A Multinodular Mass of Abdominal Splenosis: Case Report of Uncommon Images of a Rare Disease
Abstract
1. Introduction
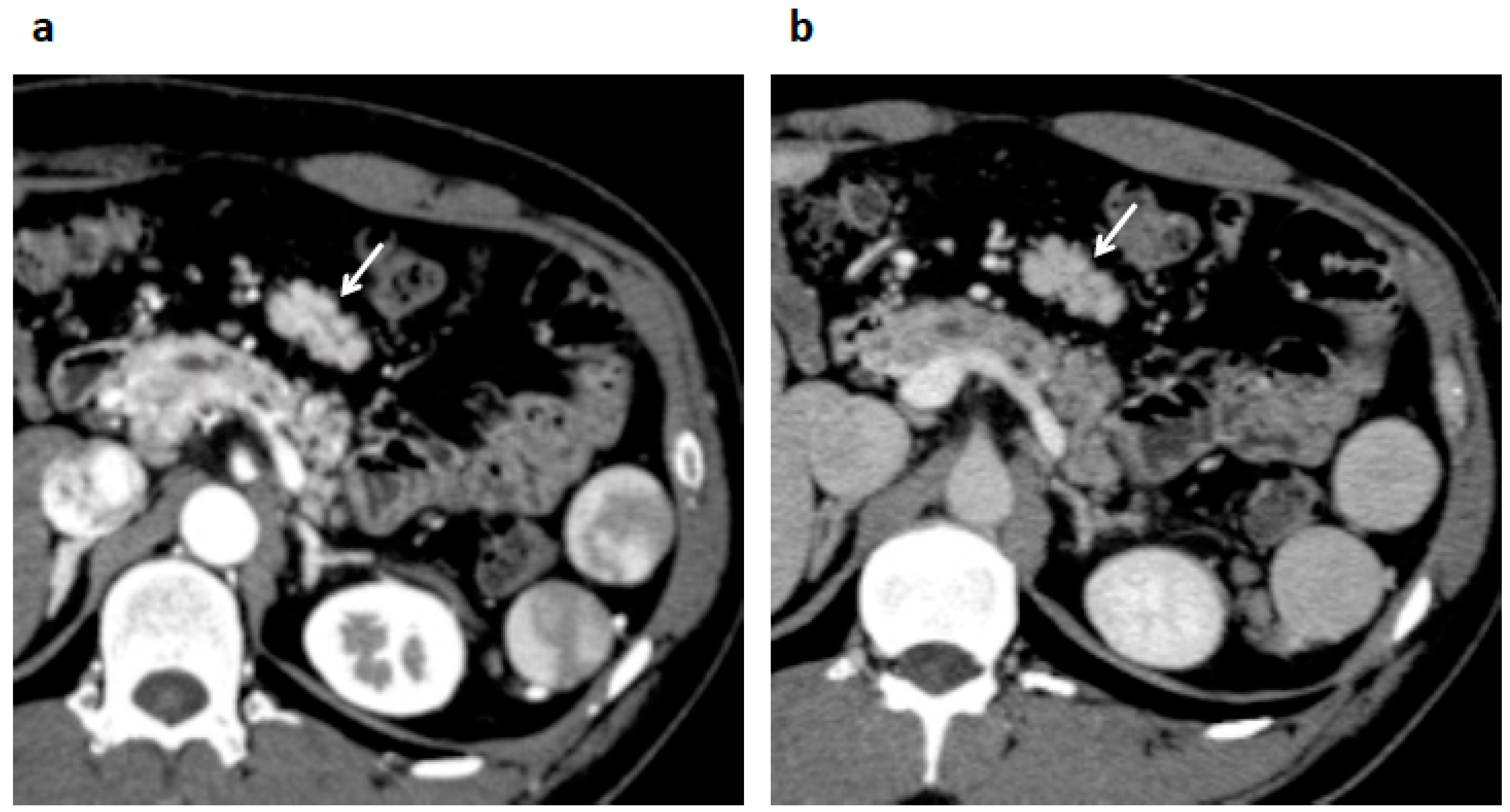
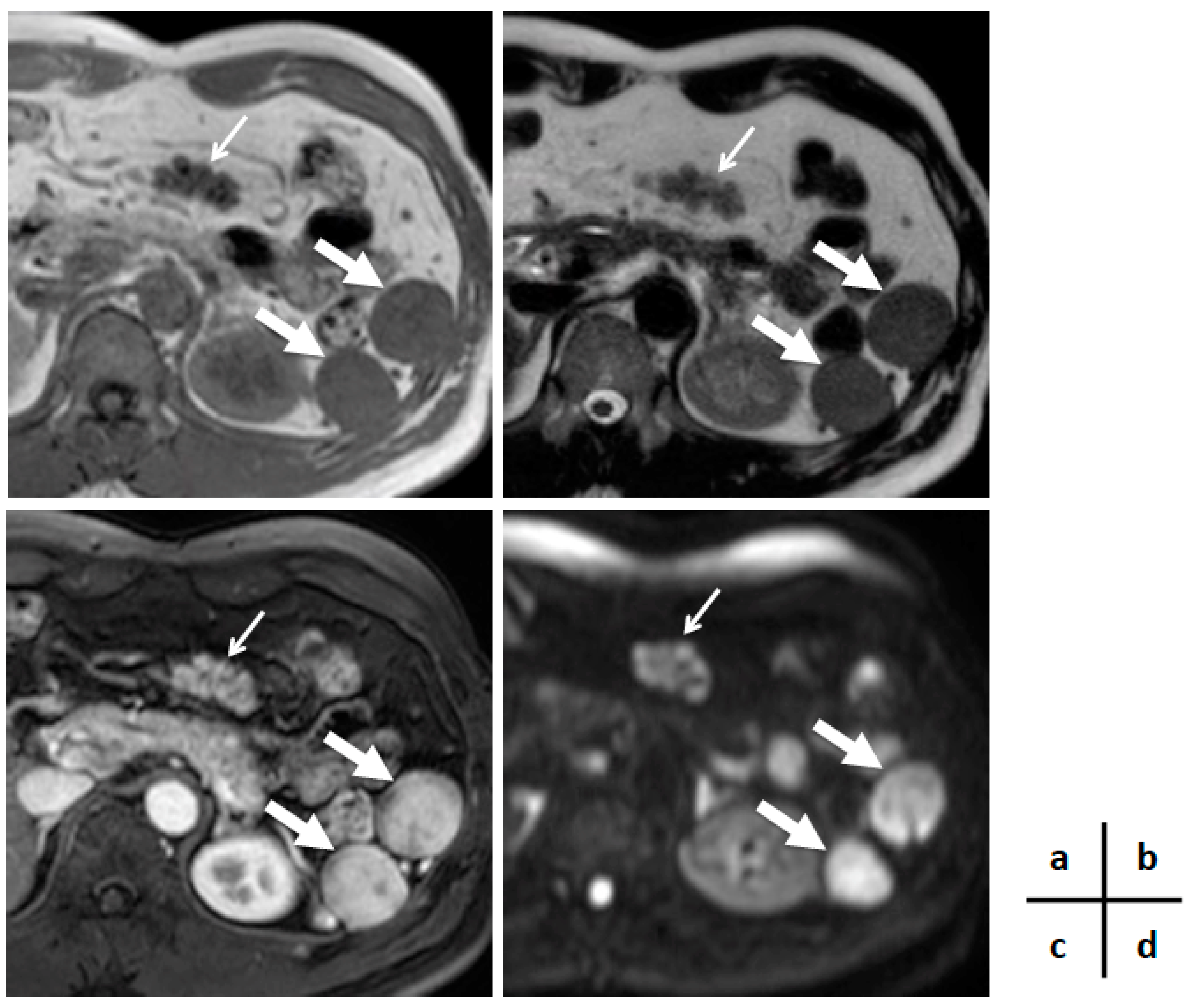
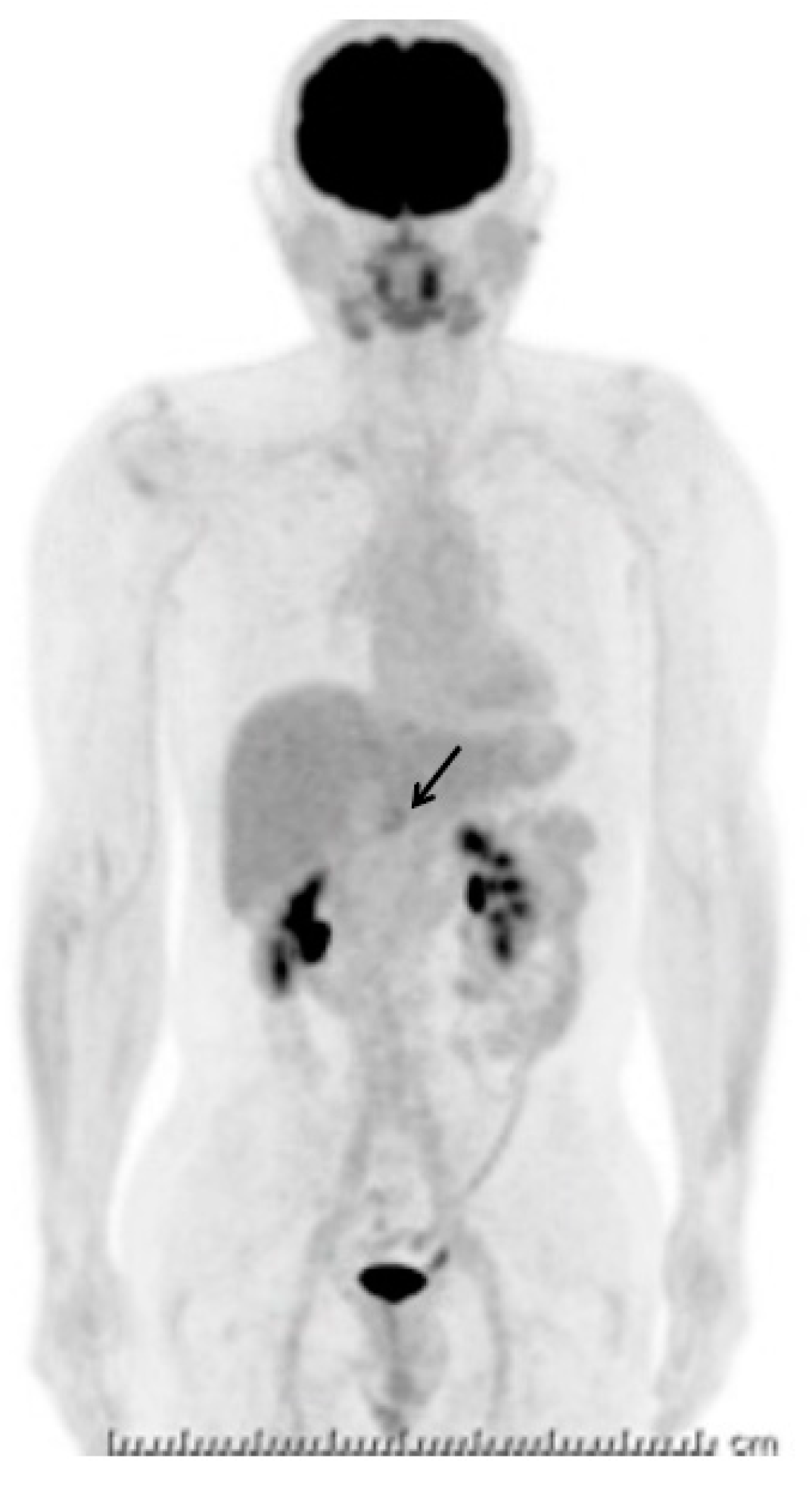
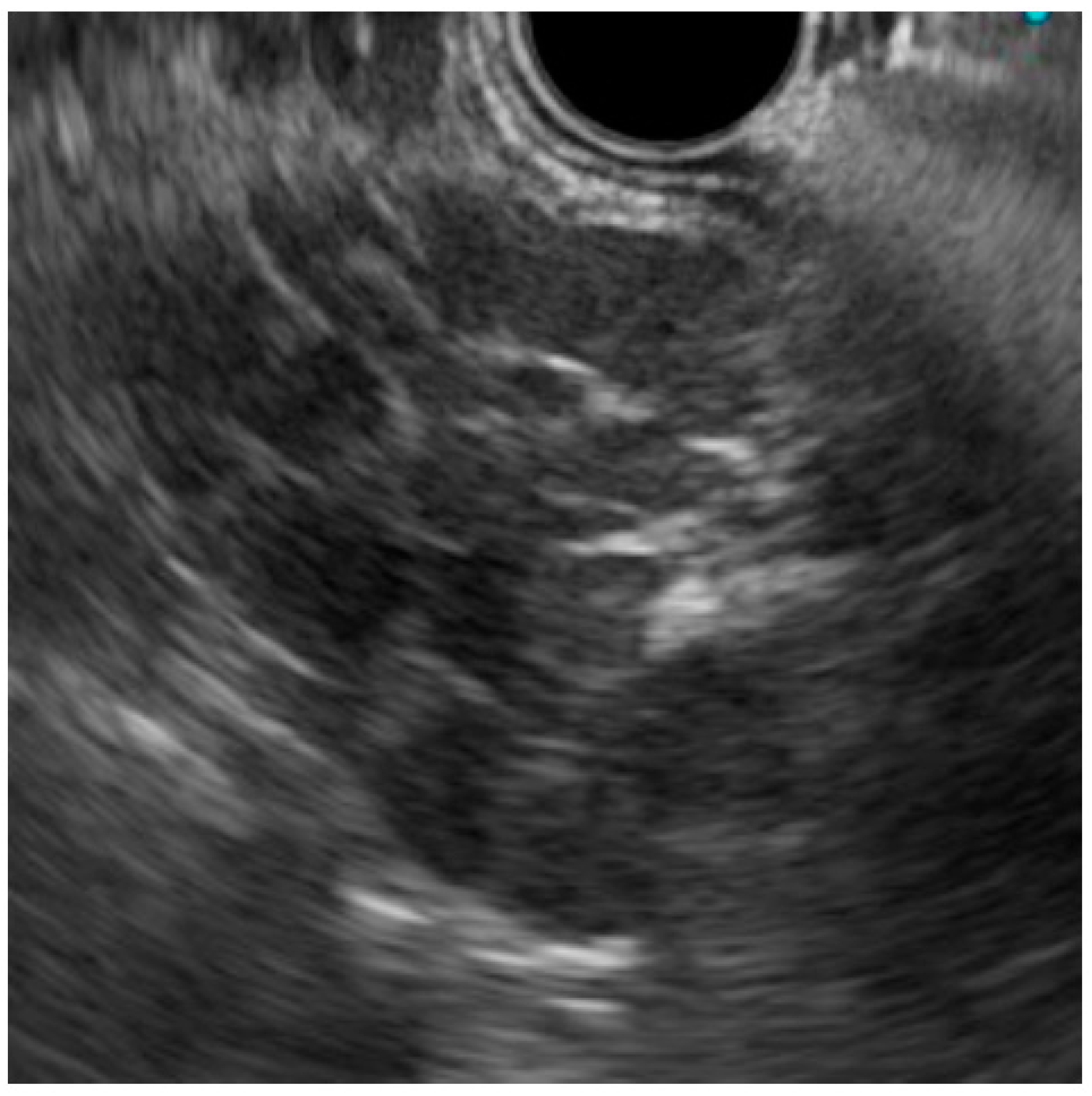
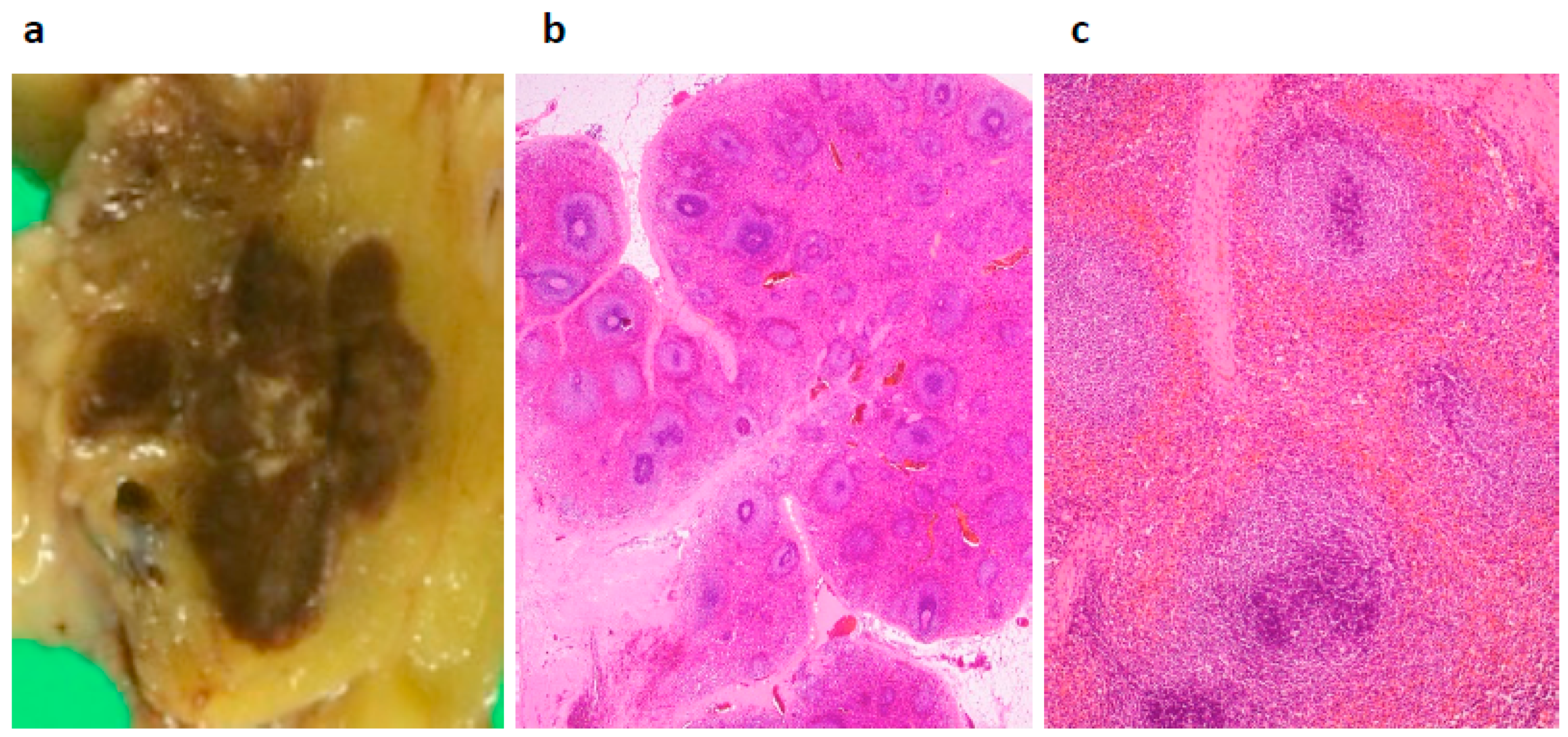
2. Case Presentation
3. Discussion
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| GIST | gastrointestinal stromal tumor |
| NET | neuroendocrine tumor |
| CT | computed tomography |
| MRI | magnetic resonance imaging |
| FDG-PET | 18F-fluorodeoxyglucose-positron emission tomography |
| EUS | endoscopic ultrasonography |
| FNA | fine needle aspiration |
| SPIO | super paramagnetic iron oxide |
References
- Leitz, E.M.; Kwan, S.W. Splenosis: A rare cause of gastrointestinal bleeding successfully treated with transarterial embolization. Clin. J. Gastroenterol. 2015, 8, 126–129. [Google Scholar] [CrossRef] [PubMed]
- Xiao, S.M.; Xu, R.; Tang, X.L.; Ding, Z.; Li, J.M.; Zhou, X. Splenosis with lower gastrointestinal bleeding mimicking colonical gastrointestinal stromal tumour. World J. Surg. Oncol. 2017, 15, 78. [Google Scholar] [CrossRef] [PubMed]
- Reinglas, J.; Perdrizet, K.; Ryan, S.E.; Patel, R.V. Splenosis involving the gastric fundus, a rare cause of massive upper gastrointestinal bleeding: A case report and review of the literature. Clin. Exp. Gastroenterol. 2016, 9, 301–305. [Google Scholar] [CrossRef] [PubMed]
- Priola, A.M.; Picciotto, G.; Priola, S.M. Diffuse abdominal splenosis: A condition mimicking abdominal lymphoma. Int. J. Hematol. 2009, 90, 543–544. [Google Scholar] [CrossRef] [PubMed]
- Tandon, Y.K.; Coppa, C.P.; Purysko, A.S. Splenosis: A great mimicker of neoplastic disease. Abdom. Radiol. 2018, 43, 3054–3059. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Paniagua, I.; Baleato-Gonzalez, S.; Garcia-Figueiras, R. Splenosis: Non-invasive diagnosis of a great mimicker. Rev. Esp. Enferm. Dig. 2016, 108, 40–41. [Google Scholar] [PubMed]
- Yang, K.; Chen, X.Z.; Liu, J.; Wu, B.; Chen, X.L.; Hu, J.K. Splenosis in gastric wall mimicking gastrointestinal stromal tumor. Endoscopy 2013, 45, E82–E83. [Google Scholar] [CrossRef] [PubMed]
- Falcao de Santana, M.; Menezes Marques, L.; Lopes Gibara, V.; Magalhaes Neto, G.E.; Gustavo De Quadros, L.; Kaiser, R.L.; Gouvea, M.A.; Filho, I.Z. Ectopic spleen mimicking hepatocellular carcinoma in the late post-operative period of bariatric surgery. Cell. Mol. Biol. (Noisy-le-Grand) 2018, 64, 113–115. [Google Scholar] [CrossRef]
- Yildiz, A.E.; Ariyurek, M.O.; Karcaaltincaba, M. Splenic anomalies of shape, size, and location: Pictorial essay. Sci. World J. 2013, 2013, 321810. [Google Scholar] [CrossRef] [PubMed]
- Kruger, R.; Freeman, S. An unusual pelvic mass: Contrast-enhanced sonographic diagnosis of pelvic splenosis. J. Clin. Ultrasound. 2019, 47. [Google Scholar] [CrossRef] [PubMed]
- Ishibashi, M.; Tanabe, Y.; Miyoshi, H.; Matusue, E.; Kaminou, T.; Ogawa, T. Intrathoracic splenosis: Evaluation by superparamagnetic iron oxide-enhanced magnetic resonance imaging and radionuclide scintigraphy. Jpn. J. Radiol. 2009, 27, 19943149. [Google Scholar] [CrossRef] [PubMed]
- Guan, B.; Li, X.H.; Wang, L.; Zhou, M.; Dong, Z.W.; Luo, G.J.; Meng, L.P.; Hu, J.; Jin, W.Y. Gastric fundus splenosis with hemangioma masquerading as a gastrointestinal stromal tumor in a patient with schistosomiasis and cirrhosis who underwent splenectomy: A case report and literature review. Medicine (Baltimore) 2018, 97, e11461. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, K.; Orita, H.; Koga, T.; Kawanaka, H.; Kono, H.; Maehara, Y. Laparoscopic treatment of splenosis: Report of a case. Surg. Today 2009, 39, 1098–1102. [Google Scholar] [CrossRef] [PubMed]
- Deutsch, J.C.; Sandhu, I.S.; Lawrence, S.P. Splenosis presenting as an ulcerated gastric mass: Endoscopic and endoscopic ultrasonographic imaging. J. Clin. Gastroenterol. 1999, 28, 266–267. [Google Scholar] [CrossRef] [PubMed]
- Tsai, L.L.; Kaliannan, K.; Mortele, K.J. Gallbladder splenosis: A hereto unreported mimicker of a gallbladder neoplasm. Clin. Imaging 2015, 39, 318–320. [Google Scholar] [CrossRef] [PubMed]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matsubayashi, H.; Bando, E.; Kagawa, H.; Sasaki, K.; Ishiwatari, H.; Ono, H. A Multinodular Mass of Abdominal Splenosis: Case Report of Uncommon Images of a Rare Disease. Diagnostics 2019, 9, 111. https://doi.org/10.3390/diagnostics9030111
Matsubayashi H, Bando E, Kagawa H, Sasaki K, Ishiwatari H, Ono H. A Multinodular Mass of Abdominal Splenosis: Case Report of Uncommon Images of a Rare Disease. Diagnostics. 2019; 9(3):111. https://doi.org/10.3390/diagnostics9030111
Chicago/Turabian StyleMatsubayashi, Hiroyuki, Etsuro Bando, Hiroyasu Kagawa, Keiko Sasaki, Hirotoshi Ishiwatari, and Hiroyuki Ono. 2019. "A Multinodular Mass of Abdominal Splenosis: Case Report of Uncommon Images of a Rare Disease" Diagnostics 9, no. 3: 111. https://doi.org/10.3390/diagnostics9030111
APA StyleMatsubayashi, H., Bando, E., Kagawa, H., Sasaki, K., Ishiwatari, H., & Ono, H. (2019). A Multinodular Mass of Abdominal Splenosis: Case Report of Uncommon Images of a Rare Disease. Diagnostics, 9(3), 111. https://doi.org/10.3390/diagnostics9030111




