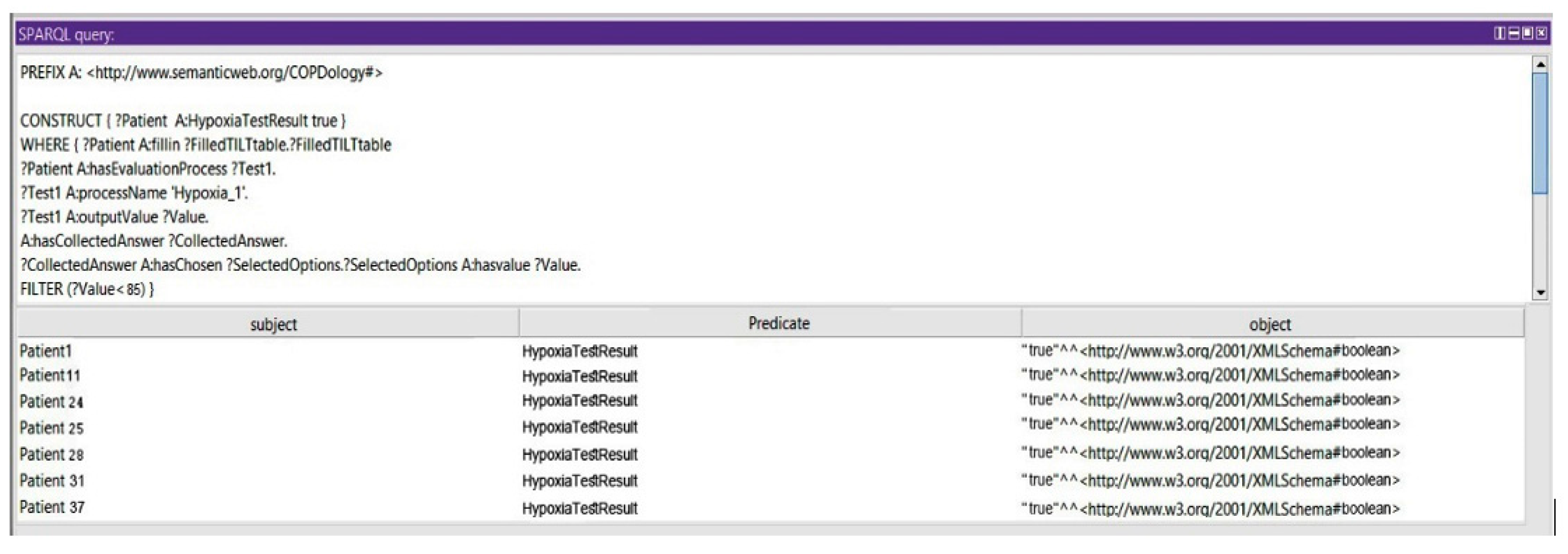
Query 4: Show the Risk of Hypoxia at High Altitude Exposure
In this query, we show that the patient could be prone to consequences of the initial exposure to high altitude: if the partial pressure and concentration of the oxygen are less than 50 mmHg and 85%, respectively, the patient cannot travel without supplemental oxygen (see
Figure 20).
7. Results
The proposed ontology is suitable for two main scenarios. First, a typical home-based telemonitoring scenario that can provide self-management service by observing physiological data regarding the general health of individuals, as well as better information about the indoor air parameters such as temperature, humidity, pollutant gases, dust, and vapor, which are considered triggering factors for COPD. The second scenario is designed for tracking patients outside the home. In the outdoor monitoring area, the system gathers contextual information or environmental stimulus that could influence the biomedical parameters.
To illustrate the use of the model, we will perform some experiments. Let us consider the scenario of a COPD patient being remotely monitored after diagnosis. The patient’s vital signs, activity, and environmental parameters are continuously monitored. The patient’s profile is shown in
Table 7.
The requirements for the supervision configured by the medical rules are depicted in each experiment. The parameters to be monitored correspond to specific limits. All these settings are described as parameters, constraints, and alarms. The defined alarms are associated with constraints configured for each profile. The activation of any of the alarms would alert both the patient and the physician; an earlier alert should be sent to the patient and pneumologist, with a visible notification and audible warning. These experiments allow for analyzing the fulfillment of the medical rules and services provided by varying both the vital signs and the activities of the patient. These experiments are classified as follows.
Experiment 1—The Main Objective of This Experiment Is to Examine the Ability of the Ontology to Detect Abnormal Changes in Physiological Parameters.
The alarms and constraints that control these changes are listed in the
Table 8.
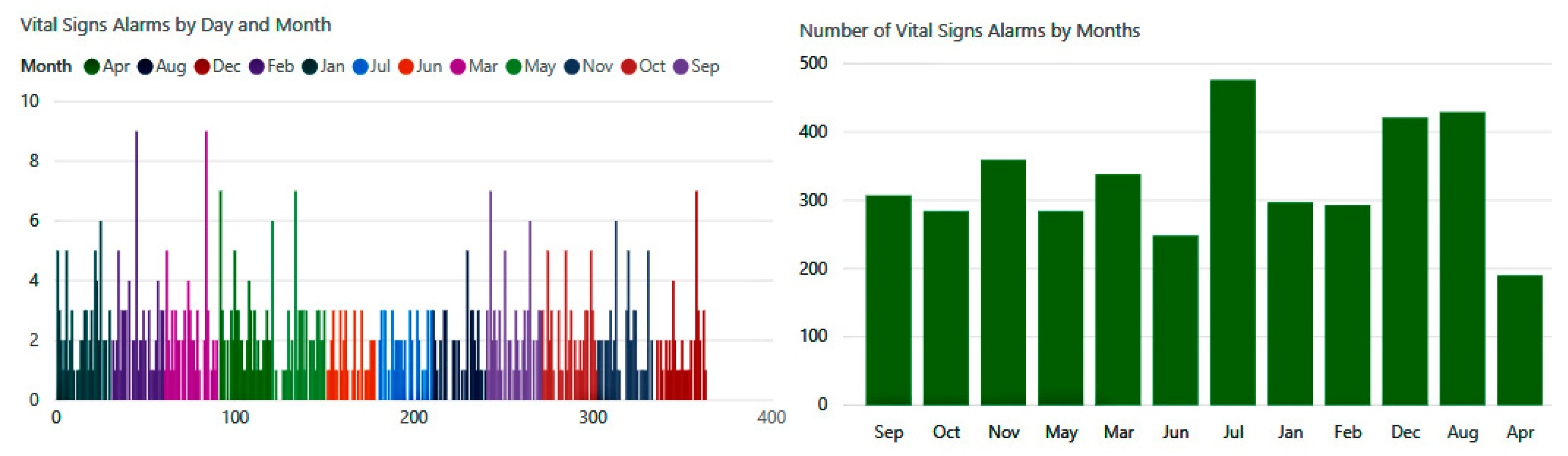
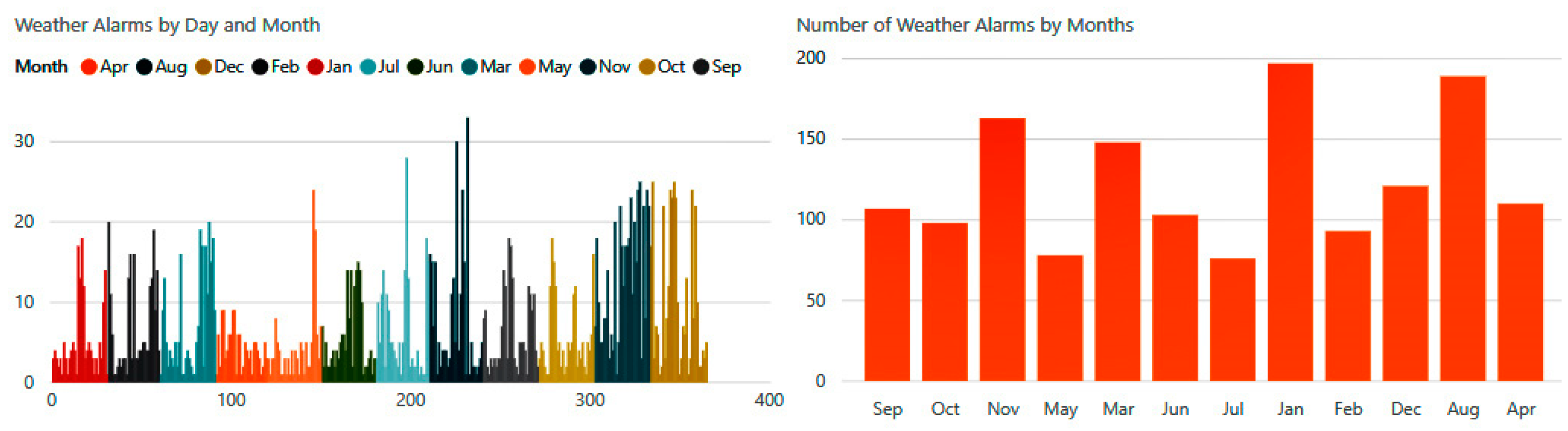
The number of biometric alerts in
Figure 21 is illustrated on a monthly basis. The experiment reveals that there were hundreds of changes in vital signs detected by our system during this year. We observe that the highest rates occurred during the months of July, August, and December, when the system detected about 1300 cases of abnormal conditions.
Experiment 2—The Aim of This Experiment Is to Determine the Protective Capacity against Indoor and Outdoor Pollutants and Guarantee the Fulfillment of the Pollution Rules Related to the Patient Profile.
As demonstrated in the
Table 9 below.
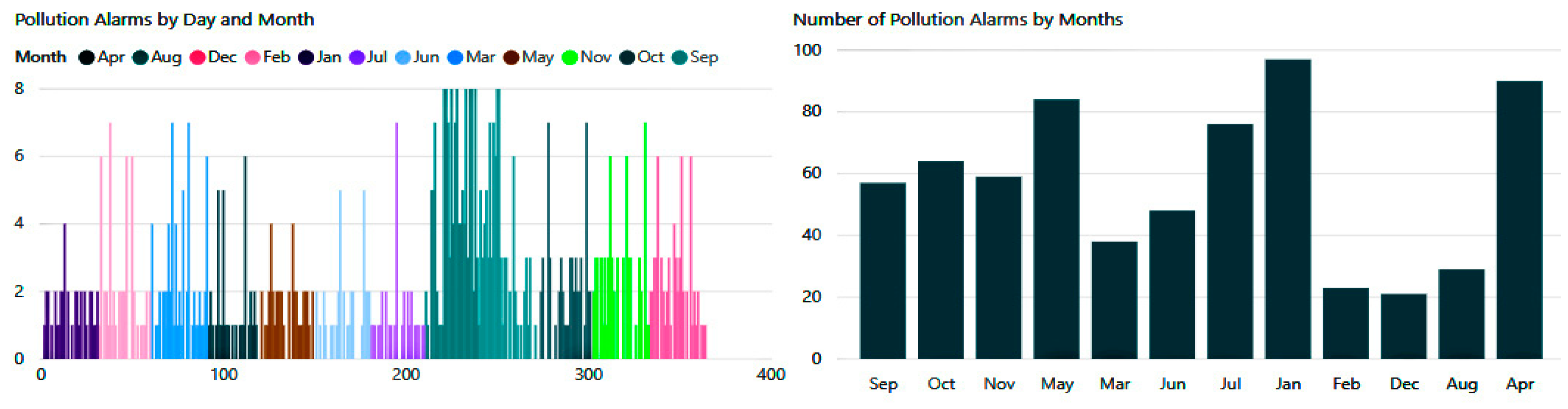
As shown in
Figure 22 below, pollution hazards in both internal and external spaces were also numerous. According to the conducted experimentation, the patient received between two and eight notification alerts per day, which would reduce the incidence of exacerbations significantly.
Experiment 3—The Main Objective of This Experiment Is to Show the Importance of the Recommendations when Patients Are Not Protected by Weather Rules.
Table 10 provides some of the alarms and constraints for climatic conditions that suit a specific profile of copd patients.
Daily weather alerts were distributed between alarms related to temperature, humidity, atmospheric pressure, wind, and precipitation.
Figure 23 shows the number of climatic notifications received by the patient during the different months of the year.
Experiment 4—The Purpose of This Experiment Is to Evaluate the Role of Activities Rules.
Table 11 provides some of the alarms and constraints for everyday activities that suit a specific profile of copd patients.
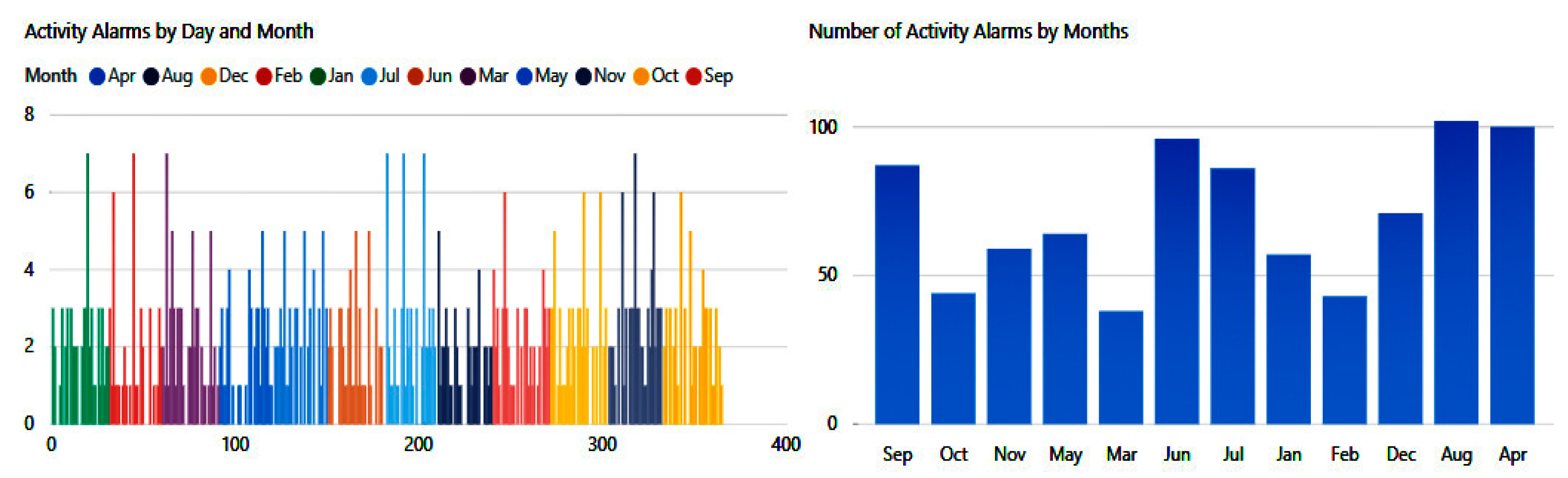
In
Figure 24, we can see the number of risks that the patient may experience because of his involvement in some unsafe activities or nonadherence to proper nutrient intake.
The presented simulations have handled a great amount of data (525,600 records) of possible situations. The generated alarms have been classified into four main categories: vital signs, activity, pollution, and weather. The system applies continuous monitoring and detects a total of 3962 abnormal situations.
Table 12 provides detailed information about the number of alarms during the different seasons.
8. Evaluation and Validation
The existing systems sought to promote their products without giving much attention to context design and performance; instead, their research focused on technology as a practice sustained by patients. It is important that the systems being used are supported by clinical practice guidelines and protocols to maintain consistency and minimize medical errors. Without a well-validated framework, the healthcare system will fail to provide any kind of protection or self-management services. Accuracy remains an important challenge for the alerts generated by the decision support systems. Practically, it is not known exactly how changes in physical parameters would affect the accuracy of the predefined alerts and their impact on patient outcomes. Improving the accuracy would enhance the overall performance of the telemonitoring systems and thus substantially reduce the disease burden.
As previously mentioned, the problem of telemonitoring systems was the impact of commercial and industrial aspects on research, which pushed hard towards blind adoption without a rigorous evaluation of the proposed designs. This lack of credible evaluation raises many questions about the feasibility and efficacy of this technology. This section will explore the performance of our ontology using a confusion matrix. Performance measurement quantifies accuracy, sensitivity, specificity, and the probability of predicting a dangerous change in physiological parameters in COPD patients. The purpose of this evaluation is to measure the accuracy of the alarms at the technical and clinical levels. A master file was created in MS Excel® that contains 1200 patient records, biometric readings for each patient (body temperature, diastolic blood pressure (DBP), systolic blood pressure (SBP), heart rate, partial pressure oxygen (PaO
2), oxygen saturation (SpO
2), partial pressure carbon dioxide (PaCO
2), oxygen consumption (VO
2), respiration rate, pH levels, concentration of hydrogen carbonate in the blood (HCO
3), and FEV1 have been extracted from the results inferred from ontology in different scenarios. This information was presented to physicians in the following format (see
Table 13):
The data are used to calculate the confusion matrices for the physician’s report outcomes. There are four possible outcomes: true positive (TP), true negative (TN), false positive (FP), and false negative (FN). The confusion matrix contains information about the predicted classifications identified by our ontology and the opinions of medical experts. The designation of the categories is as follows: (1) the alarm that is generated is based on a threshold set into the SWRL rules for each biometric parameter, (2) the values are interpreted within the ontology to identify when intervention is needed, (3) once a risk level is established the classifier is designated into one of four categories (TP, TN, FP, FN) based on physician’s report outcomes. Categories in this research are defined as follows:
TP is an alarm with a hospitalization.
FN has no alarm with a hospitalization.
TN has no alarm and no hospitalization.
FP has an alarm with no hospitalization.
The confusion matrices in
Table 14 illustrated the results of the issued alarms based on the physician’s report outcomes. The next
Table 15 summarizes the performance of our ontology include accuracy, sensitivity, specificity, the FPF, representing 1-specificity, FNF, PPV, and NPV.
The results indicate that our model reaches an accuracy of 88% in a set of 1200 clinical cases. Sensitivity and specificity have high values, denoting the ability of the ontology to detect warning signs. The positive predictive value (PPV) is defined as the probability of intervention for positive test results, while the negative predictive value (NPV) describes the probability of being healthy despite negative test results.