Multiplex Point-of-Care Tests for the Determination of Antibodies after Acellular Pertussis Vaccination
Abstract
1. Introduction
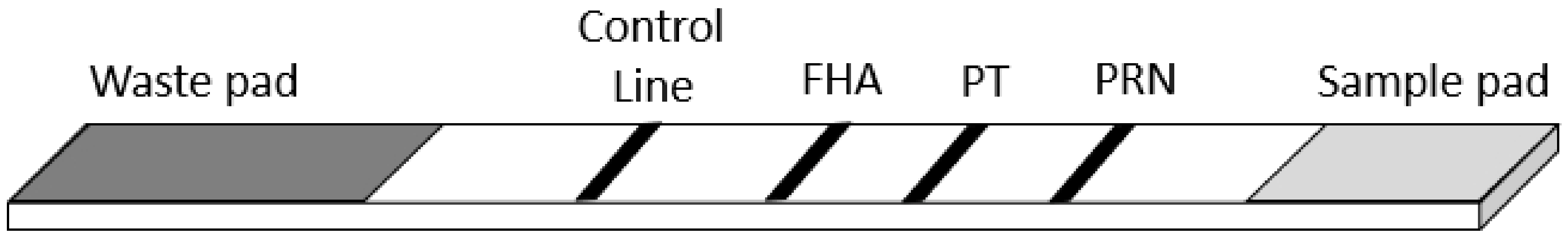
2. Materials and Methods
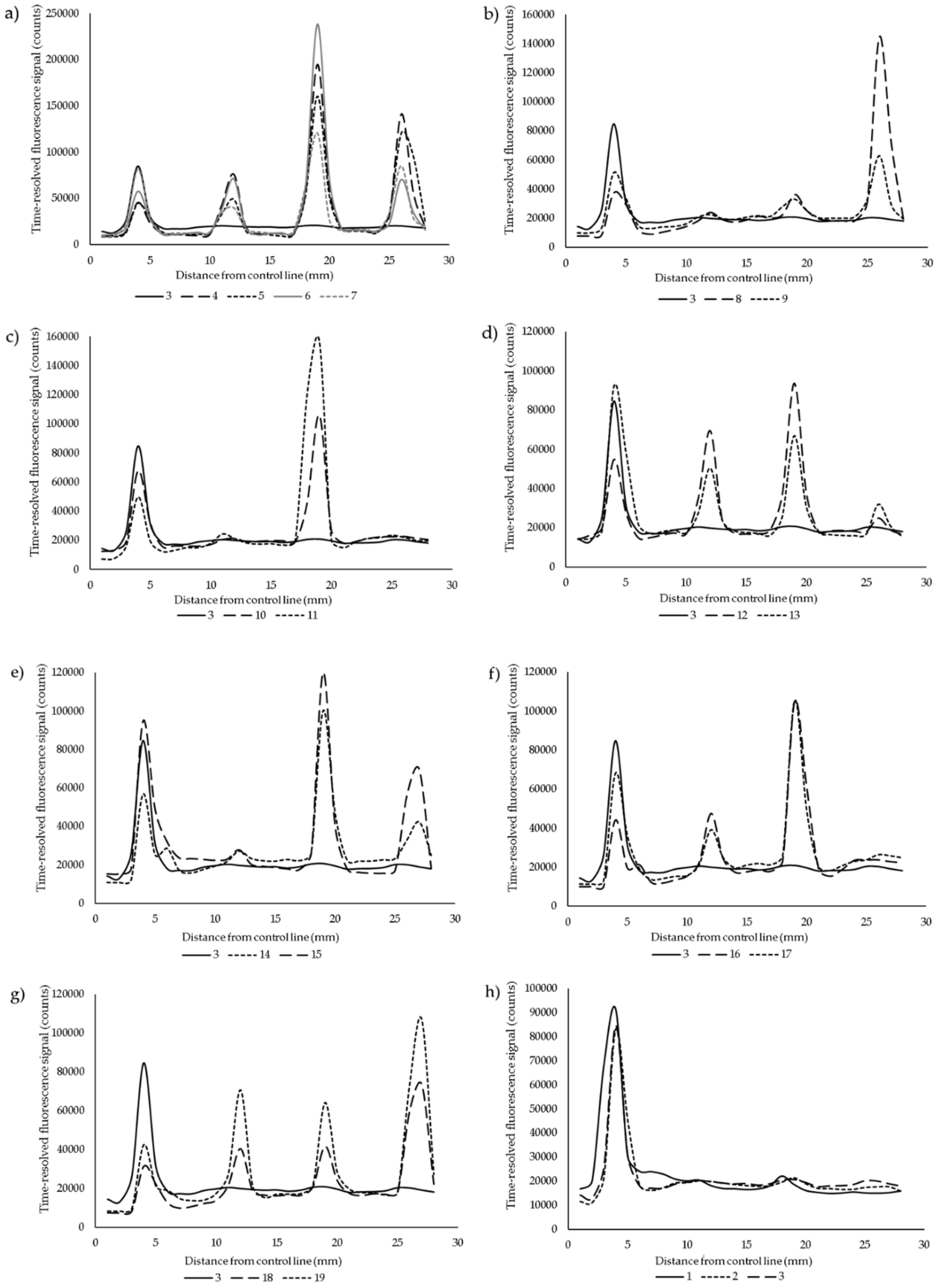
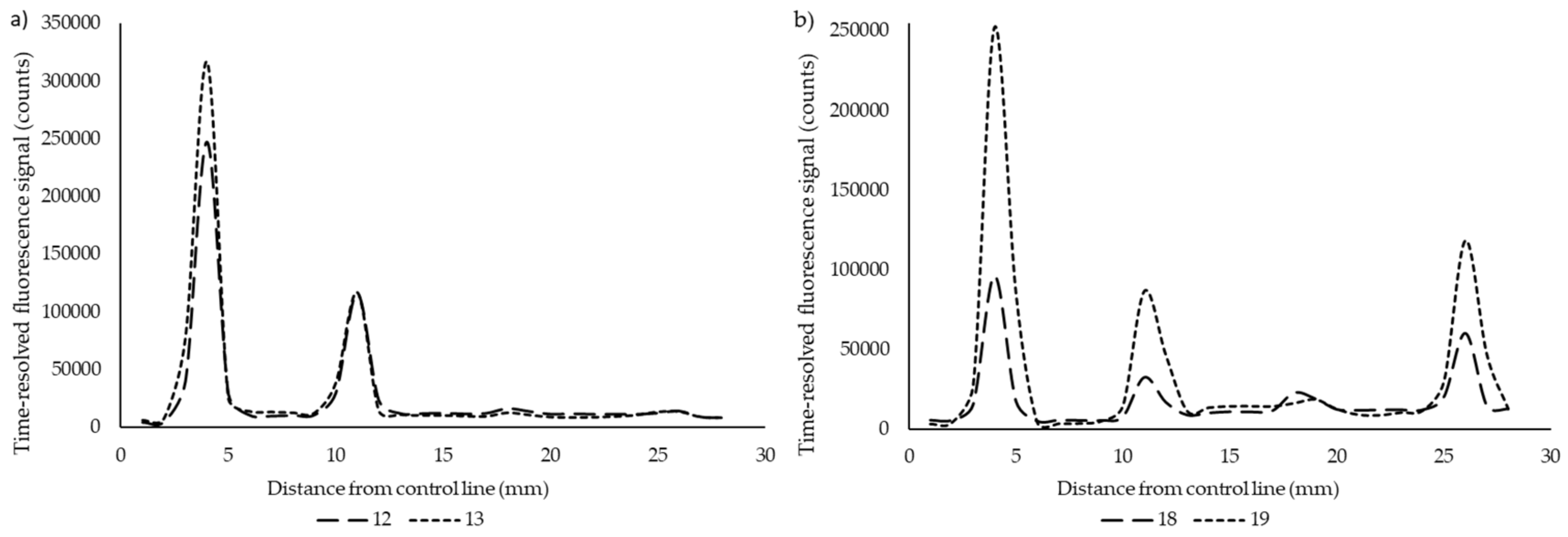
3. Results
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Barkoff, A.M.; Grondahl-Yli-Hannuksela, K.; He, Q. Seroprevalence studies of pertussis: What have we learned from different immunized populations. Pathog Dis. 2015, 73. [Google Scholar] [CrossRef] [PubMed]
- Gustafsson, L.; Hallander, H.O.; Olin, P.; Reizenstein, E.; Storsaeter, J. A controlled trial of a two-component acellular, a five-component acellular, and a whole-cell pertussis vaccine. N. Engl. J. Med. 1996, 334, 349–355. [Google Scholar] [CrossRef] [PubMed]
- Van der Zee, A.; Agterberg, C.; Peeters, M.; Mooi, F.; Schellekens, J. A clinical validation of Bordetella pertussis and Bordetella parapertussis polymerase chain reaction: Comparison with culture and serology using samples from patients with suspected whooping cough from a highly immunized population. J. Infect. Dis. 1996, 174, 89–96. [Google Scholar] [CrossRef] [PubMed]
- May, M.L.; Doi, S.A.; King, D.; Evans, J.; Robson, J.M. Prospective evaluation of an Australian pertussis toxin IgG and IgA enzyme immunoassay. Clin. Vaccine Immunol. 2012, 19, 190–197. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Dalby, T.; Petersen, J.W.; Harboe, Z.B.; Krogfelt, K.A. Antibody responses to pertussis toxin display different kinetics after clinical Bordetella pertussis infection than after vaccination with an acellular pertussis vaccine. J. Med. Microbiol. 2010, 59, 1029–1036. [Google Scholar] [CrossRef] [PubMed]
- Leber, A.L. Pertussis: Relevant species and diagnostic update. Clin. Lab. Med. 2014, 34, 237–255. [Google Scholar] [CrossRef] [PubMed]
- Barkoff, A.M.; Mertsola, J.; Pierard, D.; Dalby, T.; Hoegh, S.V.; Guillot, S.; Stefanelli, P.; van Gent, M.; Berbers, G.; Vestrheim, D.; et al. Pertactin-deficient Bordetella pertussis isolates: Evidence of increased circulation in Europe, 1998 to 2015. Euro. Surveill. 2019, 24. [Google Scholar] [CrossRef]
- Lam, C.; Octavia, S.; Ricafort, L.; Sintchenko, V.; Gilbert, G.L.; Wood, N.; Mclntyre, P.; Marshal, H.; Guiso, N.; Keil, A.D.; et al. Rapid increase in pertactin-deficient Bordetella pertussis isolates, Australia. Emerg. Infect Dis. 2014, 20, 626–633. [Google Scholar] [CrossRef]
- Pawloski, L.C.; Queenan, A.M.; Cassiday, P.K.; Lynch, A.S.; Harrison, M.J.; Shang, W.; Williams, M. Prevalence and molecular characterization of pertactin-deficient Bordetella pertussis in the United States. Clin. Vaccine Immunol. 2014, 21, 119–125. [Google Scholar] [CrossRef]
- Otsuka, N.; Han, H.J.; Toyoizumi-Ajisaka, H.; Nakamura, Y.; Arakawa, Y.; Shibayama, K.; Kamachi, K. Prevalence and genetic characterization of pertactin-deficient Bordetella pertussis in Japan. PLoS ONE 2012, 7, e31985. [Google Scholar] [CrossRef]
- Poynten, M.; Hanlon, M.; Irwig, L.; Gilbert, G.L. Serological diagnosis of pertussis: Evaluation of IgA against whole cell and specific Bordetella pertussis antigens as markers of recent infection. Epidemiol. Infect 2002, 128, 161–167. [Google Scholar] [CrossRef] [PubMed]
- Otsuka, N.; Gotoh, K.; Nishimura, N.; Ozaki, T.; Nakamura, Y.; Haga, K.; Yamazaki, M.; Gondaira, F.; Okada, K.; Miyaji, Y.; et al. A Novel IgM-capture enzyme-linked immunosorbent assay using recombinant Vag8 fusion protein for the accurate and early diagnosis of Bordetella pertussis infection. Microbiol. Immunol. 2016, 60, 326–333. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Watanabe, M.; Connelly, B.; Weiss, A.A. Characterization of serological responses to pertussis. Clin. Vaccine Immunol. 2006, 13, 341–348. [Google Scholar] [CrossRef] [PubMed]
- Subissi, L.; Rodeghiero, C.; Martini, H.; Litzroth, A.; Huygen, K.; Leroux-Roels, G.; Piérard, D.; Desombere, I. Assessment of IgA anti-PT and IgG anti-ACT reflex testing to improve Bordetella pertussis serodiagnosis in recently vaccinated subjects. Clin. Microbiol. Infect. 2019. [Google Scholar] [CrossRef]
- Storsaeter, J.; Hallander, H.O.; Gustafsson, L.; Olin, P. Levels of anti-pertussis antibodies related to protection after household exposure to Bordetella pertussis. Vaccine 1998, 16, 1907–1916. [Google Scholar] [CrossRef]
- Van Gageldonk, P.G.; van Schaijk, F.G.; van der Klis, F.R.; Berbers, G.A. Development and validation of a multiplex immunoassay for the simultaneous determination of serum antibodies to Bordetella pertussis, diphtheria and tetanus. J. Immunol. Methods 2008, 335, 79–89. [Google Scholar] [CrossRef]
- Van Twillert, I.; Bonacic Marinovic, A.A.; Kuipers, B.; van Gaans-van den Brink, J.A.; Sanders, E.A.; van Els, C.A. Impact of age and vaccination history on long-term serological responses after symptomatic B. pertussis infection, a high dimensional data analysis. Sci. Rep. 2017, 7, 40328. [Google Scholar] [CrossRef]
- Prince, H.E.; Lape-Nixon, M.; Matud, J. Evaluation of a tetraplex microsphere assay for Bordetella pertussis antibodies. Clin Vaccine Immunol. 2006, 13, 266–270. [Google Scholar] [CrossRef]
- Rajam, G.; Carlone, G.; Kim, E.; Choi, J.; Paulos, S.; Park, S.; Jeyachandran, A.; Gorantla, Y.; Wong, E.; Sabnis, A.; et al. Development and validation of a robust multiplex serological assay to quantify antibodies specific to pertussis antigens. Biologicals 2019, 57, 9–20. [Google Scholar] [CrossRef]
- Salminen, T.; Knuutila, A.; Barkoff, A.M.; Mertsola, J.; He, Q. A rapid lateral flow immunoassay for serological diagnosis of pertussis. Vaccine 2018, 36, 1429–1434. [Google Scholar] [CrossRef]
- Tran Minh, N.N.; He, Q.; Ramalho, A.; Kaufhold, A.; Viljanen, M.K.; Arvilommi, H.; Mertsola, J. Acellular vaccines containing reduced quantities of pertussis antigens as a booster in adolescents. Pediatrics 1999, 104, e70. [Google Scholar] [CrossRef]
- Goding, J.W. Use of staphylococcal protein A as an immunological reagent. J. Immunol. Methods 1978, 20, 241–253. [Google Scholar] [CrossRef]
- Sasso, E.H.; Silverman, G.J.; Mannik, M. Human IgA and IgG F(ab’)2 that bind to staphylococcal protein A belong to the VHIII subgroup. J Immunol. 1991, 147, 1877–1883. [Google Scholar] [PubMed]
- Nagel, J.; Poot-Scholtens, E.J. Serum IgA antibody to Bordetella pertussis as an indicator of infection. J. Med. Microbiol. 1983, 16, 417–426. [Google Scholar] [CrossRef]
- Thomas, M.G.; Ashworth, L.A.; Miller, E.; Lambert, H.P. Serum IgG, IgA, and IgM responses to pertussis toxin, filamentous hemagglutinin, and agglutinogens 2 and 3 after infection with Bordetella pertussis and immunization with whole-cell pertussis vaccine. J. Infect Dis. 1989, 160, 838–845. [Google Scholar] [CrossRef]
- Nagel, J.; de Graaf, S.; Schijf-Evers, D. Improved serodiagnosis of whooping cough caused by Bordetella pertussis by determination of IgG anti-LPF antibody levels. Dev. Biol. Stand 1985, 61, 325–330. [Google Scholar] [PubMed]
- Zaitsev, E.M.; Krasnoproshina, L.I.; Mazurova, I.K.; Petrova, M.S.; Popova, O.P.; Zakharova, N.S. Isotypes of anti-pertussis antibodies in pertussis patients at different stages of the disease. Zhurnal Mikrobiol. Epidemiol. Immunobiol. 2011, 3, 53–56. [Google Scholar] [PubMed]
- Nagel, J.; de Graaf, S.; Schijf-Evers, D. Serodiagnosis of whooping cough. Ned. Tijdschr. Geneeskd. 1984, 128, 1427–1429. [Google Scholar]
- Mertens, P.L.; Stals, F.S.; Steyerberg, E.W.; Richardus, J.H. Sensitivity and specificity of single IgA and IgG antibody concentrations for early diagnosis of pertussis in adults: An evaluation for outbreak management in public health practice. BMC Infect Dis. 2007, 7, 53. [Google Scholar] [CrossRef]
- Hendrikx, L.H.; Schure, R.M.; Ozturk, K.; de Rond, L.G.; de Greeff, S.C.; Sanders, E.A.; Berbers, G.A.; Buisman, A.M. Different IgG-subclass distributions after whole-cell and acellular pertussis infant primary vaccinations in healthy and pertussis infected children. Vaccine 2011, 29, 6874–6880. [Google Scholar] [CrossRef]
- Giammanco, A.; Taormina, S.; Chiarini, A.; Dardanoni, G.; Stefanelli, P.; Salmaso, S.; Mastrantonio, P. Analogous IgG subclass response to pertussis toxin in vaccinated children, healthy or affected by whooping cough. Vaccine 2003, 21, 1924–1931. [Google Scholar] [CrossRef]
- Zackrisson, G.; Lagergard, T.; Trollfors, B. Subclass compositions of immunoglobulin G to pertussis toxin in patients with whooping cough, in healthy individuals, and in recipients of a pertussis toxoid vaccine. J. Clin. Microbiol. 1989, 27, 1567–1571. [Google Scholar] [CrossRef] [PubMed]
- Zaitsev, E.M.; Mazurova, I.K.; Petrova, M.S.; Krasnoproshina, L.I.; Astakhova, T.I.; Zakharova, N.S. Distribution of specific IgG subclasses in patients with pertussis and in healthy persons. Zhurnal Mikrobiol. Epidemiol. Immunobiol. 2009, 2, 57–61. [Google Scholar] [PubMed]
- Hampl, J.; Hall, M.; Mufti, N.A.; Yao, Y.M.; MacQueen, D.B.; Wright, W.H.; Cooper, D.E. Upconverting phosphor reporters in immunochromatographic assays. Anal. Biochem. 2001, 288, 176–187. [Google Scholar] [CrossRef]
| Sample | Anti-FHA IgG | Anti-PT IgG | Anti-PRN IgG |
|---|---|---|---|
| 1 | 2.5 | 2.5 | 2.5 |
| 2 | 2.5 | 2.5 | 2.5 |
| 3 | 2.5 | 2.5 | 2.5 |
| 4 | 4205 | 582 | 1592 |
| 5 | 1364 | 580 | 1339 |
| 6 | 1730 | 578 | 314 |
| 7 | 481 | 168 | 466 |
| 8 | 66 | 2.5 | 948 |
| 9 | 63 | 2.5 | 230 |
| 10 | 14 | 68 | 6 |
| 11 | 47 | 343 | 11 |
| 12 | 1280 | 17 | 55 |
| 13 | 370 | 2.5 | 64 |
| 14 | 66 | 145 | 119 |
| 15 | 67 | 92 | 255 |
| 16 | 400 | 79 | 2.5 |
| 17 | 162 | 84 | 6 |
| 18 | 440 | 2.5 | 574 |
| 19 | 815 | 6 | 1652 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Knuutila, A.; Rautanen, C.; Mertsola, J.; He, Q. Multiplex Point-of-Care Tests for the Determination of Antibodies after Acellular Pertussis Vaccination. Diagnostics 2020, 10, 187. https://doi.org/10.3390/diagnostics10040187
Knuutila A, Rautanen C, Mertsola J, He Q. Multiplex Point-of-Care Tests for the Determination of Antibodies after Acellular Pertussis Vaccination. Diagnostics. 2020; 10(4):187. https://doi.org/10.3390/diagnostics10040187
Chicago/Turabian StyleKnuutila, Aapo, Carita Rautanen, Jussi Mertsola, and Qiushui He. 2020. "Multiplex Point-of-Care Tests for the Determination of Antibodies after Acellular Pertussis Vaccination" Diagnostics 10, no. 4: 187. https://doi.org/10.3390/diagnostics10040187
APA StyleKnuutila, A., Rautanen, C., Mertsola, J., & He, Q. (2020). Multiplex Point-of-Care Tests for the Determination of Antibodies after Acellular Pertussis Vaccination. Diagnostics, 10(4), 187. https://doi.org/10.3390/diagnostics10040187





